Review Article - Materials Science and Nanotechnology (2017) Volume 1, Issue 2
A brief overview of flexible CNT/PANI super capacitors.
Hana D Dawoud1, Talal M Altahtamouni1, Moustafa M Zagho1, Nasr Bensalah2*
1Department of Materials Science and Technology, College of Arts and Science, Qatar University, Doha, Qatar
2Department of Chemistry and Earth Sciences, College of Arts and Sciences, Qatar University, Doha, Qatar
- *Corresponding Author:
- Nasr Bensalah
Department of Chemistry and Earth Sciences
College of Arts and Sciences Qatar University
P. O. Box 2713 Doha Qatar
Tel: +97444036540
E-mail: nasr.bensalah@qu.edu.qa
Accepted date: September 26, 2017
Citation: Dawoud HD, Altahtamouni TM, et al. A brief overview of flexible CNT/PANI super capacitors. Mater Sci Nanotechnol. 2017;1(2):23-36.
DOI: 10.35841/nanotechnology.1.2.23-36
Visit for more related articles at Materials Science and NanotechnologyAbstract
The demand for flexible and sustainable energy storage devices such as solar cells, batteries or super capacitors has been increased in last decades to cover the growth of advanced electronics. Flexible super capacitors have presented promising potential because of their long cycle life, easy fabrication, high capability and low cost. However, to reach high capacitance with power and energy densities better than those of batteries and conventional capacitors, super capacitor required electrodes having thinner dielectrics and high specific surface area. Carbon nanotubes (including SWNTs and MWNTs) are favorable as electrode materials for flexible super capacitors owing to their superb electrical, mechanical, optical properties and high stability of electrochemical which are significant for producing high-performance energy devices. A conducting polymer, polyaniline (PANI) attracts the attention due to its excellent capacity and high conductivity for energy storage. This paper is a comprehensive review covering the recent articles of fabricating super capacitors by combining PANI with CNT electrode to maximize the capacitance efficiency as well as improve the stability of PANI. The characterization and future trends are also discussed.
Keywords
Nano-structures, Particle-reinforcement, Mechanical properties, Statistical properties/methods.
Introduction
Super capacitors or electrochemical capacitors gained tremendous interest over past few years for potential applications due to its sustainable, environment-friendly, high density/power energy devices, long life cycles, and its capability of charging/ discharging rapidly and effectively [1-6]. Also comparing to a standard capacitor, super capacitors can hold hundreds of times the electrical charge. Because of that, numerous applications like portable electronic devices, automobiles, and electric vehicles used the super capacitors as an effective component. Super capacitors are classified into two categories due to chargedischarge mechanism: electrochemical double layer capacitors (EDLCs) and pseudocapacitors. EDLCs rely on carbon materials with a high surface area such as graphene, carbon and carbon nanotubes and depend on the capacitance occurring from charge at the electrode/electrolyte interface for energy storage. Whereas, pseudocapacitors count on electrically conducting polymers and a transition metal such as oxides, hydroxide and sulfides [7-12].
Carbon nanotubes (CNTs)
Carbon nanotubes (CNTs) have been discovered in 1991 [13]. After its discovery, many researchers have focused their investigations on the synthesis, structure, electrochemical properties, mechanical properties and the proper applications [14-16] of CNTs. In general, carbon can be found regarding three forms: diamond, graphite and fullerenes/nanotubes. Carbon nanotubes are tiny strong cylinders with a length reaching up to 1 mm and an average diameter of 0.3 to 2.6 nm. CNT exists in two types containing more than two concentric cylindrical shells of graphite sheets; single wall nanotubes (SWNT) which have a single graphite sheet and smaller structure; and multi-wall nanotubes (MWNT) that consist of many graphene cylinders [17-19] The structure of CNT makes it unique and extraordinary material; especially the helicity of the arrangement of the carbon atoms on the surface honeycomb lattice [18]. The diameter and folding angle in CNTs can change it from a metal-like material to semiconducting material by increasing the width that will lead to decrease the band gap [20]. Both types of CNTs have been used in super capacitor applications due to their unique properties. CNTs with conducting polymers are widely used in super capacitor applications.
