Research Article - Biomedical Research (2022) Volume 33, Issue 3
A novel approach of tramadol hydrochloride ethosomal gel for topical drug delivery system
Sunayana Tyagi1*, Lovely Chaurasia1, Mojahidul Islam2, Bhumika Sagar3
1Department of Pharmaceutics, IIMT College of Medical Science, IIMT University Meerut 250001, Uttar Pradesh, India
2Department of Pharmaceutical Chemistry, IIMT College of Medical Science, IIMT University Meerut 250001, Uttar Pradesh, India
3Department of Pharmacolgy ,IIMT College of Medical Sciences, IIMT University Meerut 250001, Uttar Pradesh, India
- Corresponding Author:
- Sunayana Tyagi
Department of Pharmaceutics
IIMT College of Medical Science,
IIMT University
Meerut 250001
Uttar Pradesh
India
Accepted date: March 21, 2022
Abstract
The aim of present examination work was to create tramadol hydrochloride based ethosomal gel. Transdermal medication conveyance of the medication through the skin is the best and advantageous arrangement of the medication conveyance. Skin goes about as a significant objective just as a guideline barrier for this Transdermal medication conveyance. Regardless of the numerous benefits of this framework, the significant hindrance is the low dissemination pace of medications across the layer corneum. Despite these deterrents the vesicular framework enters into the skin and applies their activities. Among the different vesicular framework showing great entrance into the skin, ethosomes are the most valuable frameworks. Ethosomes are lipid vesicles containing phospholipids, liquor (ethanol or potentially isopropyl liquor) in generally high focus and water. Ethosomes are delicate vesicles made of phospholipids and ethanol (in higher amount) and water. The size scope of ethosomes may change from several nanometers to microns (μ). Along these lines, it tends to be an obvious end result that ethosomal definition has promising future in powerful dermal conveyance of bioactive specialists.
The communication of medication was precluded by FT-IR contemplates and no incongruence and lipids and surfactant. To upgrade the definition, factor influencing the actual appearance of Ethosomes were researched. Tramadol Hydrochloride loaded Ethosomal gel were formed utilizing soya lecithin by actual scattering strategy and described for molecule size, drug content, dependability, creation yield. Arranged ethosomal gel gave the better physical, morphological with deference grouping of the lipids, surfactant and proportion of lipid and surfactant. Six unique details of ethosomes F1-F6 were formed to acquire the upgraded plan. The (Transmission Electron Microscopy) TEM of ethosomes showed that they were adjusted shape and permeable in surface. In-vitro drug arrival of plan demonstrates that detailing F6 was chosen as upgraded definition for fuse into the erythrosomes among every one of the six details and liposome showed drug release in controlled way toward the finish of 10 hours.
Keywords
Tramadol hydrochloride, Ethosomes, In-vitro release, Topical gel, skin, Topical drug delivery.
Introduction
The clinical proof demonstrates that skin gel is a protected and successful treatment choice for use in the administration of skin related illness and utilized for nearby activity to diminish the results related with other ordinary measurements structure. Skin drug organization is a limited medication conveyance framework anyplace in the body through ophthalmic, rectal, vaginal and skin as a skin course. Skin is perhaps the most promptly available organs on human body for skin organization and is principle course of skin conveyance framework. Transdermal medication conveyance of the medications through the skin is the best and advantageous arrangement of the medication conveyance. Skin goes about as a significant objective just as a central obstruction for this skin/ Transdermal medication conveyance. Notwithstanding the numerous benefits of this framework the significant snag is the low dissemination pace of medications across the layer corneum. Disregarding these deterrents the vesicular frameworks enters into the skin and applies their activities. Among the different vesicular frameworks showing great entrance into the skin, ethosomes are the most valuable framework. Tramadol HCl is a narcotic pain relieving which is utilized in the therapy of extreme and constant agony [1,2].
Tramadol HCl is endorsed 3-4 times each day. The continuous dosing of Tramadol HCl prompts the expanded rate of results, non-compliances and improvement of resilience particularly in long haul utilized like osteoarthritis, joint inflammation, post-careful torments and so on Along these lines, to relieve the disservices related with oral treatment, skin ethosomes to expand the better entrance Tramadol HCl [3].
