Review Article - Journal of Dermatology Research and Skin Care (2017) Volume 1, Issue 1
A novel efficient and safe treatment for atopic dermatitis: Topical superoxide dismutase (SOD).
Christian Diehl*
Department of Dermatology, University of Guglielmo Marconi, Italy
- Corresponding Author:
- Christian Diehl
Department of Dermatology
University of Guglielmo Marconi
Italy
Tel: 41763992637
E-mail: chdiehl@hotmail.com
Accepted Date: August 01, 2017
Citation: Diehl C. A novel efficient and safe treatment for atopic dermatitis: Topical superoxide dismutase (SOD). Dermatol Res Skin Care. 2017;1(1):1-7
Abstract
Atopic dermatitis (AD) is a chronic inflammatory skin disease affecting more than 20% of children and 7% of adults in the world, with a wide variation worldwide. The prevalence of AD is increasing, probably due to the higher levels of pollution. Among the symptoms of AD (eczematous skin lesions with lichenification and excoriation, dry skin and a susceptibility to skin infections), pruritus is probably the major concern for the patients, as it can impact negatively their quality of life. Pathogenesis of AD, although not completely elucidated, comprises a combination of genetic, environmental and immunological factors. Involvement of oxidative stress, defined as the formation of oxidants in the cells of the human body acutely or chronically exceeding the antioxidant defence capacities, in the pathogenesis of AD has long been suggested and is now widely accepted. Superoxide dismutase (SOD) is a potent enzymatic antioxidant found in most living organisms. Besides its antioxidant properties, SOD is also displaying an anti-inflammatory and immunomodulating activity. Various clinical studies are reporting the use of topical SOD in AD, and concluded with its efficacy and tolerability in this indication. Interestingly, SOD has a potent antipruritic activity which is useful in the treatment of AD. In spite of its high molecular weight, the skin absorption of SOD was demonstrated, probably through the skin appendages.
Keywords
Atopic dermatitis, Pruritus, Eczema, Dermatitis, Chemokine, Oxidative stress.
Introduction
Skin is the largest organ of the body, it provides a protection to the organism against a broad spectrum of physical (i.e., external radiations such as ultraviolet (UV), visible light and infrared) or exogenous chemical agents. Among other factors, the skin is the first line of defence against the reactive oxygen species (ROS) which are implicated in numerous diseases, and especially in various skin disorders [1].
This article is designed to describe a novel efficient and safe topical treatment for atopic dermatitis (AD) based on the use of creams with superoxide dismutase (SOD).
Atopic Dermatitis (AD)
AD is a chronic inflammatory skin disease featuring typically distributed eczematous skin lesions with lichenification, pruritic excoriations, dry skin and a susceptibility to skin infections.
Epidemiology
Many studies have been performed to determine the prevalence of childhood AD around the world. We don’t intend to make an exhaustive report of these studies here, but only to summarize the most prominent results. Some of the best estimates of AD prevalence internationally were generated from the International Study of Asthma and Allergies in Childhood (ISAAC). Odhiambo et al. analysed data from 385,853 participants ages 6 to 7 and 663,256 participants ages 13 to 14 in the ISAAC Phase 3 study [2]. They found a wide variation in prevalence values worldwide, from 0.9% in India to 22.5% in Ecuador at ages 6 years to 7 years and from 0.2% in China to 24.6% in Colombia at ages 13 years to 14 years. Interestingly, comparison of prevalence estimates between Phases 1 and 3 of the ISAAC study (i.e., roughly between 1998 and 2008) suggests increasing prevalence of AD among 6 year olds to 7 year olds in both developing and developed nations and increasing prevalence in 13 year olds to 14 year olds in developing nations [3]. The ISAAC study along with other smaller population-based and/ or community-based studies suggests higher AD prevalence in wealthier, developed nations compared with poorer, developing nations [4]. The conventional dogma has long been that AD is a disorder of childhood, with few adults having active disease. Several recent studies suggest, however, that AD may be more common in adults than previously recognized. International studies of AD in adults found prevalence ranging from 2.0% to 6.9% prior to 2000 depending on regional and methodological differences [4]. All these data taken together emphasize the high prevalence of this disease, and the urgency of completing the current armamentarium for treating this common disorder.