Conducting polymers
Conducting polymers (CP) reported first by Weiss and coworkers in Australia in 1963. While Shirakawa [21] in 2000, got the Nobel Prize for the discovery of the conducting polymer and enhanced doping techniques to switch the electric properties of these polymers over the full range from an insulator to metal [22]. CPs is pseudocapacitive materials that show better energy densities than carbon-based super capacitors [23]. Also, CPs has significant power capability, charge density, higher conductivity compared to inorganic materials and also many of them are of low cost [24]. Furthermore, one of the advantages of using conducting polymers is their chemical structure variety and ease of synthesis [25]. Different types of conducting polymers and their transducing properties that employed in super capacitors have been studied through last decades, such as polyaniline, polypyrrole and derivatives of polythiophene [26-30].
Among all the conducting polymers, polyaniline (PANI) is considered as the one of most significant CP and it became the most widely used between the other conducting polymers because of its features like ease of synthesis, low-cost, better electrical conductive polymer, high capacitance, outstanding electrical properties, high strength and also high specific surface area that easy to manufacture [24,31,32]. Moreover, it exhibits significant redox behavior. Therefore this conducting polymer has carried out extensive studies and invested as electrode materials for many application like super capacitors and energy storage applications [33-35]. However, the biggest problem of PANI related to the efficient utilization of it, which is inherent in its lower level of conductivity in contrast with metal, poor mechanical properties and the low solubility in all available solvents [36] Of note, PANI exists in three oxidation states (pernigraniline, emeraldine and leucoemeraldine forms) that differ in the physical and chemical characteristics [37]. The oxidation state may affect conductivity and electrochemical performance of the polymer. Of these, the green protonated emeraldine has a conductivity of 100 S cm-1, higher than that of polymers (<10-9 S cm-1) but lower than that of metals (>104 S cm-1). After treatment with alkali, protonated PANI changes to a non-conducting emeraldine.
But, these issues have been solved by modifying the monomer ring [38] or doping in the right level of doping and with a suitable dopant like the use of long chain organic dopants [39,40]. Also by controlling its structure during the synthesis or by mixing it with a polymer that has better mechanical properties and processability [34,41].
There are two ways to synthesis PANI, either chemically or electrochemically. The chemical synthesis method includes mixing aniline with an acidic medium, like H2SO4 or HCl and an oxidant, such as ammonium peroxydisulfate ((NH4)2S2O8), while the electrochemical synthesis method could be implemented using potentiostat, galvanostat or potentiodynamic methods [30,42].
Along with this journey, several studies have been conducted to find the useful of CNT-PANI properties for super capacitor and energy storage application [10,12,43-45].
However, the challenges facing application of polyaniline in super capacitors which are; Low utilization in bulk state, unsatisfactory rate capability due to low electrical conductivity (at high power) and poor durability due to structural instability associated with repeated insertion and de-insertion of ions during cycling. Herein, CNT is contributed with PANI to alleviate these obstacles which proved experimentally that the presence of CNTs into polymer matrix like PANI enhances mechanical properties as well as the electric conductivity of the original polymer matrix [46,47].
CNT/PANI Composites for Super Capacitor Applications
Different synthesis methods have been developed and utilized to produce CNT/PANI electrodes including electrochemical deposition, floating catalyst chemical vapor deposition [48- 50], In situ[51,52], ex situ [53,54], drop-casting [55,56], novel vertical spinning (VS) [57] techniques and many others. The electrochemical properties of materials formed, such as specific capacity, charge/discharge, cyclability and capacity retention rate, as well as the morphological features such as surface texture, grain size, size distribution and crystallinity, are directly affected by the synthesis methods.
