Research Article - Biomedical Research (2017) Volume 28, Issue 2
A novel Glycyrrhiza uralensis polysaccharide-glycinin conjugate synthesized by maillard reaction decreases the antigenicity of glycinin
Jie Chen, Wanchen Li, Mengyuan Wang, Xiao Guo, Hongyang Yang, Xiaoqing Zhu, Kexun Lian, Yan Luo, Xinli Gu*Department of Animal Science and Technology, College of Animal Nutrition and Feed Science, Shihezi University, Shihezi, Xinjiang, China
- *Corresponding Author:
- Xinli Gu
Department of Animal Science and Technology
College of Chinese Veterinary Medicine
Shihezi University, China
Accepted date: June 22, 2016
Abstract
Glycinin is an important food allergen. In this study we aimed to develop a safe and effective method to reduce the antigenicity of glycinin. Glycyrrhiza uralensis polysaccharide (GUP) was extracted and purified. As a glycosyl donor in Maillard reaction, GUP was conjugated to glycinin with reaction conditions optimized based on amino acid binding rate and inhibition of glycinin antigenicity. The results showed that optimal reaction conditions included a ratio of 1:3 for GUP to glycinin, temperature at 55ºC, reaction time of 72 h, humidity of 72% and final concentration of mixed solution of 11%. The inhibition rate of glycinin antigenicity and the rates of allergic diarrhoea and mortality in mice were all the lowest for GUP-glycinin conjugates synthesized under these reaction conditions. The structure of GUP-glycinin conjugates was characterized by UV spectrum, FT-IRand SDS-PAGE analysis. In conclusion, using GUP as a sugar moieties donor for Maillard reaction is an effective method to reduce the antigenicity of glycinin.
Keywords
Glycyrrhiza uralensis polysaccharide, Glycinin, Conjugate, Maillard, Allergy.
Introduction
Glycyrrhiza uralensis is Leguminosae Papilionoideae herbaceous plant, and its main chemical constituents are polysaccharides, triterpene saponin compounds [1,2]. As a kind of immune modulator, Glycyrrhiza uralensis polysaccharide (GUP) has anti-allergic, anti-ulcer, anti-oxidant, protection of gastro intestine and other pharmacological activity [3-7]. In addition, GUP has been used in animal husbandry with a broad application, such as feed additive to enhance the growth of animals, improve immunity and resistance.
Soybean is one of the most important legume plants. Compared with other protein sources, soybean is widely used in the animals’ diet because of its wide source and high nutritional value [8]. The main substance in soybean is glycinin which causes allergic diarrhoea of the piglets, calves and other young animals, leading to their death. The reduction of the antigenicity of glycinin has attracted great attention in recent years. The glycosylation method is relatively simple and safe and has other advantages [9]. Vande et al. showed that dry glycosylation reactions can reduce the antigenicity of glycinin by 60%, but the reaction time is too long [10]. Tian et al. reported that Maillard reaction affected the antigenicity of soybean protein [11]. Because the glycinin was used as sugar moieties donor monosaccharide or oligosaccharide, many amino acids were involved in the reaction, and aldehyde, heterocyclic amines and other harmful intermediate products were produced [12].
In this study we aimed to develop a safe and effective protein production method, by using Chinese herbal polysaccharides as a sugar moieties donor, to reduce the antigenicity of glycinin.
Materials and Methods
Preparation of GUP
The root of G. uralensis powder was purchased from the Traditional Medicine Company in Shihezi (Xinjiang, China), and immerged with 90% ethanol for 48 h to remove impurities and small lipophilic molecules. The dried residue was extracted with water using parameters described previously [13]. After vacuum filtration, concentrated supernatant was mixed with anhydrous ethanol at a final concentration of 85% (v/v), the aqueous extract was removed. The precipitate was gathered by centrifugation (5000 r/min, 10 min), pigment was removed by macro porous resin, sevage method was used for deproteinization, and small molecular weight impurity was removed by dialysis in deionized water. The crude GUP was purified by Sephadex G-75 and DEAE-32 column chromatography (Sigma-Aldrich), and then vacuum dried at 45ºC to get GUP. The extraction rate was 15.1% and sugar content was 88.9%.