Symptoms
AD is a chronic and relapsing disease usually starting in early childhood. AD can be the initial step of the so-called atopic march, i.e., followed by allergic rhinitis and allergic asthma [5]. AD offers a wide clinical spectrum ranging from minor forms to major forms with erythrodermic rash. The essential features are pruritus, facial and extensor eczema in infants and children, flexural eczema in adults and chronic or relapsing dermatitis [6]. Frequently associated features include personal or family history of atopic disease, xerosis, cutaneous infections and nonspecific dermatitis of the hands or feet [6]. Acute and sub-acute skin lesions are often seen in children and are characterized by intensely pruritic erythematous papules associated with excoriation and serous exudate. Chronic atopic dermatitis is characterized by lichenification, papules, and excoriations [6]. The distribution and skin reaction pattern varies according to the patient’s age and disease activity. During infancy, atopic dermatitis is generally more acute and mainly affects the face, scalp, and extensor surfaces of the extremities. In older children and in those who have longstanding skin disease, the patient develops lichenification and localisation of the rash to the flexural folds of the extremities. Chronic hand eczema can be the primary manifestation of many adults with AD [6]. Dry skin is a common feature in all patients with AD, and intense pruritus is a key factor, which explains why patients suffering AD, but also their relatives, have an impaired quality of life.
Update about pathogenesis
Pathogenesis of AD is based on a combination of genetic, environmental and immunological factors. Skin barrier dysfunction is a major pathogenic factor of AD [7]. Strongest genetic association with AD has been demonstrated for loss-offunction mutations in the filaggrin (FLG) gene, which encodes the important barrier protein (pro-)filaggrin [8]. Filaggrin aggregates keratin filaments into compact bundles and modifies the composition of keratinocytes and the granular cell layer [9]. It interacts with lamellar bodies and reduced availability of filaggrin metabolites leads to changes of skin hydration and skin pH [10]. Environmental factors including skin irritation and mechanical damage, low humidity but also the cytokine milieu in the skin with reduction of filaggrin expression by Th2 cytokines, IL-17, IL-22, IL-25 or IL-31 as well as micro-organisms colonizing the skin and topical and systemic treatment are capable to modulate filaggrin expression secondarily [10]. Expression and function of pattern recognition receptors on skin cells in AD is altered due to genetic modification but also due to secondary factors such as cytokines and chemokines in the micromilieu [7]. Tolllike receptors (TLR) signaling is not only important for host defence mechanisms of the innate immune system but also for the epidermal barrier function [11]. A recent study demonstrates that activation of TLR2 on dendritic cells (DC) converts Th2 type dermatitis to chronic cutaneous inflammation in a mouse model [12]. Inflammatory AD skin contains vast numbers of resident and infiltrated immune cells, such as Langerhans cells (LC), inflammatory epidermal dendritic cells (IDECs), monocytes (MC), neutrophils, basophils, eosinophil, innate lymphoid cells (ILCs), natural killer cells, fibroblast and various T cell subsets [7]. Among skin immune cells, DCs are the most important cell type to initiate Th2 immune responses [13]. Inflammatory DCs are regarded as main amplifier of allergic inflammation on the level of DCs [7]. In vitro studies demonstrated that allergen challenge of inflammatory DC subtypes increases the release of pro-inflammatory cytokines and chemokine’s [7]. Current results support a model in which LCs provide important regulatory functions in a steady state, while they are also capable to contribute to T cell responses upon activation [7]. Attenuate IFN-? and TGF-ß signaling and responsiveness of human monocytes from AD patients might promote Th2 immune responses due to decreased TGF-ß signaling on one hand and impaired Th1 cytokine IFN-? stimulation on the other hand [7]. MCs are the key cells to mediate type I hypersensitivity reactions. ILCs have also been observed to infiltrate AD skin and to release Th2 type cytokines IL-5, IL-9 and IL-13 to promote local inflammation after stimulation with allergen, TSLP or IL-33 [14]. Upon stimulation with allergens, skin barrier derived-TSLP stimulates DCs to derive Th2 responses, and infiltration of Th2 T cells into acute AD skin, together with moderate infiltration with Th22 cells and few Th17 cells was reported [7].