Structural characterization of CNT/PANI synthesized have been done by using different techniques like Scanning Electron Microscopy (SEM), infrared (IR), Energy Dispersive X-Ray Analysis (EDX), X-ray Diffraction (XRD), Transmission Electron Microscopy (TEM), Fourier Transform Infrared Spectroscopy (FTIR), Field emission scanning electron microscopy (FESEM), Brunauer-Emmett-Teller (BET) and Raman spectroscopy. These techniques have been utilized to determine crystal structures, chemical and phase compositions, surface morphological characteristics, and microstructural features of synthesized materials. From another hand, the electrochemical properties of CNT/PANI composites have been studied by using Cyclic Voltammetry (CV), Galvanostatic charge/discharge and electrochemical impedance spectroscopy (EIS) measurements.
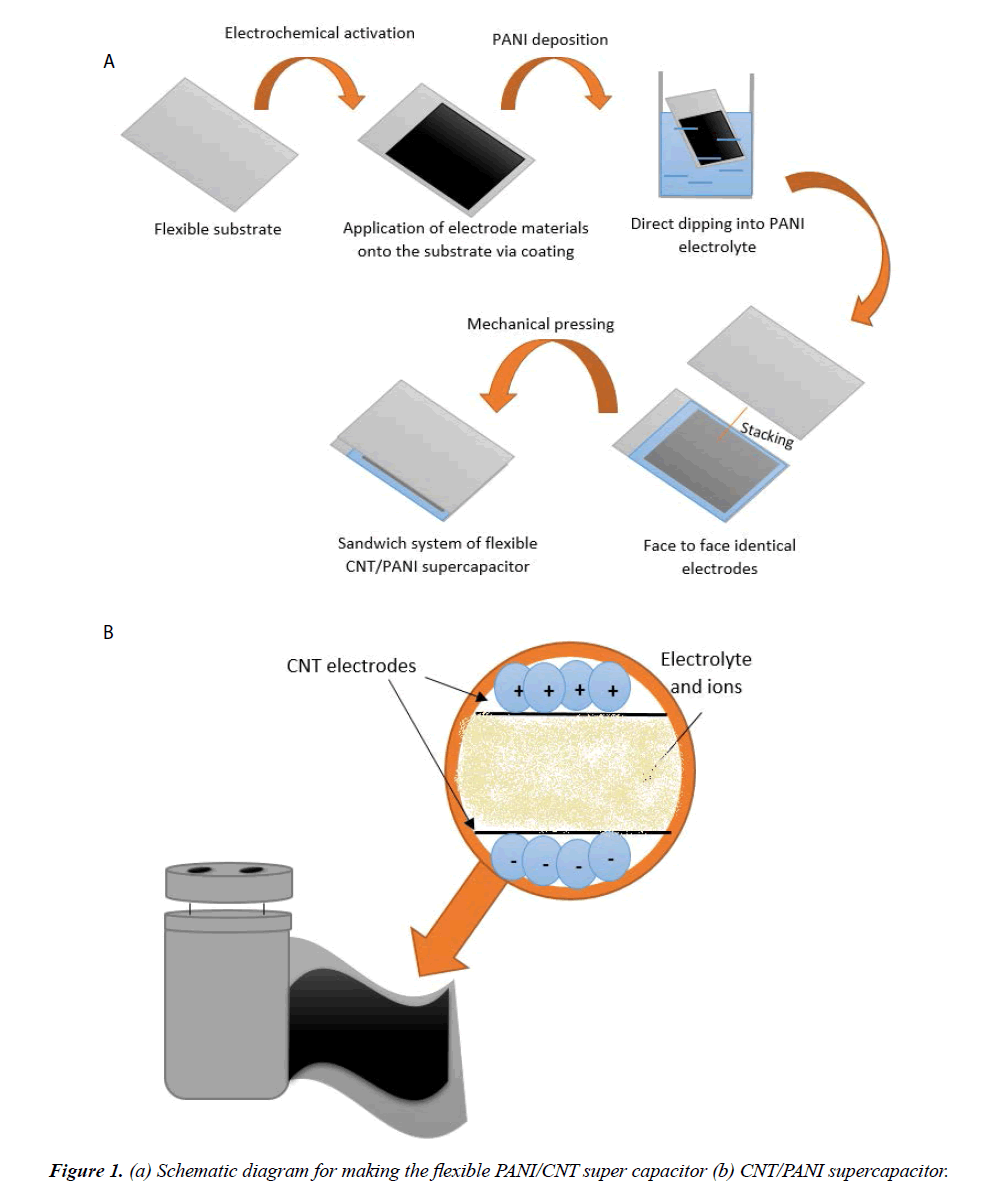
In this review, a comprehensive review of carbon nanotube and polyaniline electrodes proposed or used for super capacitors is attempted. The fabrication method of flexible CNT/PANI super capacitor is described in Figure 1.
