Review Article - Journal of Medical Oncology and Therapeutics (2020) Volume 5, Issue 1
A comprehensive review of synthesized derivatives of methotrexate in relation to their anticancer potential.
Nemat A1, Iqbal M1*, Mehmood T21Department of Chemistry, School of Natural Sciences (SNS), National University of Sciences and Technology (NUST), Islamabad, Pakistan
2Institute of Biochemistry and Biotechnology, University of Veterinary and Animal Sciences-UVAS, Lahore, Pakistan
- *Corresponding Author:
- Iqbal M
Department of Chemistry
School of Natural Sciences (SNS)
National University of Sciences and Technology (NUST)
Islamabad, Pakistan
Tel: +92 300 6005249
E-mail: mudassir.iqbal@sns.nust.edu.pk
Accepted date: February 11, 2020
Citation: Nemat A, Iqbal M, Mehmood T. A comprehensive review of synthesized derivatives of methotrexate in relation to their anticancer potential.J Med Oncl Ther. 2020;5(1):04-20.
DOI: 10.35841/medical-oncology.5.1.4-20
Visit for more related articles at Journal of Medical Oncology and TherapeuticsAbstract
Methotrexate (MTX) is a chemotherapeutic agent with certain side effects. Efforts were made to synthesize analogues of MTX to enhance the activity and reduce side effects. Various derivatives were synthesized by structural modification of parent drug and mainly assayed for anticancer activity and binding affinity with dihydrofolatereductase (DHFR) and folylpolyglutamate synthetase (FPGS). In vitro study was carried out on cell lines CCRF. CEM, ATCC8, L. Casei, P388, L1210, L1210/R81, and H35. These synthesized derivatives have been assayed for human squamous cell carcinoma namely SSC25, SSC68, SSC78, SSC25/R1, SSC68/R1, and SSC78/R1. Some modification proved to enhance anticancer activity while others are detrimental. Some of derivatives were also tested for other biological activity like anti-malarial, anti-bacterial and anti-diabetic and show better activity. This article deals with a comprehensive overview of the synthesized derivatives by structural modifications and the impact of these modifications on its activity.
Keywords
Methotrexate, Derivatives, Anticancer activity, Review.
Introduction
Methotrexate is known as amethopterin which suppresses the immune system and used as chemotherapeutic agents. It was synthesized in 1947 and in 1956 it proved to cure as for metastatic cancer [1].
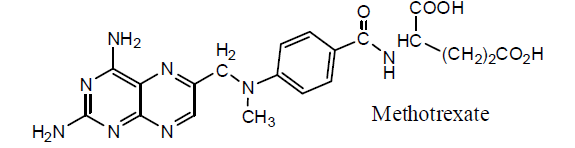
There is another analogue of folic acid and methotrexate known as aminopterin. It is also used in chemotherapy as it supresses the production of folic acid. It has comparable activity with methotrexate.
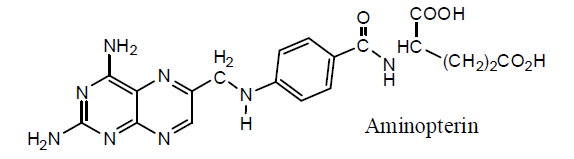
Methotrexate effects folic acid pathway [2], as it closely resembles folic acid, acting as antimetabolite and used in the treatment of various diseases like arthritis and cancer [3]. Although methotrexate has many applications but it has certain disadvantages, e.g. fast metabolism and low selectivity for tumor cell [4]. The mechanism of inhibition of cancer cells by MTX starts from inhibition of DNA and RNA synthesis, particularly in rapidly dividing cancerous cells thus acting as anticancer drug [5]. Dihydrofolatereductase (DHFR) is used to catalyse the dihydrofolate (DHF) to tetrahydrofolate (THF). THF is necessary for folate reduction and hence its needed for DNA synthesis [6]. Methotrexate is used to treat rheumatoid arthritis [7], psoriasis [8], cancer [9] and ectopic pregnancy [10]. Keeping in view all the uses of methotrexate it has certain disadvantages as it causes life threatening side effects on vital organs [11]. There are a number of derivatives synthesized by modification of MTX showing different activity and binding affinity.
Literature Review
Lysine and ornithine derivatives of MTX were synthesized and both derivatives have same binding affinity as MTX and lysine have the same potency as MTX. Reaction mechanism involved protection and de-protection of amino acid and deprotected derivative is eight times more potent. Lysyl derivative has the same potency in protected and de-protected form. Lysyl derivative was more potent than ornithine derivatives. Presence of Cbz group didn’t affect activity but removal improved activity 3 folds in ornithine. Inhibition and DHFR binding were tested in liver of chicken. Results are summarized in (Table 1).
| Comp | R1 | R2 | n | I50 × 108Ma |
|---|---|---|---|---|
| 1(MTX) | H | H | 2 | 9 |
| 2 | t-Bµ | Cbz | 3 | 310 |
| 3 | t-Bµ | Cbz | 4 | 95 |
| 4 | H | Cbz | 3 | 33 |
| 5 | H | Cbz | 4 | 38 |
| 6 | H | H | 3 | 25 |
| 7 | H | H | 4 | 13 |
| a=The enzyme concentration was 18.4 × 10-8 M | ||||
Table 1: Lysine and ornithine derivatives and their I50 valµes [12].