Materials and Methods Introduction
Drug Tramadol Hydrochloride, Carbopol, Methyl paraben and propyl paraben was purchased from Central Drug House (CDH) Pvt. Ltd, New Delhi, India. Soya lecithin and ethanol is obtained from SIGMA-ALDRICH Chemical, Mumbai, RFCL Limited, New Delhi (INDIA) respectively.
Preparation of unsonicated ethosomes
The above preparation consists of ethosomes after stirring on the magnetic stirrer for 5 min. These ethosome are called unsonicated ethosomes. These are now transferred to the sonicator and are sonicated in 3 cycles of 5 minutes each at 4ºC. These are now left aside for 45 minutes.
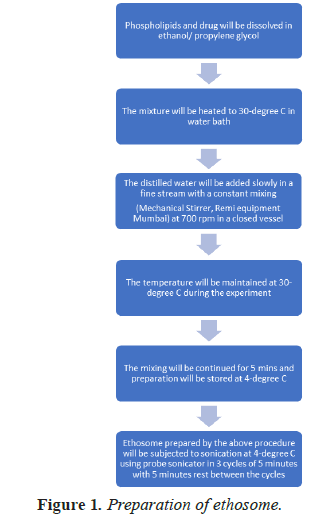
Formulation chart is shown in Table 1 and flow chart of preparation of ethosome is shown in Figure 1.
| S.no | Soya Lecithin | Ethanol(ml) | Propylene glycol (mg) | Tramadol Hydrochloride (mg) | Double distilled |
|---|---|---|---|---|---|
| 1. | 300 | 2.5 | 600 | 100 | 10 |
| 2. | 300 | 3.0 | 600 | 100 | 10 |
| 3. | 300 | 3.5 | 600 | 100 | 10 |
| 4. | 300 | 4.0 | 600 | 100 | 10 |
| 5. | 300 | 4.5 | 600 | 100 | 10 |
Table 1. Formulation chart.
Preparation of gel base
Ethosomes of Tramadol Hydrochloride were prepared with the use of phospholipids, ethanol, PG and distilled water. Here, phospholipids (soya lecithin) were used as vesicle forming agent, ethanol as penetration enhancer 2% W/W gel base was prepared by dispersing carbapol in distilled water containing 0.2% methyl paraben and 0.02% propyl parabens, using magnetic stirrer. Here, carbapol was used as gelling agent and methyl parabens and propyl paraben were used as preservatives.
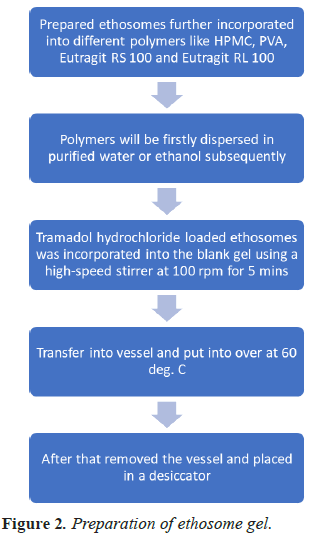
Preparation of ethosomal gel in shown in Figure 2 in the form of flow chart.
Results and Discussion
Physical characteristics
Tramadol hydrochloride is a white to off white crystalline powdered extract which is pungent in taste (Table 2).
| c | Physical characteristics | Observation |
|---|---|---|
| 1 | color | White to off white |
| 2 | order | Strong pungent |
| 3 | taste | pungent |
Table 2. Physical characteristics.
Melting point range
Dissolving point of medication was controlled by slender combination technique. For this limited quantity of Tramadol hydrochloride was taken in a hair like cylinder shut down toward one side. An advanced softening point contraption was taken and afterward slim cylinder was put in it. Temperature was recorded at which the medication began softening. This was acted in three-fold and a normal worth at noted [4] (Table 3) (Figure 3).
| S.no | Melting point | Reference value | Observation |
|---|---|---|---|
| 1 | Tramadol hydrochloride | 316 ºC-320ºC | 318ºC |
Table 3. Melting point.
FT-IR study (drug-excipient compatibility studies)
The pure drug and the excipients were mixed separately with IR grade KBr in a ratio of 100:1 and corresponding discs were prepared by applying 5.5 metric tons of pressure in a hydraulic press. The discs were scanned over a wave number range of 4,000-400 cm-1[5].