Preparation of GUP-glycinin conjugate (GPG)
GUP was conjugated to glycinin using Maillard reaction as described previously [12]. Glycinin was purchased from TransGen Biotech (Beijing, China). Glycinin and GUP were dissolved in distilled water with a ratio of GUP to glycinin at 1:1, 1:3 and 1:5, respectively, and the solution concentration ranged from 8% to 14%. The frozen dried sample was put into the dryer with saturated KBr solution to keep the relative humidity at 65-75%, temperature at 45ºC, 50ºC, or 55ºC, for different reaction time to prepare GPG conjugates.
Formula for calculating the binding rate of amino acids: Y %=(Cbefore-Cafter)/Cbefore × 100%. Type:Y:Amino acid binding rate (%); Cbefore: Occurrence of amino acid content before conjugation reactions (μg/ml); Cafter: Amino acid content occurred after conjugation reactions (μg/ml).
Formula for calculating the inhibition rate of glycinin antigenicity: Inhibition rate of glycinin antigenicity%=(1- ODsample)/ODblank × 100%. Type: ODsample: The sample absorbance value; ODblank: No system of competition absorption value.
UV spectrum scanning
GPG was dissolved in Tris-HCL (50 mmol/l, pH 7.0) at a final concentration of 0.1%. To observe the changes of protein structure, the wavelength scanning was performed in the range of 190-350 nm.
Fourier infrared spectroscopic analysis
GPG samples were prepared as powder and pressed into thin slices. The samples were analysed by Fourier transform infrared point’s spectrophotometric analysis of protein secondary structure of the full band (4000-400 cm-1) scanning.
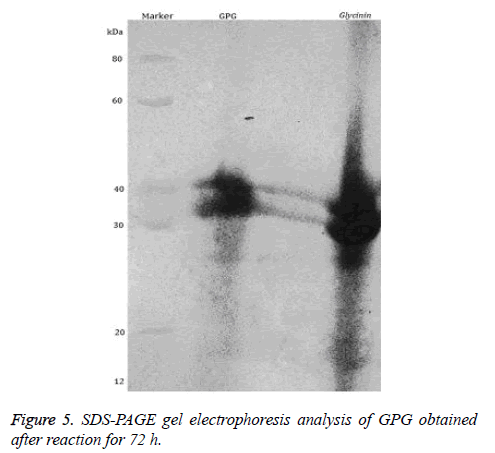
SDS-PAGE gel electrophoresis
SDS-PAGE was performed to separate and identify protein purity. The concentration of separation gel and concentrated gel was 12% and 4%, respectively, gel thickness 1 mm, sample volume 8 μl. The gel was stained by Coomassie brilliant blue R-250.
Evaluation of allergy in the mice
70 healthy female BALB/c mice aged 3 weeks were selected and randomized into 7 groups (n=10). The mice were given free drinking water and treated for seven consecutive days with GPG with different reaction time or saline as the control. Allergy symptoms were observed for 30 to 40 minutes a week later after the mice were orally sensitized three times in each day with 10 mg/ml glycinin.
Statistical analysis
All the data were expressed as mean ± standard deviation. The Tukey method for the multiple comparisons among the groups was used to determine the significant differences. All the samples were measured for three times, and statistical analyses were carried out using SPSS version 17.0.