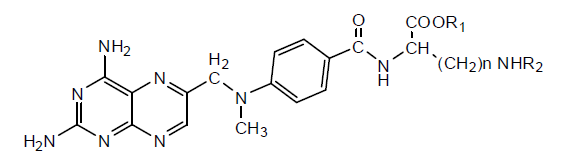
Various MTX derivatives have been synthesized having a different group by replacing γ-COOH and only a few were tested for anti-proliferative activity. These derivatives have been tested against DHFR, FPGS, L1210 and L1210/R81. Ornithine derivative was found to be more potent against DHFR and FPGS (Table 2).
| Comp | R | n | X | DHFR IC50 µM | FPGS Ki µM | L1210 Ki µM | L1210/R81 IC50 µM |
|---|---|---|---|---|---|---|---|
| MTX | Me | 2 | COOH | 0.035 | - | 0.002 | 220 |
| AMT | H | 2 | COOH | 0.035 | - | 0.002 | 84 |
| 2 | Me | 4 | NH2 | 0.065 | - | 0.4 | 220 |
| 3 | Me | 3 | NH2 | 0.16 | 20.4 | 1.3 | 86 |
| 4 | Me | 2 | NH2 | 0.12 | - | 2.42 | 290 |
| 5 | Me | 1 | NH2 | 0.18 | - | 0.44 | 405 |
| 6 | H | 3 | NH2 | 0.072 | 0.15 | 1.3 | 32 |
Table 2: Analogµes of MTX and their IC50 valµes [13].
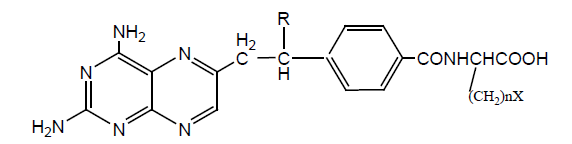
Various Derivatives of MTX have been synthesized by modification of γ-COOH like MTX-N (idoacetyl) L-lysine, another with Cbz, and NH2 Group. Bonding Affinity for DHFR tested against L-Casei and L1210. N (idoacetyl) L-lysine shows the best activity for L. Casei than MTX, but less activity for L1210. All other derivatives exhibit less activity and possible reason is the presence of charged groups at the end of chain lessens binding affinity results mentioned in (Table 3).
| Comp | R | L. casei (ID 50)b | L1210 (ID 50)b |
|---|---|---|---|
| MTX |  |
6.2 | 2.7 |
| 1 | 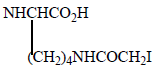 |
4.5 | 31 |
| 4 | 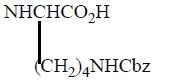 |
14 | 11 |
| 5 | 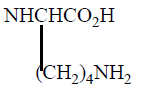 |
23 | 11 |
| b=Competitive [3H] MTX binding assay affinity for DHFR | |||
Table 3: Analogμes of MTX and their binding affinity [14].
Lysyl derivatives of methotrexate have been synthesized by reaction of γ-COOH with the amine group of lysine. These derivatives vary due to the number of Lysyl groups attached. It was found that synthesized derivatives show less affinity to DHFR from 2-3 folds as well as decrease in cytotoxic activity by 30-120 folds. Minimum activity was shown by derivative with three Lysyl groups; moreover, an increase in the number of Lysyl derivative does not affect activity. Activity was tested against L1210 and H35 cell line and these showed same result (Table 4).
| Comp | L1210 DHFR IC50 nM | L1210 cells IC50 µM | H35 Cells IC50 µM |
|---|---|---|---|
| MTX | 50 | 0.024 | 0.01 |
| 1 | 87 | 0.76 | 0.4 |
| 2 | 86 | 1.8 | 0.5 |
| 3 | 140 | 2.9 | 0.56 |
Table 4: Dihydrofolate redµctase inhibitory activity and cytotoxicity of lysyl derivatives of MTX [15].