Role of ROS in AD
Oxidative stress is defined as the formation of oxidants in the cells of the human body that acutely or chronically exceeds the antioxidant defense capacity, and involvement of ROS in the pathogenesis of AD has long been suggested. Oxidative stress has been implicated in atopic dermatitis for more than 15 years, mainly in the following three aspects: (1) the presence of oxidative stress; (2) increased oxidative stress during AD exacerbation; and (3) decreased antioxidant capability [15]. Urine markers of oxidative stress are altered in children with AD, including 8-OHdG, nitrite/nitrate and selenium, and those marker levels are higher in children with AD than that in non- AD children [16]. In preschool age children with AD, blood total antioxidant capacity was found to be significantly less, while malondialdehyde was higher in those patients [17]. In case-control studies on eczema patients with healthy individuals as controls, it was found that, compared to the control group, patients with eczema had a significantly higher level of lipid peroxidation by measuring serum malondialdehyde (MDA), and lower levels of antioxidants including vitamins A, C, and E [18]. In children with acute exacerbation of AD, urinary glycosylation end products and bilirubin oxidative metabolites were significantly higher in AD children compared with controls. Further, response to treatment was associated with significant falls in the concentrations of these markers [19]. Nakai et al. [20] also demonstrated that urine nitrate and MDA levels correlate with the severity of AD. A first exogenous cause of oxidative stress is air-pollution and not surprisingly, atopic dermatitis is increasing worldwide in correlation with air pollution. Recently, it was demonstrated that activation of aryl hydrocarbon receptor (AhR) in keratinocytes was induced by air pollutants, namely polycyclic aromatic hydrocarbons (PAHs), the main organic constituents of particulate matter, and that this was sufficient to induce various AD-like phenotypes, including percutaneous sensitization and systemic complications of AD [21]. A relationship was also demonstrated between AD occurrence and gestational exposure to CO, where exposure during the first trimester seemed to be the most important [22]. Another source of air pollution is smoking. A systematic review and meta-analysis of observational studies concluded that active and passive exposure to smoke was associated with increased AD prevalence [23]. Another source of oxidative stress might be skin microbes; the normal as well as diseased skin of AD patients is markedly colonized with Staphylococcus aureus [15].
Monocytes from patients with atopic dermatitis are primed to generate ROS in response to zymosan, a Toll-like receptor 2 (TLR2) ligand, suggesting that Staphylococcus aureus may damage lesional skin of the disease by production of ROS [24]. Psychological stress is a well-known cause of oxidative stress [25] and causes abnormal skin barrier function in humans and is a frequent cause of AD flares. The hallmark of AD is dermal inflammation in affected areas, which could be enhanced by oxidative stress [15]. It is known that oxidative stress can activate nuclear factor kappa-B (NF-κB) pathways, inducing expression of pro-inflammatory cytokines, such as IL-6, IL-8, IL-9, and IL-33, which in turn enhances dermal inflammatory infiltrate and histamine release in the affected skin to worsen symptoms [26]. Oxidative stress can directly cause damage to epidermal keratinocytes by DNA damage, damage of cellular enzymes, or damage to cell membrane structures through lipid oxidation [15].
Superoxide dismutase (SOD)
Given the association of oxidative stress with other factors in the development and maintenance of AD, it is worthwhile to consider incorporating strategies in reducing oxidative stress in managing AD. This is possible through the use of topical antioxidants, such as SOD.
Generalities about SOD
Fridovich and McCord discovered the enzymatic activity of superoxide dismutase in 1968. There are three major families of superoxide dismutase, depending on the protein fold and the metal cofactor: the Cu/Zn type (which binds both copper and zinc), Fe and Mn types (which bind either iron or manganese), and the Ni type (which binds nickel). Copper and zinc are most commonly used by eukaryotes, including humans. Iron or manganese is used by prokaryotes and protists, and in mitochondria and chloroplasts. Nickel is a prokaryotic form. Three forms of superoxide dismutase are present in humans and in all other mammals: SOD1 is located in the cytoplasm, SOD2 in the mitochondria, and SOD3 is extracellular.
SOD as an anti-oxidant
Superoxide dismutase (SOD) is an ubiquitous antioxidant enzyme, defined as the first line of antioxidant defence of the body, known as a primary antioxidant. As an enzyme, SOD exhibits a very high catalytic rate of reaction and is constantly renewing itself [27]. This mode of action is radically different from the so-called secondary antioxidants (vitamin C, vitamin E, glutathione, carotenoids, polyphenols, mineral, etc.), which are quickly exhausted with no possibility of renewal [28]. SOD converts the extremely reactive superoxide anion O2•− into hydrogen peroxide H2O2. By disrupting O2•−, SOD prevents the liberation of free iron ions and, thus, the formation of harmful ROS such as OH•. At the same time, SOD protects the vascular signalisation from NO• by preventing its reaction with O2•− and the formation of peroxinitrite ONOO–, a harmful reactive nitrogen species (RNS) [27].