Synthesis of CNT/PANI composites
Preparing polyaniline (PANI) as a conductive polymer with carbon nanotube (CNT) has been tremendous fundamental, technological importance and studied by many scientists due to its high electrical and mechanical properties, its great influence at super capacitors performance and their wide applications in electrochemical capacitors [58-60], disease detection [19], gas sensing [25,61] and so on. Usually 1 to 25 wt% of CNT are added to the aniline to form PANI/CNT composite [62-65].
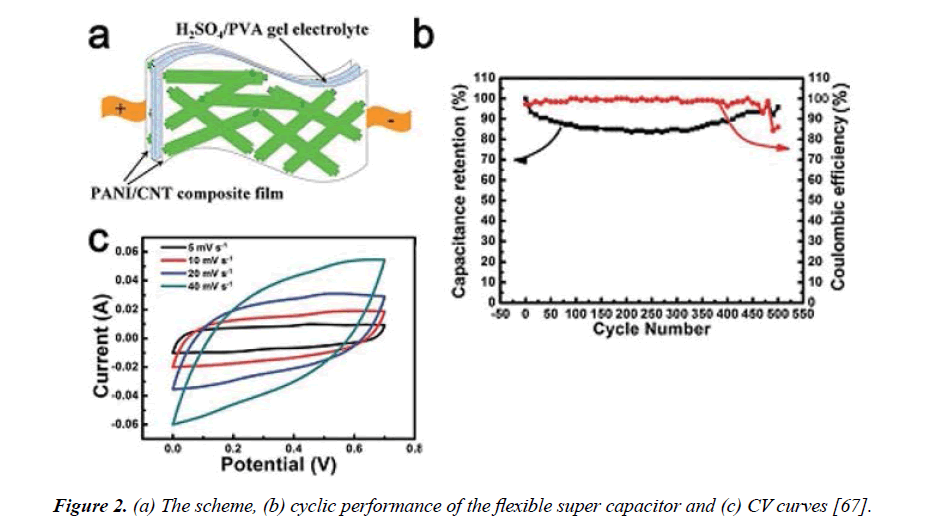
In situ electropolymerization technique: The critical volumetric alteration of conducting polymers during cycling charging-discharging procedure leads to decreasing the capacitance and degrading the electrodes. To avoid this,In situ electropolymerization technique was used to deposit the PANI chains inside the channels of CNTs. A case in point, Wang et al. [66] synthesized PANI nanowires within a ten nm-diameter multiwall carbon nanotube (MWCNT) porous membrane for a flexible super capacitor device. In situ electropolymerization technique applied by depositing the PANI nanowires inside the hollow channels of MWCNTs. The electrochemical measurements of cyclic voltammetry and galvanostatic charging/ discharging measurements proved that the hybrid fabric has a good electrochemical capacitance (≈ 296 F/g at J=1.6 A/g) with excellent cycle life (after 2000 charging-discharging cycles, capacitance loss is less than 5%). CV, Galvanostatic chargedischarge (GCD) and EIS measurements used to evaluate the electrochemical performances of the sheet. Zeng et al. [67] loaded PANI after synthesized the CNT sheet, using a floating catalyst chemical vapor deposition method in solid-state super capacitor, found that, the sheet of the electrochemically invented CNT hydrogel film with PANI deposition had maximum capacitance 680 mF.cm-2 at a particular area equal to 1 mA cm-2 with 400 cycles of PANI deposition which was higher than many similar super capacitors (Figure 2).