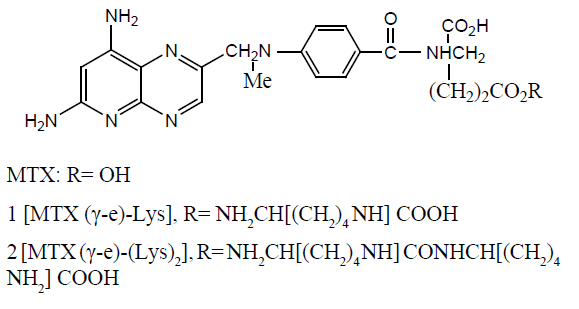
3 [MTX (γ-e)-(Lys)3],R= NH2CH[(CH2)4NH] CONHCH[(CH2)4NH2]CONHCH[(CH2)4 NH2]
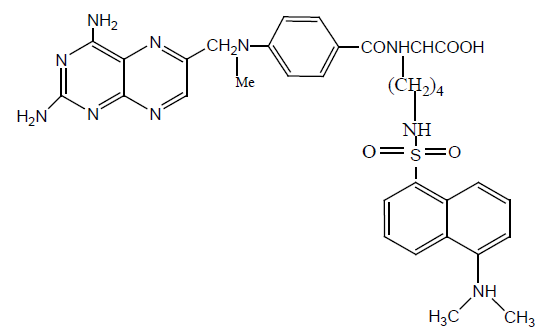
A fluorescent derivative of MTX has been synthesized by the lysine derivative of MTX to dansyl analogue and this analogue show higher activity against DHFR of L. Casei [16].
Protected dilysine and trislysine analogues of methotrexate have been synthesized. Two schemes were used to synthesize lysine derivatives. In Scheme (I) the substituted L-glutamyl-L-lysine intermediate 6 was formed stepwise from dilysine, while in the scheme (II) synthesis was done from two N-terminal lysine joined in 6 steps [17].
Shams A. Nadhum et al 2015 synthesized a conjugate of silibinin and MTX in order to enhance efficacy than parent drugs and to minimize their side effects. Scheme of reaction consists of 6 steps in which firstly imine derivative formed. This imine derivative is converted into imine-MTX-cysteine and after 2-3 steps imine conjugate of MTX and silibinin. Imine conjugate hydrolyzed to get conjugate of MTX and silibinin. Anticancer activity was tested against HEP-2 cell lines from human epidermoid larynx carcinoma for 24 hr and 48 hr. General trend shows compound 5 and 6 have a high inhibition rate on high concentration dose and decreased at low concentration. Silibinin shows 33.1 % inhibition MTX-silibinin conjugate shows 41.2% [17].
Andre et al 1984 synthesized a polygultamate derivative bind as well as MTX and leave mammalian cell more slowly. This analogue contained gama-SO3H instead of COOH and was synthesized in 78% yield. Binding affinity is measure of interaction by which a molecule (protein) binds with drug and in turn potency of drug can be found which actually drugs activity, for anticancer activity cell lines of humane lymphoblastic leukemia cells were used for measuring binding and anticancer efficacy, MTX used as positive control. The binding affinity of analogue was same as of MTX. Three experiments were conducted using same quantity and different doses and it revealed that by increasing frequency increase in molar potency of the drug shown in (Table 5) [18].
| Comp | X | L1210DHFR IC50 (nM) | L. Casei DHFR IC50 (nM) | L1210 cells IC50 (nM) |
| MTX | CO2H | 1 | 17 | 0.3 |
| mAPA-HCysA | SO2H | 0.95 | 10 | 0.01 |
Table 5: Dihydrofolate redµctase (DHFR) binding and cytotoxicity of glµtamate derivatives of MTX [19].
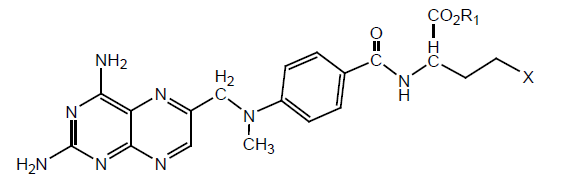
γ-Hydrazide, γ-n-butylamide, γ-benzylamide and γ-tert-butyl ester analogues of MTX have been synthesized. The binding affinity of synthesized derivatives was tested for DHFR from L1210 mouse leukemia cells and Lactobacillus casei. It has been found that γ-terminal region of MTX is site for modifications. Binding affinity has been tested to DHFR of L. Casei and L1210 by spectrophotometric assay and radio-ligand assay respectively. It was found that gamma substituted compound binds effectively than α-Substituted. γ-tert-butyl ester shows 1.9 times higher binding affinity than MTX. γ-tert-butyl ester and γ hydrazide showed same affinity as MTX. γ-n-butyl amide showed slightly same activity as MTX. γ-benzyl amide showed less activity (Table 6).
| Comp | R1 | R2 | L. Casei ID50 µm | L. Casei (Binding affinity) ID50 µm | L1210 (Binding Assay) ID50 µm | L1210 ID50 µm |
|---|---|---|---|---|---|---|
| 1 | H | O-t-Bµ | 0.025 | 0.012 | 0.0029 | 0.0029 |
| 2 | H | NHNH2 | 0.0021 | 0.013 | 0.012 | 0.012 |
| 3 | H | NH-n-Bµ | 0.053 | 0.036 | 0.0033 | 0.0033 |
| 4 | H | NHCH2C6H5 | 0.055 | 0.022 | 0.003 | 0.003 |
| 6 | CH3 | -t-Bµ | 0.33 | 3.9 | 0.011 | 0.011 |
| 7 | -t-Bµ | CH3 | 0.2 | 2.2 | 0.019 | 0.019 |
| 8 | -t-Bµ | NHNH2 | 0.17 | 2.2 | ND | ND |
| 9 | -t-Bµ | H | 0.036 | 0.21 | 0.035 | 0.035 |
| 10 | CH2Ph | NH-nC4H9 | ND | 0.25 | ND | ND |
Table 6: Binding affinity of side chain modified MTX derivatives for analogµes of methotrexate [20].