SOD as an anti-inflammatory factor
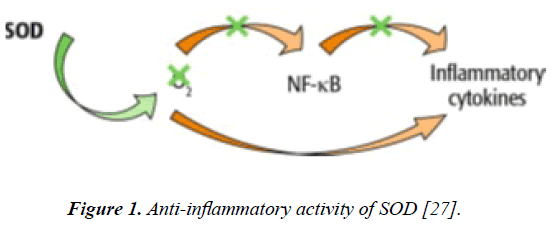
There are numerous studies reporting the anti-inflammatory properties of SOD. One mechanism of action of SOD against inflammation is related to modulation of the expression and distribution of angiogenic factors and inflammatory mediators [29]. More precisely, these authors showed that the antiangiogenic and anti-inflammatory effects of SOD might be due to suppression of hypoxia-inducible factor-1a (HIF-1α), protein kinase C (PKC) and NF-κB expression (Figure 1). Thanks to this specific mechanism of action, SOD is able to inhibit expression of VEGF, MMPs (by increasing the expression of tissue inhibitor of matrix metalloproteinases (TIMP)) and other proangiogenic and pro-inflammatory mediators [27]. Consistently, NF-κB-dependent expression of pro-inflammatory enzymes such as COX-2 and iNOS is also decreased in the skin [30].
SOD in immunity process
Kim et al. [29] and Kwon et al. [30] also demonstrated that SOD participates in the immune response by blocking immune cell infiltration and inhibiting leukocyte–endothelium adhesion. SOD blocks this cascade by inhibiting NADPH oxidase activity and the subsequent O2•− production. Consequently, TLR4-mediated NF-kB trans-activation and the expression of inflammatory cytokines is impaired, alleviating skin inflammation. In addition, SOD (SOD3) may control the adaptive immune response by inhibiting dendritic cell maturation [30]. It is likely that SOD may control T-cell-mediated immune diseases, including and in particular inflammatory diseases.
Clinical Trials with topical SOD in AD
For the above mentioned reasons, it was legitimate testing topical SOD in AD patients. To the best of our knowledge, two studies were published.
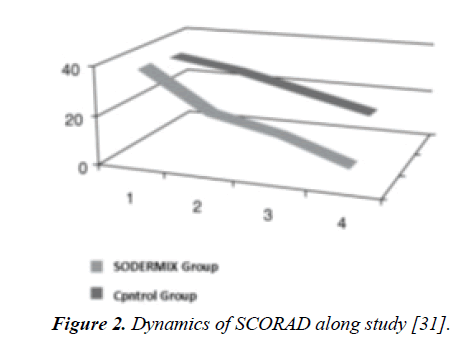
Study by Umanets et al. [31]: In this trial performed in Ukraine, the aim was to examine the efficacy and tolerability of a cream with SOD (SODERMIXTM) in combination treatment of children with AD. This was an open, randomized; parallelgroup clinical trial involving 67 children aged 2-8 years with AD at mild and moderate stage. Screening of patients (at the first visit) included assessment of the main criteria for inclusion: children older than 2 years with documented diagnosis of mild to moderate severity AD, isolated or complemented by other allergic disorders (bronchial asthma, allergic rhinitis), AD severity SCORAD index > 40 points and signed informed parental consent to participate in the study. All children included in the study were randomized into two groups: study and control. The main group of children (35 patients) was applied an emollient and the product under study 2xday. In the control group (32 children) emollient alone was applied. All children included in the study were viewed once a week for 4-week course of treatment. The criteria of treatment efficacy were as follows: (1) the dynamics of improvement of symptoms scored on international scale SCORAD [32] and the index of overall assessment by investigators IGA (Investigator's Global Assessment). Tolerability of the product was also assessed. Clinical features of AD in the children included in this study corresponded to erased erythematous form in the majority (86.6%) of children and was characterized by the presence in the background of severe dry skin, itch, papules, and secondary elements (crusts, peeling, skin excoriations) signs of lichenification in the elbow and popliteal folds. Among the children, the combination treatment topical SOD + emollient contributed to gross significant regression of the main symptoms of AD already in the first week of treatment due to significant reduction of itching of the skin (in 27 (77.1%) children at the 4-5th day of application); decrease in the intensity of skin lesions and improvement of dry skin (in 30 (85.7%) children at the 5-6th day of treatment), reduction in the number and size of papules (in 29 (82.9%) children after 2 weeks of treatment), and disappearance of rash in 100.0% of children at the end of treatment. The children in the control group also noted positive dynamics of clinical symptoms of AD, but less pronounced than the children of the main group: persistent itching and rashes, and new occurrences requiring treatment with topical corticosteroids in 2/3 children in this group. Positive dynamics of clinical symptoms observed in AD children was confirmed by evaluation of SCORAD index, which was significantly reduced only in children with combination treatment (from baseline 38.0 ± 1.3 to 6.9 ± 1.2, p< 0.05) compared with children in the control group (from baseline 36.5 ± 2.1 to 20.0 ± 1.4), (Figure 2). A similar trend was observed with respect to IGA index (from baseline 1.50 ± 0.02 to 0.90 ± 0.01 in combination treatment group, compared to baseline 1.60 ± 0.02 to 1.20 ± 0.01 in control group). When monitored for four weeks the treatment with topical SOD showed good tolerability of the cream as evidenced by the absence of side effects. The authors concluded that the inclusion of combination treatment with topical SOD cream in children with AD helps to increase the effectiveness of the treatment directed to control itching and improved the reparation of the skin.