Lin [68] prepared a MWCNTs composition electrode with dry-drawing approach and synthesizing PANI using electrochemical deposition for fabricating transparent and flexible super capacitors, the result showed a high specific area capacitance of 233 F/g at 1 A/g current density that is greater by 23 times of pure PANI and 36 time of blank MWCNT sheet. To characterize the MWCNT/PANI composite film, CV and Galvanostatic charge/discharge measurements of super capacitors were used to characterize the electrochemical property of aligned MWCNT sheet and MWCNT/PANI composite films [68]. In another study, In situelectrochemical polymerization used to synthesis MWCNT/PANI composite electrodes, cyclic voltammetry and AC impedance spectroscopy were applied to illustrate the highest capacitance (reached 500 F/g), lower resistance and better cyclic stability of the composition film comparing to pure PANI film [69]. Similarly, Deng et al. applied In situemulsion polymerization to the synthesized hybrid material of CNTs/PANI, which supplied high conductivity and thermal stability [70].
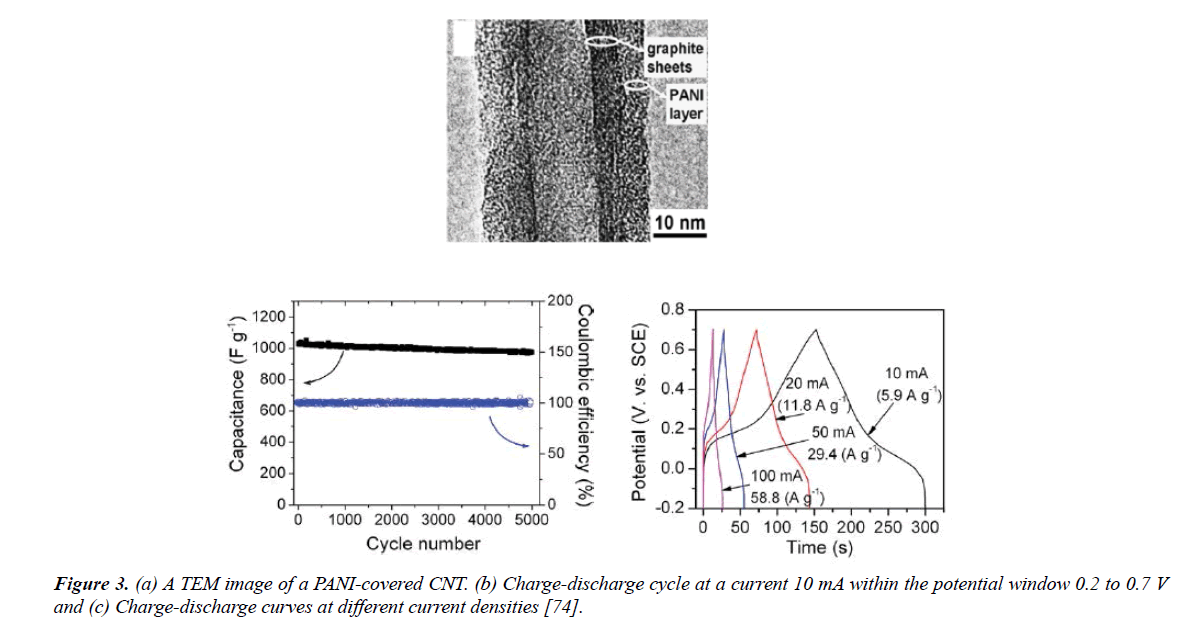
Nanostructured PANI studied by Hui N. et al. (2016) synthesized PANI nanowire arrays using In situ galvanostatic electropolymerization on the glassy MWCNTs electrodes. They offered great electrocatalytic and electrochemical properties with high specific capacitance and superb cycling stability at a current density of 0.1 mM.cm-2. EIS, CV and galvanostatic charge/discharge measurements were performed to study the electrochemical properties of CNT/PANI electrode [71]. Studying the impact of the surface modification of CNTs on the electrical conductivity and the dispersion stability of CNT/PANI composites were investigated by Park et al. [72], CNTs were treated first with acid mixtures, then potassium persulfate (KPS) displayed better dispersion stability while the CNTs/PANI composites treated with acid mixture provided the highest conductivity compared to the other surfacetreated of CNTs (≈ 16.5 S/cm). Guo and Li [73] added MWCNT to aniline to well-dispersed in aniline monomer by electrochemical polymerization. The composite film fabricated in an excellent uniformity and dispersion for super capacitor electrode. CV and EIS all show the enhancements made in the various properties of the present MWNTs are expected to improve the application potential of the conducting polymer without obstructing its chemical properties [73]. Zhang et al. [74] testified the use of PANI on a carbon nanotube array (CNTA) by synthesizing using electrodeposition technique and connecting directly to the current collector (Ta foil) as a supporter to produce composite electrodes with hierarchical porous structures. The electrochemical studies have shown that a superior specific capacitance of around 1000 F/g with great stability (95% capacity retention after 5000 cycles and at 118 A/g). CV and galvanostatic charge/discharge were applied for the electrochemical properties [74]. Figure 3 illustrate the image of TEM of a PANI-covered with CNT, displayed the charge–discharge cycle at a current 10mA and also the charge– discharge curves at different current densities.