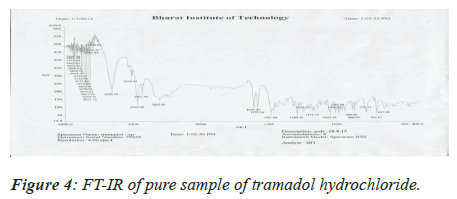
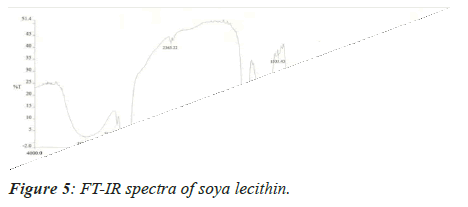
Medication Excipient similarity was done by utilizing FTIR spectrophotometer. The FT-IR spectra of unadulterated medication test alone, polymer tests and actual blend with polymer following 15 days for study the any association between them. The FT-IR spectra drug, soya-lectin and both mix combination were shown in Figures 4-6 and Tables 4 and 5.
| S. no | Functional Group | Reported Frequencies (cm-1) | Observed Frequencies (cm-1) |
|---|---|---|---|
| 1 | N-H | 3500-3100 | 3055.70 |
| 2 | C=O(Carboxylic Acid) | 1725-1705 | 1726 |
| 3 | OH(Carboxylic Acid) | 3400-2400 | 2461 |
| 4 | C-H(Aromatic) | 3150-3050 | 3000 |
| 5 | F | 1400-1000 | 1094.19 |
| 6 | C-H (Alkane) | 3400-2400 | 2703.5 |
Table 4. FT-IR studies of pure drug.
| S.no | Region in cm-1 | Type of Vibration | Functional group |
|---|---|---|---|
| 1 | 3250 | Stretching | C-N |
| 2 | 2950 | Stretching | C-H |
| 3 | 1380 | Stretching | O-H |
| 4 | 1257 | Stretching | C-O |
Table 5. Drug-Excipients compatibility studies.
UV spectrum analysis of tramadol hydrochloride
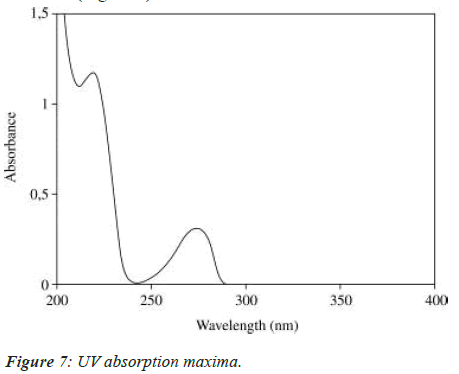
The solution was scanned in the range of 200 to 400 nm to fix the maximum wave length and UV spectrum was obtained (Figure 7).
Preparation of standard stock solution of Tramadol Hydrochloride
Exact weighed 100 mg Tramadol hydrochloride and was dissolved in 100 ml phosphate buffer saline of pH 6.8,7.2,7.4 separately where its concentration is 1000 μg/ ml, from this stock solution 10 ml was withdrawn and transferred into 100 ml volumetric flask. Volume was made with phosphate buffer saline in order to get standard stock solution containing 100 μg/ml [6].
1. Standard solution of Tramadol hydrochloride prepared buffer saline of pH 6.8.
2. Standard solution of Tramadol hydrochloride prepared buffer saline of pH 7.2.
3. Standard solution of Tramadol hydrochloride prepared buffer saline of pH 7.4.
Standard graph of tramadol hydrochloride
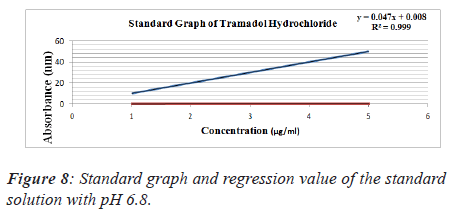
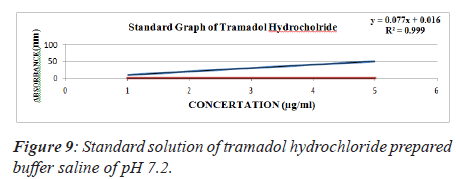
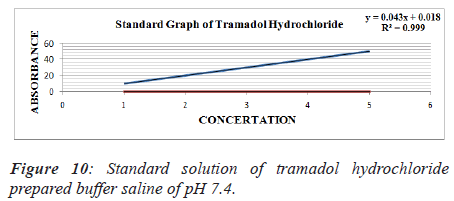
From standard stock solution a series of dilution (10, 20, 30, 40, 50 μg/ml) were prepared using phosphate buffer saline of pH 6.8, 7.2, 7.4 separately. The absorbance of these solutions was measured spectrophotometrically against blank of PBS of pH 6.8, 7.2, 7.4 respectively at 271 nm for Tramadol Hydrochloride [7] (Figures 8-10 and Tables 6-9).