Figure 2: Dynamics of SCORAD along study [31].
Study by Protsenko et al. [33]: This open comparative clinical study included observations on 61 patients aged between 12 and 44 years, including 24 patients with plaque-type psoriasis and 37 patients with AD at light or moderate stage, receiving NB- 311nm phototherapy. The sequence of the diagnostic algorithm includes the following steps: history and analysis of previous therapy; checking of comorbidities and stages of their activity; dermatological examination with the determination of skin condition and severity of dermatosis and then calculating the SCORAD index for patients with AD or PASI in patients with psoriasis. Narrowband phototherapy was performed using «GH- 8ST» equipment (Germany) in the following way: 3-4 sessions a week for a course of 16-24 procedures. Starting dose of 0.231 J/cm2, the dose was increased at each subsequent procedure by 0.1-0.2 J/cm2 until reaching 2,957 J/cm2. The patients were divided into two groups. The first group - 29 patients - (PS - 10, AD - 19), was submitted to a dual therapy which included, along with narrowband phototherapy, a SOD cream. The control group, 32 patients (PS – 14, AD - 18), was submitted to narrowband phototherapy combined with a soothing baby cream. The tolerability of this method, its time-efficiency on itching, rashes, inflammatory component, infiltration and the onset of clinical remission were evaluated. Regarding AD, 37 patients were included (12 men and 25 women aged 12 to 28 years, mean age 21,5 ± 2,8 years). 19 patients were treated with the combination treatment and 18 were constituting the control group. A significant reduction in inflammation rashes and itching were noted at the termination of 5-day treatment in 12 (63.15%) patients under dual therapy that involved the use of topical SOD, and only 10 (55.55%) patients in the control group. Clinical remission on the 14th day of treatment was achieved in 13 (68.4%) patients of the study group vs. 8 patients (44.4%) in the control group. At D21 of treatment, clinical remission was achieved in 18 of 19 patients of the study group (94.7%) and in 14 of 18 patients (77.8%) in the control group. Similarly to the first study, no side effect was reported. The authors concluded that topical SOD provides high efficiency in the complex treatment of patients with mild and moderate forms of AD, enhances the therapeutic effect of phototherapy, is well tolerated and can be widely used in cosmetic and dermatological outpatient practices, especially in children and patients with steroidophoby.