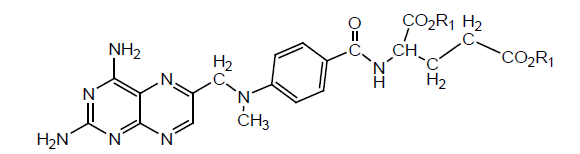
Several MTX derivatives have been synthesized by modification of the glutamyl moiety with peptide side chain. Nine different amino acids were used for modification of the side chain. Various intermediate peptides were also separated. Biological activity was tested for L1210 leukemia and W25 carcinoma in mouse and rat respectively. It has been found that α-COOH is more important for activity than gamma(γ)-COOH. All derivatives were inactive, which show glutamyl moiety is necessary for activity. If α-COOH is present, an increase in length of the alkyl chain restores activity (Table 7).
| Comp | R | L1210 Dose (mg/kg) × no. of administration | L1210 %ILS |
|---|---|---|---|
| 1a | Glycine | 23 × 7 | 0 |
| 1b | DL-alanine | 33 × 6 | 0 |
| 1c | Β-alanine | 25 × 6 | 19 |
| 1d | Sarcosine | 50 × 6 | 0 |
| 1e | DL-α-aminobµtyric acid | 78 × 9 | 16 |
| 1f | γ- aminobµtyric acid | 10 × 8 | 0 |
| 1g | DL-Valine | 20 × 7 | 0 |
| 1h | L-leµcine | 90 × 9 | 24 |
| 1i | L-phenylalanine | 45 × 10 | 14 |
Table 7: Biological data of analogµes of methotrexate [21].
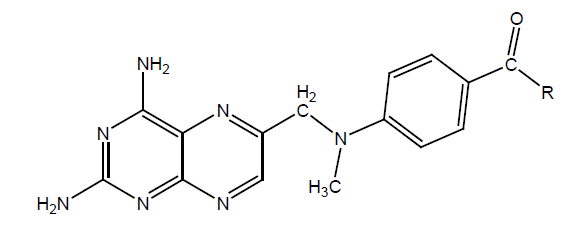
Different derivatives of MTX have been synthesized by alkylation of the side chain. DHFR affinity and antiproliferating activity was tested against L1210 in mice. It was found that those derivatives have a high inhibition rate, which was structurally closed to MTX. Minute changes in Structure such as derivatization of pyridine ring, chlorination gave good results. But the substitution of aliphatic group decreased the activity greatly. Introducing the carbon around the benzene ring decreased binding affinity, but between –COOH enhances the binding affinity, but none of the synthesized derivatives show significant anti-proliferative activity (Tables 8a-8e).
| No | R | Inhibition of DHFR I50 µm | Cytotoxicity to KB cells ED50 µg/µL | Inhibition of leµkemia cells L1210 |
|---|---|---|---|---|
| 1 | -NMe- (MTX) | 0.026 | 0.004 | 1.5 |
| 2 | CH(CO2H)(CH2)2CO2H | 0.026 | 0.003 | 0.67 |
| 26 | CH(CO2H)(CH2)4CO2H | 0.013 | 0.025 | 20 |
| 27 | (CH2)3CO2H | 0.38 | >100 | 25 |
| 28 | CH2CO2H | 1.1 | 88 | 80 |
Table 8a: Biological data for derivatives of MTX [22].
| No | R | Inhibition of DHFR I50 µm | Cytotoxicity to KB cells ED50 µg/µL | Inhibition of leµkemia cells L1210 |
|---|---|---|---|---|
| 29 | CH2COGlµ | 0.38 | 41 | 500 |
| 30 | CH2COAsp | 1 | >100 | 200 |
| 31 | (CH2)2COGlµ | 0.46 | 37 | 500 |
| 32 | SO2Glµ | 1.1 | 45 | 100 |
Table 8b: Biological data for derivatives of MTX [22].
| No | R | Inhibition of DHFR I50 µm | Cytotoxicity to KB cells ED50 µg/µL | Inhibition of leµkemia cells L1210 |
|---|---|---|---|---|
| 33 | Et | 0.050 | 0.022 | |
| 34 | H | 0.030 | 0.006 | |
| 35 | Me | 20 | >100 |
Table 8c: Biological data for derivatives of MTX [22].