| S.no | Concentration(µg/ml) | Absorbance(271 nm) |
|---|---|---|
| 1 | 10 | 0.055 |
| 2 | 20 | 0.103 |
| 3 | 30 | 0.154 |
| 4 | 40 | 0.198 |
| 5 | 50 | 0.246 |
Table 6. Standard solution of tramadol hydrochloride prepared buffer saline of pH 6.8.
| S.NO | Concentration (µg/ml) | Absorbance (nm) |
|---|---|---|
| 1 | 10 | 0.098 |
| 2 | 20 | 0.167 |
| 3 | 30 | 0.251 |
| 4 | 40 | 0.328 |
| 5 | 50 | 0.406 |
Table 7. Standard solution of tramadol hydrochloride prepared buffer saline of pH 7.2.
| S.no | Concentration (µg/ml) | Absorbance (nm) |
|---|---|---|
| 1 | 10 | 0.063 |
| 2 | 20 | 0.103 |
| 3 | 30 | 0.152 |
| 4 | 40 | 0.195 |
| 5 | 50 | 0.236 |
Table 8. Standard solution of tramadol hydrochloride prepared buffer saline of pH 7.4.
| Solvent | Absorbance | Concentration |
|---|---|---|
| Water | 4 | 36.79 |
| n-Octanol | 1.865 | 17.16 |
Table 9. Evaluation parameter of partition coefficient.
Preparation of gel base
2% W/W gel base was prepared by dispersing carbopol in distilled water containing 0.2% methyl paraben and 0.02% propyl parabens, using magnetic stirrer. Here, carbapol was used as gelling agent and methyl paraben and propyl paraben were used as preservatives.
Preparation of ethosomal gel
Ethosomal formulation was subjected to 2000 rpm for 20 min at 40ºC .The sediments were collected and incorporated into the prepared gel base to get ethosomal gel [8].
Evaluation of drug loaded ethosomal gel
pH: The pH of detailing inside the worthy reaches which demonstrate there is no conceivable disturbance up on instillation.
Appearances: Tramadol Hydrochloride is the white crystalline powder (Table 10).
| S.no | Parameters | Appearance | pH | Gelation Capacity | Gelation Temperature |
Drug Content |
|---|---|---|---|---|---|---|
| 1 | F1 | Transparent | 7.4 | ++ | 37ºC | 98.20 |
| 2 | F2 | Transparent | 7.0 | ++ | 36ºC | 101.02 |
| 3 | F3 | Transparent | 7.0 | ++ | 37ºC | 98.34 |
| 4 | F4 | Transparent | 6.8 | ++ | 35ºC | 98.25 |
| 5 | F5 | Transparent | 6.4 | ++ | 36ºC | 101.4 |
Table 10. Different characterization parameters of tramadol hydrochloride ethosomal gel.