Results
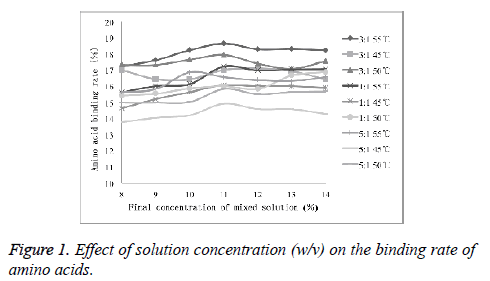
Optimization of the concentration of mixed solution
As shown in Figure 1, with the increasing concentration of the solution, the amino acid binding rate did not change significantly. It was observed that the effect of the final concentration of the mixed solution on conjugation reaction was relatively small. Therefore, 11% was selected as ideal final concentration for conjugation experiments.
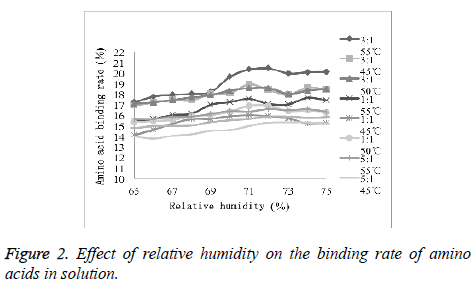
Optimization of reaction humidity
As shown in Figure 2, with the increasing relative humidity, the amino acid binding rate did not change significantly. It was observed that the effect of the final concentration of the mixed solution on conjugation reaction was relatively small. Therefore, 72% was selected as ideal humidity for conjugation experiments.
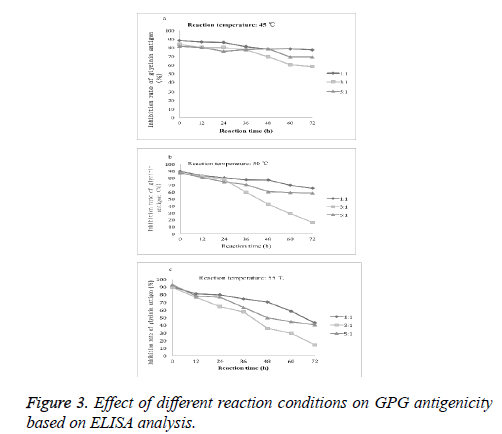
Effect of different conjugate conditions on the antigenicity of glycinin
The reaction temperature, reaction time and the proportion of protein and polysaccharide affect antigenic change of the glycosylated complex. As shown in Figure 3, with the increase of the glycosylation reaction time, the antigenicity of glycinin glycosylated conjugates decreased. At reaction temperature at 45ºC, 50ºC and 55ºC, the lowest inhibition rates of glycinin antigenicity were 57.77%, 16.30% and 14.44%, respectively. Therefore, we chose the best reaction conditions as follows: glycinin and GUP mass ratio at 3:1, reaction temperature at 55ºC and the reaction time of 72 h.
The effects of GPG with different reaction time on glycinin induced allergy of mice
We observed the effects of GPG with different reaction time on glycinin induced allergy of mice Table 1. The diarrhoea incidence rate and mortality rate were the lowest with GPG of the reaction time of 60 h or 72 h.
| Diarrhoea rate (%) | Mortality (%) | |||||||
| Groups (Days) | 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| Glycinin | 65 | 80 | 95 | 100 | 100 | 100 | 100 | 70 |
| GPG (12 h) | 35 | 35 | 30 | 30 | 55 | 55 | 55 | 20 |
| GPG (24 h) | 15 | 20 | 15 | 15 | 20 | 20 | 20 | 15 |
| GPG (36 h) | 15 | 15 | 15 | 10 | 30 | 30 | 20 | 20 |
| GPG (48 h) | 0 | 15 | 0 | 20 | 10 | 0 | 0 | 10 |
| GPG (60 h) | 10 | 0 | 0 | 0 | 0 | 20 | 30 | 5 |
| GPG (72 h) | 0 | 0 | 20 | 15 | 10 | 0 | 0 | 5 |
Table 1: The rate of allergic diarrhea and mortality in the mice of different groups.