| No | R | Inhibition of DHFR I50 µm | Cytotoxicity to KB cells ED50 µg/µL | Inhibition of leµkemia cells L1210 |
|---|---|---|---|---|
| 2 | -NH- | 0.026 | 0.003 | 0.67 |
| 1 | -NMe- | 0.026 | 0.004 | 1.5 |
| 36 | -NEt- | 0.045 | 0.005 | 10 |
| 37 | -NBn- | 14 | >100 | 80 |
| 38 | -NPhe- | 0.28 | 26 | 80 |
| 39 | -NMeCH2- | 0.75 | 0.043 | 50 |
| 40 | -O- | 0.51 | 3.5 | 1.3 |
Table 8d: Biological data for derivatives of MTX [22].
| No | R | Inhibition of DHFR I50 µm | Cytotoxicity to KB cells ED50 µg/µL | Inhibition of leµkemia cells L1210 |
|---|---|---|---|---|
| 41 | H | 0.63 | 0.05 | 100 |
| 42 | Br | 2.5 | 76 | 200 |
| 43 | NO2 | 38 | >100 | 200 |
Table 8e: Biological data for derivatives of MTX [22].
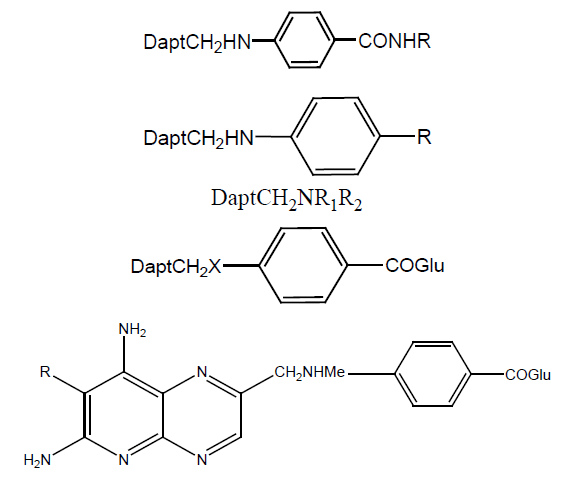
In a study [23] α and γ-monoesters of MTX were formed and anticancer activity checked against lymphoblastic leukemia (CCRF-CEM) cell lines. γ–monoesters were inhibitory than α-isomer and difference was about 10 folds, but with increase in chain length this difference reduced 2 to 2.5 folds. Monoesters showed a less inhibitory effect than diesters. Diethyl ester was 516 times more activity than α-monoethyl ester and 48 times than γ-monoester. Dibutyl esters show the same result as diethyl esters (Table 9).
| Comp | CCRF-CEM ID50 mol/L |
|---|---|
| MTX | 0.006 × 10-6 |
| MTX- γ-monomethyl ester | 0.43 × 10-6 |
| MTX-dimethyl ester | 0.40 × 10-6 |
| MTX- α-monoethyl ester | 6.2 × 10-6 |
| MTX- γ-monoethyl ester | 0.58 × 10-6 |
| MTX-diethyl ester | 0.012 × 10-6 |
| MTX- α-monobµtyl ester | 2.0 × 10-6 |
| MTX- γ-monobµtyl ester | 0.76 × 10-6 |
| MTX-dibµtyl ester | 0.057 × 10-6 |
Table 9: In vitro biological data for ester derivatives of MTX [23].
In this work numbers of alkyl esters derivatives of MTX were prepared by the direct esterification method. But with 2º alcohols or 1º alcohols, reaction at room temperature gives a poor yield. So, reaction mixture heated at 55-60ºC. In vitro growth inhibitory activity was also studied with two murine leukemia L1210 in hybrid mice and p1534 leukemia in mice. Dibutyl ester showed a 25% increase in life span. Binding affinity was tested with DHFR of Lactobacillus casei ATCC 7469 and it shows 1000 times less tightly than MTX (Table 10).
| Comp | R | X | Sµrvival in L1210 |
|---|---|---|---|
| ID50 µM | |||
| 1 | C2H5 | H | 51% |
| 5 | n-C4H9 | H | 25% |
| 6 | n-C4H9 | Cl | 66% |
| 12 | n-C8H17 | H | Inactive |
| 13 | n-C8H17 | Cl | Inactive |
Table 10: In vitro growth inhibitory activity alkyl ester derivatives of MTX [24].