Rheological studies
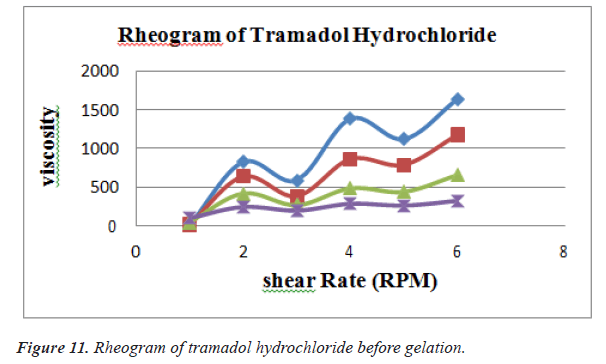
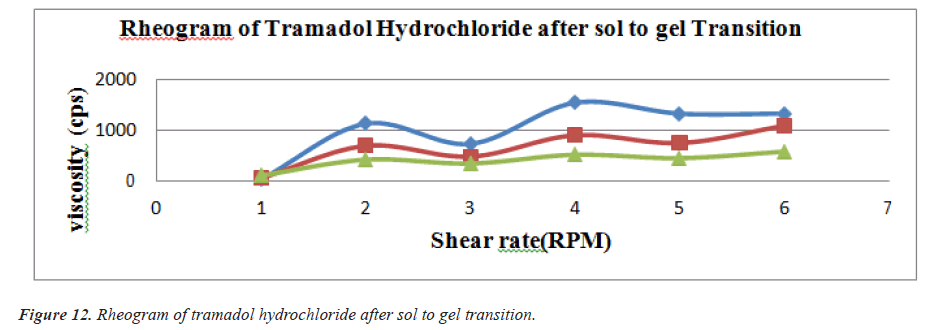
Viscosity of instilled formulation is an important factor determining residence time of drugs on the target site. Rheological studies were performed using Brookfield Viscometer .Prepared ethosomal gels were evaluated for their viscosities using suitable spindles at varying RPM [9] (Figures 11 and 12) (Tables 11 and 12).
| Shear Rate (RPM) | Viscosity of the formulation (cps) | ||||
|---|---|---|---|---|---|
| F1 | F2 | F3 | F4 | F5 | |
| 10 | 825 | 589 | 1384 | 1120 | 1639 |
| 20 | 640 | 384 | 865 | 790 | 1178 |
| 50 | 420 | 268 | 487 | 441 | 659 |
| 100 | 245 | 198 | 286 | 263 | 324 |
Table 11. Shear rate of different formulation before gelation.
| Shear Rate (RPM) | Viscosity of the formulation(cps) | ||||
|---|---|---|---|---|---|
| F1 | F2 | F3 | F4 | F5 | |
| 10 | 1405 | 1328 | 2024 | 2610 | 2610 |
| 20 | 1124 | 725 | 1540 | 1324 | 1324 |
| 50 | 689 | 475 | 898 | 745 | 1084 |
| 100 | 415 | 340 | 512 | 446 | 574 |
Table 12. Shear rate of different formulation after gelation.
Drug entrapment efficiency
To take drug content example take 20 mg of arranged items and put in 25 ml of volumetric carafe and added 10 ml of methanol under sonication for 30 min. the volume was made up to stamp with methanol and afterward went through whatman channel paper no. 41. Consequently arrangement was reasonably weakened with a similar dissolvable and medication content spectrophotometric tested at specific frequency. The rate drug content was determined for all clusters utilizing the condition [10].
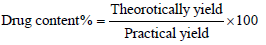
Evaluation of tramadol hydrochloride efficiency
Stacking effectiveness of ethosomes was dictated by centrifugation strategy. About 2 mg of lyophilized definition was weighed precisely and taken in a 2 ml of miniature axis tube. To it 2 ml of ethanol-PBS blend was taken and plan was suspended by vortex. At that point it was sonicated for few moments and centrifuged at 15000 rpm for 15 min. After centrifugation clear supernatant was gathered, the absorbance of the supernatant arrangement was estimated in UV/VIS Spectrophotometer against the clear at the frequency of 432 nm. The medication content was resolved from the standard bend [11] (Table 13).
| Formulation code | Absorbance (271 nm) | %Entrapment |
|---|---|---|
| F1 | 0.305 | 99.01% |
| F2 | 0.223 | 99.25% |
| F3 | 0.483 | 98.3% |
| F4 | 1.488 | 94.5% |
| F5 | 0.291 | 98.9% |
Table 13. Drug entrapment efficiency.