SOD in pruritus
As we know, pruritus is a common hallmark in AD. Controlling pruritus is an important but still challenging issue in the management of AD, as this is one of the major factors in the impairment of the quality of life in the patients with AD and their relatives. We had previously investigated the antipruritic effect of topical SOD in a sensory evaluation [34]. In this randomized intra-individual study including 15 volunteers, a SOD cream was evaluated by recording thermal sensitivity levels using a Cutaneous Thermal Sensitivity Analyzer (CTSA) before and 30 and 90 minutes after the product application. Thus the antipruritic effect was determined by comparison of variations in the period of pruritus occurrence, its length and intensity before and after the application of the product versus placebo zone without any cream. In summary, SOD cream significantly delayed the onset of pruritus 30 minutes after application; pruritus appeared 35 seconds (mean) later on the treated zone then on the non-treated one (p=0.040). Length of pruritus was also significantly reduced at 30 minutes from the application; the length of pruritus was reduced by 41 seconds (mean) on the treated zone compared with control (p=0.042). Intensity of pruritus was also significantly decreased at 90 minutes from the application; the intensity of pruritus was less pronounced (-1±0.4) on the treated zone (p=0.026) than on control area. According to the results of this study, we demonstrated the antipruritic effect of SOD cream, which could prove useful for local supportive therapy of pruritogenic dermatoses. In another paper we intended understand the mechanism of action of topical SOD in reducing pruritus [35]. It seems that this antipruritic activity is due to the action of SOD on various factors responsible for the occurrence and maintenance of pruritus. It was shown on mice that oxidative stress by different oxidants can induce profound scratching behaviour, which is largely histamine and TRPV1-independent but TRPA1-dependent [36]. By the examination of maternal plasma levels of various antioxidants in intrahepatic cholestasis of pregnancy (ICP), characterized by skin pruritus and normal pregnancy controls, it was observed that the clinical severity of ICP was closely related to the degree of lipid peroxidation, and suggested that the natural antioxidant system might fail to work effectively in the presence of lipid peroxidation damage in ICP [37]. Another study in rats provided the evidence that oxidative stress upregulates proteinase-activated receptors-2 (PAR 2) in endothelial cells [38]. It was also shown that antioxidants such as N-acetyl-L cysteine (NAC) and N-tertbutyl- a-phenylnitrone (PBN) significantly alleviated compound 48/80- and chloroquine-induced acute itch in a dose-dependent manner, attenuated dry skin-induced chronic itch, and suppressed oxidative stress in the affected skin [39]. There are no literature data permitting to suspect any activity or interference of SOD with H1- or H4-receptors, or with PAR-2, or with any other receptors involved in pruritus. On the contrary, a lot of possible interferences of SOD with numerous mediators of pruritus can be detected from the published data. In neurogenic plasma exudation enhanced by substance P (SP) in guinea pig lungs, SOD was found to significantly inhibit this neurogenic plasma leakage, suspecting a possible antagonist effect of SOD on neurokinin-1 (NK-1) receptors which could be in favour of alleviating pruritus [40]. It is of great relevance to note that SOD was shown to clearly decrease the CGRP mRNA expression in a rat model of ischemic facial paralysis [41], strongly suggesting a regulatory role of SOD on calcitonin gene-related peptide (CGRP), a well identified mediator of pruritus. Regarding neurotrophins, there are no data on a direct influence of SOD on nerve growth factor (NGF) but it was demonstrated that potent pro-inflammatory cytokines like TNF-α, IL-1 and interferon-? (IFN-?) can up-regulate the cutaneous expression of NGF, and may thus contribute to the vicious cycle of proinflammatory events maintaining and promoting inflammatory skin diseases, besides pruritus [42]. As all these proinflammatory cytokines are down-regulated by SOD [35], SOD will indirectly contribute to the decrease of NGF levels. There is also an obvious efficacy of SOD in reducing or even suppressing NO production and diminishing iNOS expression, being established that NO has a key role in the occurrence and maintenance of pruritus [35].
What about the cutaneous absorption of SOD?
Cutaneous absorption is a major concern when we consider topical treatment. SOD is a big molecule, whose molecular weight ranges around 32.5 kDa. Dermatologists argue that the molecular weight of a compound must be fewer than 500 Dalton to allow skin absorption and that larger molecules cannot pass the corneal layer [43]. Arguments for this ‘‘500 Dalton rule’’ are; 1) virtually all common contact allergens are under 500 Dalton, larger molecules are not known as contact sensitizers; 2) the most commonly used pharmacological agents applied in topical dermatotherapy are all under 500 Dalton; 3) all known topical drugs used in transdermal drug-delivery systems are under 500 Dalton [43]. However, using fluorescent labelling of the SOD with fluorescein isothiocyanate, epifluorescence microscopy and digital imaging processing, Emerit et al. [44] showed that SOD applied to the skin surface penetrates through the follicular appendages, as well as through the unbroken stratum corneum.