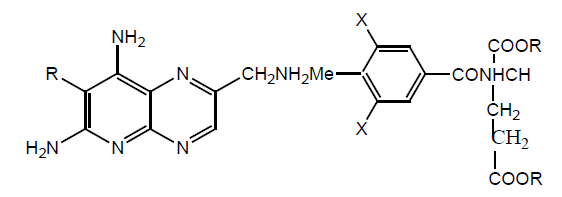
Several derivatives of MTX have been synthesized by substitution of alkyl group at 7-position. Inhibitory activity against Streptococcus faecium ATCC 8043 showed that synthesized derivatives were 1000 times less potent than the parent drug. The inhibitory action against p388 murine leukemia shows similar results. The lack of activity of both derivatives against DHFR showed that it might be due to steric effect of –CH3. Studies against L1210 leukemia in mouse showed that these derivatives were inactive (Table 11).
| R | X | S. faeciµm | L1210-FR8 | L. case | P388 | CCRF-CEM |
|---|---|---|---|---|---|---|
| ATCC8043 ID50 | ID50 mol/l | ID50 mol/l | ID50 mol/l | ID50 mol/l | ||
| H(MTX) | H | 0.002 | 1.5×10-9 | 3×10-9 | 0.01 | 0.018 |
| CH3 | H | 0.17 | 7×10-6 | 1× 10-5 | 0.1 | 0.1 |
| H | Cl | 0.01 | 1×10-9 | 3×10-9 | 0.007 | 0.009 |
| CH3 | Cl | 1 | 4×10-6 | 9×10-6 | 0.1 | 0.1 |
Table 11: Biological data for alkyl derivatives of MTX [25].
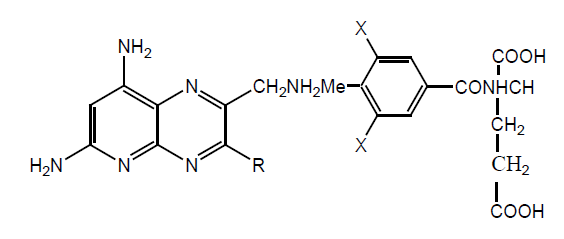
Many MTX derivatives were synthesized by MTX diethyl esters and various amines. The procedure involves the use of an excess of amines without solvent. Four of synthesized derivatives have been tested for CCRF-CEM and three for RBL (Rat basophilic leukemia) cells to make a comparison between two cell lines. Inhibitory activity against lymphoblastic leukemia CCRF-CEM showed that bis-amides derivatives were less active than MTX or MTX esters. Bis (benzylamide) showed higher activity in vivo against L1210 in mice, and this activity considered as bis (benzylamide) derivative release free MTX at site other than serum. 1h show most significant activity and IL%. Derivatives show better result for RBL than CCRF-CEM (Table 12).
| Comp | R | CCRF-CEM | RBL |
|---|---|---|---|
| ID50 | ID50 | ||
| MTX | H | 0.003 | 0.003 |
| 1a | NH2 | - | - |
| 1b | NH-n-C3H7 | 7 | - |
| 1c | NH-n-C4H9 | - | - |
| 1d | NH-s-C4H9 | - | - |
| 1e | NH-s-C6H13 | 10 | - |
| 1f | NH-c-C6H11 | 1 | 0.22 |
| 1g | c-NC4H8 | - | - |
| 1h | NHCH2C6H5 | 7.6 | 2.5 |
| 1i | NH CH2CH2C6H5 | - | - |
| 1j | NHCH2CH2C6H3-3,4 (OMe)2 | - | - |
| 1k | NHCH2CH2CH2OH | - | - |
| 1l | NHCH2CH2NMe2 | - | - |
| 1m | NHCH2CH2CH2NMe2 | - | - |
Table 12: Growth inhibitory activity of selected bisamide derivatives of MTX [26].
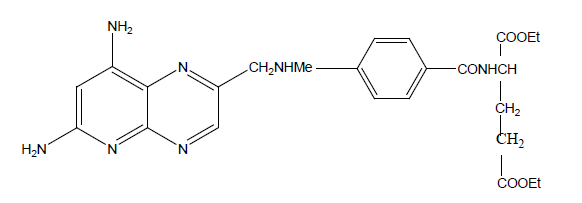
Various derivatives of MTX were synthesized having general formula 8-alkyl-7,8-dihydromethotrexate. Synthetic pathway includes alkylation of 7,8-dihydromethotrexate. In vitro studies tested against Lactobacillus casei, thymidylate synthetase, and DHFR. All derivatives were less potent for DHFR than MTX but more potent for thymidylate synthetase. In vitro inhibitory activity tested against CCRF-CEM show all derivatives have less inhibitory activity than MTX. Derivative having H at 8-position has the same activity as MTX but it was inactive for l1210 leukemia (Table 13).
| Comp | R | L. casei (×10-11)a | DHFR (×10-11)a | Thymidylate Synthetase (X 10-6)a | CCRF-CEM ID50 µg/mL |
|---|---|---|---|---|---|
| - | MTX | 4 | 0.3 | >100 | 0.018 |
| 7 | H | 2 | 2 | 1 | 0.021 |
| 8 | Methyl | 10 | 14 | 4 | 0.23 |
| 9 | Ethyl | 35 | 90 | 20 | 0.27 |
| 10 | n-Bµtyl | 120 | 38 | 19 | 0.23 |
| 11 | n-hexyl | 27 | 180 | 43 | 0.58 |
| 12 | Cyclohexylmethyl | 350 | 87 | 17 | 0.14 |
| 13 | Benzyl | 35 | 74 | 8 | 0.78 |
| 14 | 3,4 dichlorobenzyl | 1940 | 360 | 13 | >1.0 |
| 15 | 1-Naphthylmethyl | 47 | 450 | 16 | >1.0 |
| a=Molar conc. for 50% inhibition | |||||
Table 13: In Vitro biological data of MTX analogµes [27].