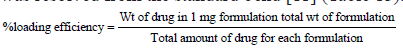
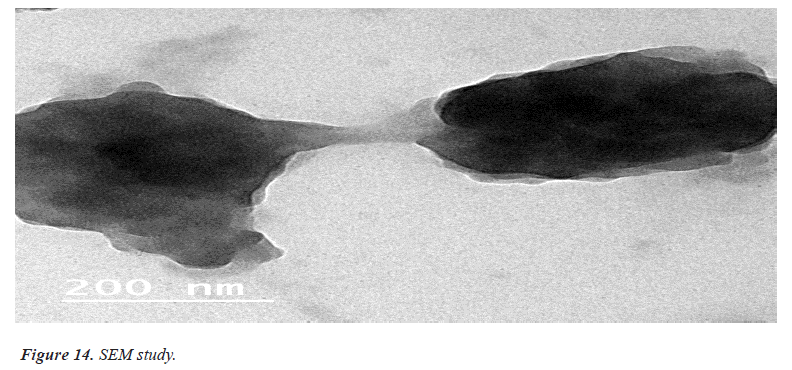
Surface morphology of ethosomal Gel
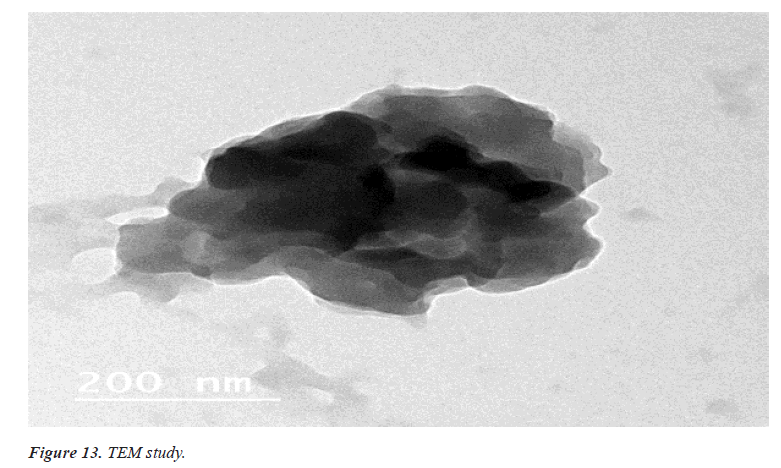
Imaging technique that provides photographic images and elemental information TEM is useful for characterization the morphology and size of microscopic specimens with particle size as low as Nano meter to decameter.
The sample is placed in an evaluated chamber and scanned in a controlled pattern by an electron beam. Interaction of the electron beam with the specimen produces a variety of physical phenomena that, when detected, are used to form images and provides elemental information about the specimens [12] (Figures 13 and 14).
In vitro Diffusion Study
Release kinetics
The energy and component of medication discharge was resolved utilizing distinctive motor models. The active models utilized were zero request as total measure of medication discharge versus time, first request as log aggregate level of medication remaining versus time, Higuchi model as total level of medication discharge versus square foundation of time, Korsmeyer and Peppas as log aggregate percent drug discharge versus log time [13] (Table 14).
| Time | F1 | F2 | F3 | F4 | F5 |
|---|---|---|---|---|---|
| 1 | 31.55 | 21.5 | 18.29 | 14.35 | 13.32 |
| 2 | 43.21 | 33.56 | 32.45 | 26.01 | 27.23 |
| 3 | 51.56 | 44.93 | 40.32 | 34.27 | 36.35 |
| 4 | 67.13 | 55.27 | 48.10 | 44.85 | 44.67 |
| 5 | 75.11 | 63.16 | 61.1 | 54.38 | 56.59 |
| 6 | 80.24 | 70.51 | 69.47 | 62.34 | 65.67 |
| 7 | 88.43 | 79.5 | 74.86 | 68.20 | 70.23 |
| 8 | 91.36 | 88.87 | 84.34 | 78.27 | 74.86 |
Table 14. In vitro diffusion study.
In vitro drug release kinetics
In vitro drug release through cellophane membrane
Cellophane film (0.45 μm, got from sigma synthetics) was utilized for this examination. An example of 1 g of the planning was filled in cellophane film recently splashed for the time being in the delivery medium. The stacked film was solidly positioned in disintegration medium (Phosphate support, pH 7.4, 900 ml) at 37ºC ± 0.5ºC. Aliquots every one of 5 ml were removed from the delivery medium at determined time spans. Removed examples were supplanted by equivalent volumes of new delivery medium. The examples were tested spectrophotometrically at λ max 240 nm and the centralization of the medication was resolved from the recently built adjustment bend. Every information point addressed the normal of three judgments. In vitro discharge reads were recorded for a 12 hour period (Tables 15 and 16).