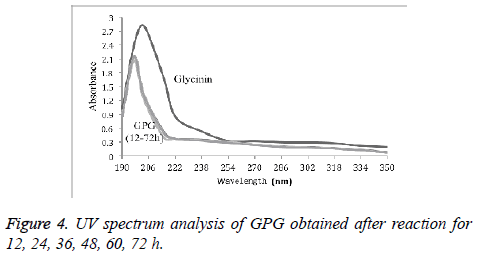
Structure characteristics of GPG
UV spectrum analysis of GPG with different reaction time (12 h, 24 h, 36 h, 48 h, 60 h, and 72 h) was shown in Figure 4. It was found that the UV scanning curves of conjugates with different reaction time were similar compared to that of glycinin protein. There was no absorption peak near 260-280 nm, and the absorption peak near 210 nm was shifted to the short wave direction.
FT-IR is commonly used to detect the change in the peptide structure in the absorption spectra for the protein characteristics of 1600-1700 cm-1 range. 1600-1640 cm-1 was the β-sheet structure; 1640-1650 cm-1 was the disordered structure; 1650-1660 was the α-helix structure; 1660-1700 cm-1 was the β-turn structure. Using Peak Fit 4.20 software we analysed structure change of GPG as shown in Table 2. Compared with glycinin, the content of α-helix, β-sheet and disordered structure of GPG decreased, but the content of the β-turn structure increased.
| Structural changes in the model proteins. | ||||
| Samples | α-helix | β-sheet | β-turn | Disordered |
| structure | ||||
| Glycinin | 14.31 | 35.26 | 37.11 | 13.32 |
| GPG (12 h) | 13.61 | 31.65 | 43.14 | 10.3 |
| GPG (24 h) | 13.37 | 32.42 | 44.27 | 9.94 |
| GPG (36 h) | 13.52 | 32.51 | 44.03 | 9.94 |
| GPG (48 h) | 13.93 | 32.74 | 42.36 | 10.97 |
| GPG (60 h) | 13.03 | 34.69 | 41.29 | 10.99 |
| GPG (72 h) | 13.01 | 35.01 | 41.88 | 10.1 |
Table 2: Analysis of GPG structure based on FT-IR.
Finally, we analysed the protein structure of GPG by SDS-PAGE. We observed two bands of about 42 kDa and 38 kDa as shown in Figure 5. Therefore, GPG is a complex containing two types of subunits.
Discussion
To reduce the antigenicity of protein, masking effect of the introduction of sugar chains on the epitope and heating process to change protein structure could both affect the epitope. In this study we optimized the reaction time, temperature as well as the ratio of glycinin and GUP, and examined the change of the antigenicity of glycosylated products. In general, when the concentration of protein was 8-14% and the relative humidity was 65-75%, the activity of the amino acids in the solution was the best, and the binding rate of amino acids was relatively high, therefore, we chose these conditions.
FT-IR analysis of the conjugates showed that the secondary structures of protein such as β-sheet and α-helix structure were slightly lower. In theory, with the increase of the heating time, the structure of the protein can be disordered. Protein α-helix and β-sheet structures are usually embedded in the interior of polypeptide chain, cross-link with sugars leads to spatial structure change, shielding IgE binding epitopes and changing the antigenicity of soybean protein isolate [11].
SDS-PAGE electrophoresis showed that glycinin had the molecular weight of six polymers at 30-36 kDa, containing acidic subunits (A1a, A1b, A2, A3, A4, A5) and basic subunits (B1a, B1b, B2, B3, B4) and other 11 subunits with two disulphide bonds formed by the combination of two pairs of dimer [14,15]. Coomassie brilliant blue staining showed the obvious migration of GPG and glycinin, and molecular weight of GPG product increased compared to glycinin. These results suggest that each subunit is involved in glycosylation reactions, and the GPG conjugates formed will hide some IgE binding epitopes, thereby reducing glycinin antigenicity of the glycosylation products.