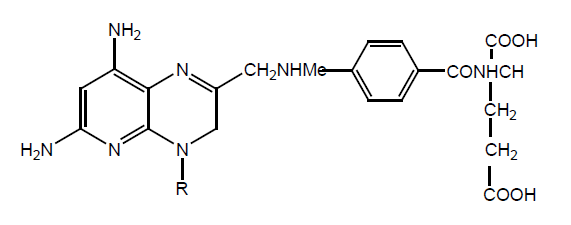
MTX γ-L-glutamate diethyl ester, MTX α-L-glutamate esters and α- γ-bis (L-glutamate tetraethyl esters) derivatives were synthesized. Major product obtained was γ-isomer. Further esterification of both isomers gave triethyl esters. Antiproliferating activity tested against L1210 leukemia. The increase in life span due to γ and α diethyl ester is 40% and 10% respectively while MTX has +60%. Growth inhibition activity again CCRF-CEM show γ-isomer 10 times more activity but α-isomer didn’t show same result. L isomer show higher activity than D isomer (Table 14).
| Comp | Hµman Lymphoblastic Leµkemia Cells (CCRF-CEM)ID50 µg/mL |
|---|---|
| MTX | 0.003 |
| 1 | 0.88 |
| 2 | 10+ |
| 3 (LLL) | 3.8 |
| 3(DLL) | 9 |
| 4 | 1.8 |
| 5 | 0.85 |
Table 14: Growth inhibitory activity of glµtamate ester derivatives of MTX [28].
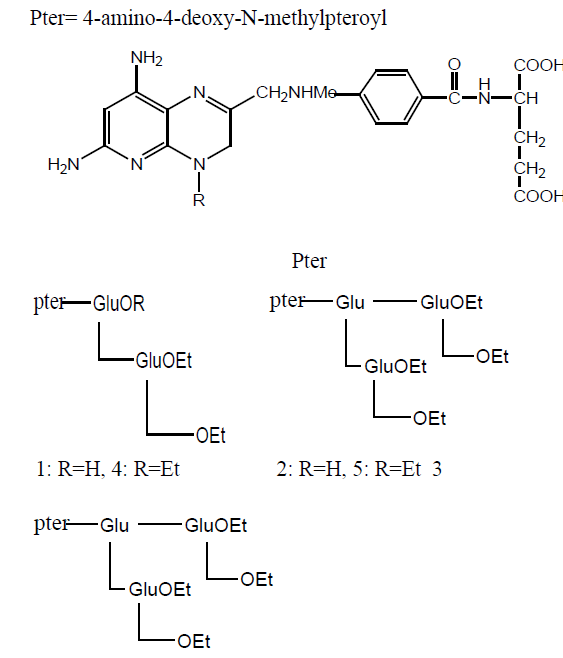
Aza derivative of MTX has been synthesized by additional N-atom between phenyl and carbonyl of the side chains. Photochemical method was used to insert additional N-atom. Inhibitory activity was tested against DHFR and thymidylate synthetase of Lactobacillus casei and CCRF-CEM and found to be less cytotoxic. In vivo inhibition was tested against L-1210 leukemia (mice) and show significant activity than MTX in the order of 55% and 88% respectively. (Table 15) summarizes the obtained results.
| No. | Comp | L. casei ng/mL |
DHFR M X (10-8) |
Thymidylate synthetase M X (10-6) |
|---|---|---|---|---|
| MTX | - | 0.01 | 0.3 | 100 |
| 5 | 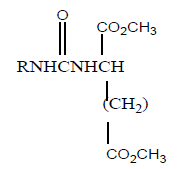 |
6 | 60 | - |
| 6 | 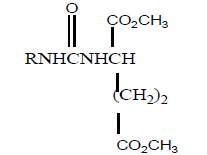 |
6 | 30 | - |
| 7 | 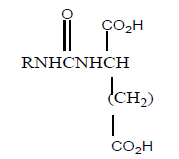 |
150 | 5 | 190 |
| 8 | 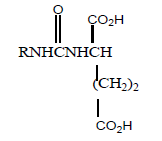 |
8 | 8 | 250 |
| 10 | 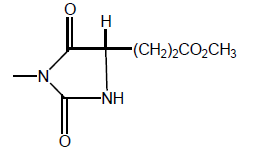 |
104 | 400 | - |
Table 15: Inhibition of L. casei (ATCC 7469) growth and DHFR and thymidylate synthetase by MTX derivatives [29].