| Formulation code | Zero order | First order | Higuchi | Korsmeyer peppas | Best fitting model | |
|---|---|---|---|---|---|---|
| R2 | R2 | R2 | R2 | n | ||
| 1 | 0.991 | 0.994 | 0.947 | 0.921 | 0.543 | First order |
| 2 | 0.980 | 0.993 | 0.971 | 0.948 | 0.648 | First order |
| 3 | 0.960 | 0.997 | 0.987 | 0.941 | 0.724 | First order |
| 4 | 0.939 | 0.992 | 0.996 | 0.933 | 0.766 | Higuchi |
| 5 | 0.925 | 0.994 | 0.996 | 0.920 | 0.820 | Higuchi |
Table 15. Release kinetics data for tramadol hydrochloride ethosomal gel preparations.
| Formulation code | Average score | ||
|---|---|---|---|
| DAY-1 | DAY-4 | DAY-8 | |
| Control | 0 | 0 | 0 |
| Std | 0 | 1.8 | 3.5 |
| GF2 | 0 | 0 | 1.2 |
Table 16. Skin irritation study.
Stability studies
Stability Studies are quite possibly the main investigations completed over the span of the improvement cycle. An item steady in the enormous scope of temperature and dampness climate is accepted to an ideal item. As the completed items might be transported into different ecological zones, their strength should be ensured. Every one of the details were exposed to sped up soundness testing. Details were dissected for the visual appearance, lucidity, pH and % drug remaining. 4 weeks of security affirmed that there was no adjustment of the visual appearance, clearness, pH and % drug remaining. a month of steadiness affirmed that there was no adjustment of the visual appearance and lucidity. Anyway slight changes were accounted for in the pH of the plan, yet were in the satisfactory reach (± 0.5). Percent Drug remaining was likewise found acceptable during the security study (Tables 17-20).
| S.no | Number of days | Visual Appearance | Clarity | pH | |||
|---|---|---|---|---|---|---|---|
| RT | 40ºC | RT | 40ºC | RT | 40ºC | ||
| 1 | 0 | Transparent | Transparent | Clear | Clear | 7.4 | 7.4 |
| 2 | 7 | Transparent | Transparent | Clear | Clear | 7.4 | 7.4 |
| 3 | 14 | Transparent | Transparent | Clear | Clear | 7.4 | 7.5 |
| 4 | 21 | Transparent | Transparent | Clear | Clear | 7.4 | 7.5 |
| 5 | 28 | Transparent | Transparent | Clear | Clear | 7.4 | 7.5 |
Table 17. Stability study result on formulation F1.
Where, GF2=Gel Formulation
0=no irritation
0.5%=Faint, barely perceptible and slight dryness
1=Definite erythema but no eruption or brain skin
1.5=Well defined erythema with dryness and epidermal fissuring
2=Moderate erythema
2.5=Moderate erythema with barely perceptible edema
3=Sever erythema
3.5=Moderate to severe erythema with escher formation
4=Moderate to server erythema with escher formation and edema extending the applied area.
| S.no | Number of days | F2 | F3 | F4 | F5 | ||||
|---|---|---|---|---|---|---|---|---|---|
| pH | pH | pH | pH | ||||||
| RT | 40ºC | RT | 40ºC | RT | 40ºC | RT | 40ºC | ||
| 1 | 0 | 7.0 | 7.0 | 7.0 | 7.0 | 6.8 | 6.8 | 6.4 | 6.4 |
| 2 | 7 | 7.0 | 7.0 | 7.0 | 7.0 | 6.8 | 6.9 | 6.4 | 6.5 |
| 3 | 14 | 7.0 | 7.1 | 7.0 | 7.1 | 6.8 | 6.8 | 6.4 | 6.5 |
| 4 | 21 | 7.0 | 7.1 | 7.0 | 7.1 | 6.8 | 6.9 | 6.4 | 6.4 |
| 5 | 28 | 7.0 | 7.2 | 7.0 | 7.2 | 6.8 | 6.8 | 6.4 | 6.5 |
Table 18. Stability study result on formulation F2, F3, F4, F5.