In conclusion, we demonstrate that using GUP as a sugar moieties donor to reduce the antigenicity of glycinin by Maillard reaction is an effective method and has remarkable effect. However, further studies are needed to understand the mechanism by which the antigenicity is reduced and identify the glycosylation sites of Maillard reaction.
Acknowledgements
This study was supported by a grant-in-aid for Youth Graduate Student Innovation Fund from Xinjiang (No. XJGRI2014057). We thank all staffs in the Institute of Animal Nutrition And Feed Science and College of Chinese Veterinary Medicine, Shihezi University, Xinjiang for their assistances in the experiments.
References
- Hayashi H, Inoue K, Ozaki K, Watanabe H. Comparative analysis of ten strains of Glycyrrhiza uralensis cultivated in Japan. Biol Pharm Bull 2005; 28: 1113-1116.
- He J, Chen L, Heber D, Shi WY, Lu QY. Antibacterial compounds from Glycyrrhiza uralensis. J Nat Prod 2006; 69: 121-124.
- Cheng AW, Wan FC, Jin ZY, Xu XM. Nitrite oxide and inducible nitric oxide synthase production was regulated by polysaccharides isolated from Glycyrrhiza uralensis Fisch. J Ethnopharmacol 2008; 118: 59-64.
- Cheng AW, Wan FC, Wang JQ, Jin ZY. Macrophage immunomodulatory activity of polysaccharides isolated from Glycyrrhiza uralensis Fisch Int Immunopharmacol 2008; 8: 43-50.
- Cinatl J, Morgenstern B, Auer GB, Chandra P, Rabenau H, Doerr H. Glycyrrhizin an active component of liquorice roots, and replication of SARS-associated coronavirus. Lancet 2003; 361: 2045-2046.
- Yin SS, Yao Z, Gao WY, Wang J, Man SL, Liu H. Effects of nitrogen source and phosphate concentration on biomass and metabolites accumulation in adventitious root culture of Glycyrrhiza uralensis Fisch Acta Physiol Plant 2014; 35: 1579-1585.
- Wan FC, Cheng AW. Polysaccharide isolated from Glycyrrhiza uralensis Fisch induces intracellular enzyme activity of macrophages. Mediterr J Nutr Metab 2009; 1: 165-169.
- Bu GH, Zhu TW, Chen FS. Antigenicity and structural properties of soybean protein-glucose conjugates. Moder Food Sci Technol 2014; 11: 28-33.
- Li Z, Luo YK, Feng G, Liu XY, Zhang Y. Study on preparation and antigenicity of wpi-isomalto-oligosaccharide conjugate. J China Agr Univ 2011; 16: 133-137.
- Vande LJ, Manuel SFJ, Javier M, Agustin O. In vitro glycation and antigenicity of soy proteins. Food Res Int 2007; 40: 153-160.
- Tian SJ, Chen J, Small DM. Enhancement of solubility and emulsifying properties of soy protein isolates by glucose conjugation. J Food Process Pres 2011; 35: 80-95.
- Sun Y, Hayakawa S, Puangmanee S, Izumori K. Chemical properties and antioxidative activity of glycated α-lactalbumin with a rare sugar, D-allose, by Maillard reaction. Food Chem 2006; 95: 509-517.
- Cui GT, Zhang WX, Wang QJ, Zhang AM, Mu HB, Bai HJ, Duan JY. Extraction optimization, characterization and immunity activity of polysaccharides from Fructus Jujubae. Carbohydr Polym 2014; 111: 245-255.
- Guo PE, Piao S, Cao YH, Ou D, Li D. Recombinant soybean protein B-conglycininA-subunit expression and induced hyper sensitivity reaction in rats. Int Arch Allergy Immunol 2008; 145: 102-110.
- Hao Y, Li DF, Piao XL. Forsythia suspensa extract alleviates hypersensitivity induced by soybean beta-conglycinin in weaned piglets. J Ethnopharmacol 2010; 128: 412-418.