Conclusion
Skin is a highly metabolic tissue with the largest surface area in the body. The skin tissue is exposed to a variety of damaging threats coming from the outer environment, among them oxidative stress. Oxidative stress and altered antioxidant defences are involved in the pathophysiology of acute exacerbation of AD. As a potent antioxidant, but also anti-inflammatory, topical SOD appeared to be a potential candidate for the treatment of AD. On the other hand, one of the most disturbing symptoms of AD is pruritus and it was previously shown that topical SOD possessed a potent antipruritic activity. The clinical trials currently available using topical SOD in AD patients show its efficacy and tolerability. In an indication where there is paucity of really satisfactory and efficient treatments, the use of topical SOD is worth of interest and more studies, with a larger number of patients, would be welcomed.
References
- Briganti S, Picardo M. Antioxidant activity, lipid peroxidation and skin diseases. What's new. J Eur Acad Dermatol Venereol.2003;17(6):663-9.
- Odhiambo JA, Williams HC, Clayton TO, et al. Global variations in prevalence of eczema symptoms in children from ISAAC Phase Three. J Allergy Clin Immunol.2009;124(6):1251-8.
- Williams H, Stewart A, Von Mutius E, et al. Is eczema really on the increase worldwide? J Allergy Clin Immunol. 2008;121(4):947-54.
- Silverberg JI. Public health burden and epidemiology of atopic dermatitis. Dermatologic Clinics. 2017; 35(3):283-9.
- Bieber T. Atopic dermatitis. Ann Dermatol 2010; 22(2):125-137.
- Leung DYM, Bieber T. Atopic dermatitis. Lancet 2003; 361: 151–60.
- Peng W, Novak N. Pathogenesis of atopic dermatitis. Clin Exp Allergy. 2015; 45(3):566-74.
- Irvine AD, McLean WI, Leung DY. Filaggrin mutations associated with skin and allergic diseases.. 2011 N Engl J Med;365 (14):1315-27.
- Thyssen JP. Atopic dermatitis, filaggrin mutations and irritant contact dermatitis. Br J Dermatol. 2013;168(2):233-4.
- Thyssen JP, Kezic S. Causes of epidermal filaggrin reduction and their role in the pathogenesis of atopic dermatitis. J Allergy Clin Immunol 2014; 134:792–799.
- Volz T, Skabytska Y, Guenova E, et al. Nonpathogenic bacteria alleviating atopic dermatitis inflammation induce IL-10-producing dendritic cells and regulatory Tr1 cells. J Invest Dermatol.2014; 134:96–104.
- Kaesler S, Volz T, Skabytska Y, et al. Toll-like receptor 2 ligands promote chronic atopic dermatitis through IL-4-mediated suppression of IL-10. J Allergy Clin Immunol 2014; 134:92–99.
- Novak N. An update on the role of human dendritic cells in patients with atopic dermatitis. J Allergy Clin Immunol 2012; 129:879–886.
- Imai Y, Yasuda K, Sakaguchi Y, et al. Skin-specific expression of IL-33 activates group 2 innate lymphoid cells and elicits atopic dermatitis-like inflammation in mice. Proc Natl Acad Sci. 2013; 110:13921–13926.
- Ji H, Li XK. Oxidative stress in atopic dermatitis. Oxid Med and Cell Long 2016; 2721469:1-8.
- Omata N, Tsukahara H, Ito S, et al. Increased oxidative stress in childhood atopic dermatitis. Life Sciences 2001; 69(2):223–228.
- Chung J,Oh SY, Shin YK. Association of glutathione-S-transferase polymorphisms with atopic dermatitis risk in preschool age children. Clin Chem Lab Med. 2009; 47(12):1475-81.
- Amin MN, Liza KF, Sarwar MS et al. Effect of lipid peroxidation, antioxidants, macro minerals and trace elements on eczema. Arch Dermatol Res 2015; 307(7):617–623.
- Tsukahara H, Shibata R, Ohshima Y, et al. Oxidative stress and altered antioxidant defenses in children with acute exacerbation of atopic dermatitis. Life Sci. 2003; 72(22):2509-16.
- Nakai K, Yoneda K, Maeda R, et al. Urinary biomarker of oxidative stress in patients with psoriasis vulgaris and atopic dermatitis. J Eur Acad Dermatol Venereol. 2009; 23(12):1405-8.
- Hidaka T, Ogawa E, Kobayashi EH, et al. The aryl hydrocarbon receptor AhR links atopic dermatitis and air pollution via induction of the neurotrophic factor artemin. Nat Immunol. 2017; 18(1):64-73.
- Huang CC, Wen HJ, Chen PC, et al. Prenatal air pollutant exposure and occurrence of atopic dermatitis. Br J Dermatol. 2015; 173(4):981-8.