| S.no | Number of days | Percentage Drug Remaining | ||||
|---|---|---|---|---|---|---|
| F1 | F2 | F3 | F4 | F5 | ||
| 1 | 0 | 98.90 | 101.2 | 98.34 | 98.25 | 101.4 |
| 2 | 7 | 98.18 | 101.2 | 98.32 | 98.21 | 101.01 |
| 3 | 14 | 97.95 | 100.34 | 98.28 | 98.17 | 100.94 |
| 4 | 21 | 97.78 | 100.15 | 98.24 | 98.13 | 100.67 |
| 5 | 28 | 97.68 | 99.99 | 98.16 | 98.07 | 100.43 |
Table 19. Stability study of all formulation at room temperature.
| S.no | Number of days | Percentage Drug Remaining | ||||
|---|---|---|---|---|---|---|
| F1 | F2 | F3 | F4 | F5 | ||
| 1 | 0 | 98.20 | 101.2 | 98.34 | 98.25 | 101.4 |
| 2 | 7 | 98.13 | 101.1 | 98.29 | 98.19 | 101.1 |
| 3 | 14 | 97.87 | 99.94 | 98.21 | 98.15 | 100.74 |
| 4 | 21 | 97.73 | 99.89 | 98.17 | 98.09 | 100.57 |
| 5 | 28 | 97.64 | 99.84 | 98.13 | 98.03 | 100.33 |
Table 20. Stability study of all formulation at 40ºC.
Conclusion
It was conclude that Tramadol Hydrochloride ethosomal gel was prepared and evaluated for various parameters like vesicle shape, % drug entrapment efficiency, in vitro diffusion study, skin irritation study and stability studies so as to find out the formulation with optimum drug release and efficiency. Formulation 2 was found the best with the spherical vesicle shape and 99.25% drug entrapment efficiency. It showing the non-irritant to the skin when tested on animals and was also stable at 40ºC ± 50ºC and room temperature even after two months.
References
- Robert L. Polymer-controlled drug delivery systems. Acc Chem Res 1993; 26: 537-542.
- Jong-HoKim, Kyeongsoon Park, Hae Yun Nam, Seulki Lee, Kwangmeyung Kim, Ick ChanKwon. Polymers for bioimaging. Prog Poly Sci 2007; 32: 1031-1053.
- Thacharodi D, Panduranga Rao K. Development and in vitro evaluation of chitosan based transdermal drug delivery systems for the controlled delivery of propranolol hydrochloride. Biomaterials 1995; 16: 145-148.
[Crossref] [Google Scholar] [PubMed]
- Davis Stanley S, Lisbeth Illum. Drug delivery systems for challenging molecules. Int J Pharm 1998; 176: 1-8.
- Cross SE, Michael S. Roberts. Targeting local tissues by transdermal application: Understanding drug physicochemical properties that best exploit protein binding and blood flow effects. Drug Develop Res 1999; 46: 309-315.
- Finnin CB. Transdermal drug delivery-What to expect in the near future. Business Briefing: Pharma Tech 2003: 192-193.
- Kumar, Ritesh, Anil Philip. Modified transdermal technologies: Breaking the barriers of drug permeation via the skin. Tropical J Pharma Res 2007; 6: 633-644.
- Martin RJ, Stephen PD, Jonathan Hadgraft. Skin metabolism of topically applied compounds. Int J Pharm 1987; 39: 23-32.
- Denyer SP, Richard H Guy, Jonathan Hadgraft, Barry WH. The microbial degradation of topically applied drugs. Int J Pharm 1985; 26: 89-97.
- Pannatier AP, Jenner B Testa, JC Etter. The skin as a drug-metabolizing organ. Drug metabolism reviews 8, no. 2 (1978): 319-343.
[Crossref] [Google Scholar] [PubMed]
- Chien Yie W. Transdermal controlled systemic medications. Drugs and the Pharmaceutical Sciences 1987; 31: 416-440.
- Singh UV, Pandey S, Udupa N. Preparation and evaluation of flurbiprofen and diclofenac sodium transdermal films. Indian J Pharma Sci 1993; 55: 145-147.
- Garala KC, Anil JS, Pratik H Shah. Formulation and in vitro characterization of monolithic matrix transdermal systems using HPMC/Eudragit S 100 polymer blends. Int J Pharm Pharma Sci 2009; 1: 108-120.