Tripepetide derivatives of methotrexate have been synthesized by reacting with four different amino acids. These synthesized derivatives have been tested for anti-proliferative activity against W256 (rat) and L 1210 (mouse) leukemia. In this work an extra amino acid added between glutamic acid and amino benzoyl portion. This insertion of amino acid made these derivatives to shows borderline activity. Generally, an increase in dose increase in life spam (%ILS) (Table 16).
| Comp | R | X | n | L 1210 mg/kg | %ILS |
|---|---|---|---|---|---|
| Dose (mg/kg) × no. of admin | |||||
| 1a | H | Gly | 1 | 40×10 | 69 |
| 1b | H | Gly | 2 | 33×6 | 14 |
| 1c | CH3 | Gly | 1 | 100×8 | 40 |
| 1d | CH3 | Gly | 2 | 100×8 | 0 |
| 1e | CH3 | DL-ala | 2 | 50×6 | 19 |
| 1f | CH3 | Sar | 2 | 100×6 | 0 |
| 1g | CH3 | L-Leµ | 2 | 50×8 | 0 |
| 1h | CH3 | L-phe | 1 | 100×7 | 0 |
| 1i | CH3 | L-phe | 2 | 50×10 | 25 |
Table 16: Anti proliferative activity of peptide derivatives of MTX [30].
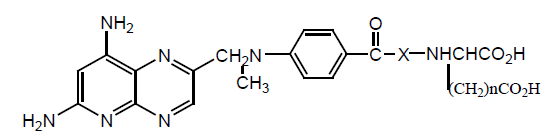
Various diesters of MTX and DCM have been prepared by the reaction of acid catalysed esterification. Neutral esterification also carries out using Cs2CO3. In vitro anticancer activity was tested against L1210 in mice. Different doses injected to check the increase in median life span [31].
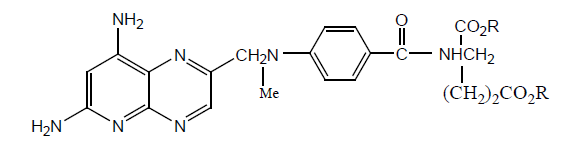
Various γ-monoamides of the AMT and MTX have been synthesized by modification of coupling method of mixed carboxylic- anhydride method. All derivatives have been tested in vitro against L1210 leukemia, wild type L1210 and sublime (CEM/MTX). In vitro γ-N-aryl alkyl and γ-N-aryl derivatives show higher potency than γ-N-tert-alkyl analogues. Results show that AMT derivatives have more potency than MTX derivatives and all MTX derivatives show more potency against sublime L1210/R81 than MTX. It was also found that all derivatives show more activity against cell lines than human cells. In vivo studies showed γ-N-tert-alkyl analogues inactive however significant activity was shown by γ-N-aryl alkyl and γ-N-aryl derivatives. The results are shown in (Table 17).
| Comp | R2 | R4 | R5 | DHFR | L1210 | L1210/R81 |
|---|---|---|---|---|---|---|
| IC50 µm | IC50 µm | IC50 µm | ||||
| MTX | Me | H | H | 0.02 | 0.01-0.03 | 220 |
| 3a | H | H | t-BµNH | 0.044 | 0.12 | 22 |
| 3b | H | H | (1-admantyl)NH | 0.1 | 0.68 | 25 |
| 3c | H | H | C6H5CH2NH | 0.087 | 0.005 | 17 |
| 3d | H | H | 3,4-Cl2 C6H3CH2NH | 0.13 | 0.046 | 32 |
| 3e | H | H | 2,6-Cl2 C6H3 CH2NH | 0.08 | 0.0037 | 61 |
| 3f | H | H | C6H5NH | 0.06 | 0.0035 | 10 |
| 3g | H | H | 3,4-(OCH2O) C6H3NH | 0.045 | 0.0032 | 28 |
| 3h | Me | H | 3,4-(OCH2O) C6H3NH | 0.042 | nd | 25 |
| 3i | H | H | 3,4-(OH)2 C6H3NH | 0.057 | 0.037 | 23 |
| 3j | Me | H | 3,4-(OH)2 C6H3NH | 0.031 | 0.03 | 38 |
| AMT | H | H | H | 0.02 | 0.003 | 84 |
Table 17: In Vitro biological data for monoamides derivatives of MTX [32].
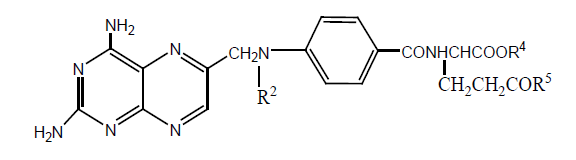
Various derivatives of MTX and AMT have been synthesized by replacing glutamate moiety with DL-2-aminoalkanedioic acid having up to 10 CH2 alkyl group. All derivatives have been tested against L1210 leukemia, CEM leukemia (human), CEM/ MTX, and L1210/R81. Derivative with 9 CH2 alkyl group show higher activity with CEM, and with 6 CH2 alkyl group against L1210 cell lines (Table 18).