- Kantor R, Kim A, Thyssen JP, et al. Association of atopic dermatitis with smoking: A systematic review and meta-analysis. J Am Acad Dermatol. 2016; 75(6):1119-1125.
- Okayama Y. Oxidative stress in allergic and inflammatory skin diseases. Curr Drug Targets Inflamm Allergy.2005; 4(4):517-9.
- Wright RJ, Cohen RT, Cohen S. The impact of stress on the development and expression of atopy. Curr Opin Allergy Clin Immunol. 2005; 5(1):23–29.
- Wullaert A, Bonnet MC, Pasparakis M. NF-κB in the regulation of epithelial homeostasis and inflammation. Cell Res. 2011; 21(1):146–158.
- Le Quéré S, Lacan D, Lemaire B. The role of superoxide dismutase (SOD) in skin disorders:A review. Nutrafoods 2014; 13:13-27.
- Bafana A, Dutt S, Kumar A et al. The basic and applied aspects of superoxide dismutase. J Molec Catal B 2011; 68(2):129–138.
- Kim Y, Kim BH, Lee H, et al. Regulation of skin inflammation and angiogenesis by EC-SOD via HIF-1α and NF-κB pathways. Free Radic Biol Med 2011; 51(11):1985–1995.
- Kwon M-J, Kim B, Lee YS, et al. Role of superoxide dismutase 3 in skin inflammation. J Dermatol Sci 2012; 67(2):81–87.
- Umanets TR, Lapshin VF, Tzhertzh LM, et al. Atopic dermatitis in children: Optimization of antipruritic therapy. Perinatol Paediatr .2013; 3(55):1-5.
- Kunz B, Oranje AP, Labreze L, et al. Clinical validation and guidelines for the SCORAD index: Consensus report of the European Task Force on Atopic Dermatitis. Dermatol.1997;195(1):10-9.
- Protsenko TV, Protsenko OA, Chornovil AC, et al. Ability to control skin condition in patients with chronic dermatoses with a background of phototherapyClin Immunol. Allergol. Infectiology. Special Issue ? 1. 2014; 1-3.
- Diehl C, Vaurillon E, Lipozenci? J. Antipruritic effect of natural superoxide dismutase--sensory evaluation. Acta Dermatovenerol Croat. 2009; 17(3):217-25.
- Diehl C, Lipozenci? J, Ledi?-Drvar D. The basis of topical superoxide dismutase antipruritic activity. Acta Dermatovenerol Croat. 2009; 17(1):25-39.
- Liu T, Ji RR. Oxidative stress induces itch via activation of transient receptor potential subtype ankyrin 1 in mice. Neurosci Bull. 2012; 28(2):145-54.
- Hu YY, Liu JC, Xing AY. Oxidative stress markers in intrahepatic cholestasis of pregnancy: a prospective controlled study. Eur Rev Med Pharmacol Sci. 2015; 19(17):3181-6.
- Aman M, Hirano M, Kanaide, et al. Upregulation of proteinase-activated receptor-2 and increased response to trypsin in endothelial cells after exposure to oxidative stress in rat aortas. J Vasc Res. 2010; 47(6):494-506.
- Zhou FM, Cheng RX, Wang S, et al. Antioxidants Attenuate Acute and Chronic Itch: Peripheral and Central Mechanisms of Oxidative Stress in Pruritus. Neurosci Bull. 2017; 33(4):423-435.
- Lin SM, Hwang KH, Lin HC, et al. Endotoxemia augments neurogenic plasma exudation in guinea pig lungs. Chang Gung Med J. 2000;23(1):14-21.
- Mohri D, Satomi F, Kondo E, et al. Change in gene expression in facial nerve nuclei and the effect of superoxide dismutase in a rat model of ischemic facial paralysis. Brain Res. 2001;893(1):227-36.
- Bläsing H, Hendrix S, Paus R. Proinflammatory cytokines upregulate the skin immunoreactivity for NGF, NT-3, NT-4 and their receptor, p75NTR, in vivo. A preliminary report. Arch Dermatol Res 2005; 96:580-4.
- Bos JD, Meinardi MM. The 500 Dalton rule for the skin penetration of chemical compounds and drugs. Exp Dermatol. 2000; 9(3):165-9.
- Emerit I, Filipe P, Freitas J, et al. Protective effect of superoxide dismutase against hair graying in a mouse model. Photochem Photobiol. 2004; 80(3):579-82.