The presence of CNT/ unzipped carbon nanotube (UCNT) and deposited PANI onto it by using In situchemical oxidative polymerization method, led to improving the mechanical strength. This composite can significantly simplify the electrolyte ions accessibility to the electrode material during charge/discharge process. The composition of PANI/CNT showed great specific capacitance 762 F/g in contrast of pure PANI 295 F/g at scan rate 30 mV/s and better cycling stability by losing only 20% capacitance after 1000 cycles. The electrochemical properties of the composite electrodes have been studied by CV, EIS and galvanostatic charge/discharge techniques in 1 M HCl aqueous solution [75].
Zhang et al. [76] studied the redox electrolytes of hydroquinone (HQ) and PANI/CNT composite redox electrode that reveals a specific capacitance of 7926 F/g at 0.2 A/g in a three-electrode system and the maximum energy density reaches of 114 Wh/kg at 1 A/g in 2 M HQ-1 M H2SO4 solution in two-electrode symmetric configuration. Furthermore, the specific capacitance retention of 96% after 1000 galvanostatic charge/discharge cycles shows an excellent cyclic stability [76]. PPy coated carbon nanocoils (CNCs) and PANI were employed in super capacitors which were efficient binderfree electrode materials. PANI/CNCs and PPy/CNCs exhibited specific capacitance up to 360 and 202 F.g-1, respectively. The PANI/CNC based super capacitors showed the maximum stored energy of 44.61 W.h.kg-1. These devices lost capacitance initially due to the instability of the polymer. However they become stable after 500 charge-discharge cycles [77]. Multiwalled CNT reinforced PANI, PPy and PEDOT composites were prepared by In situchemical polymerization for using these composites super capacitor applications. These composites were optimized by using different varying ratios of ECPs to MWCNT. All the materials exhibited good energy storage performance. PPy/MWCNT composites showed superior super capacitor characteristics than other composites. Liu et al. [78] incorporated various types of nanomaterials, such as silicon nanoparticles, carbon nanotubes, titanium oxide particles and graphene flakes into the conducting polymer PANI to form nanocomposite materials for making flexible super capacitor sheets. Incorporation of nanomaterials in PANI has significantly improved the energy and power capabilities of the capacitor [78] (Figure 4).
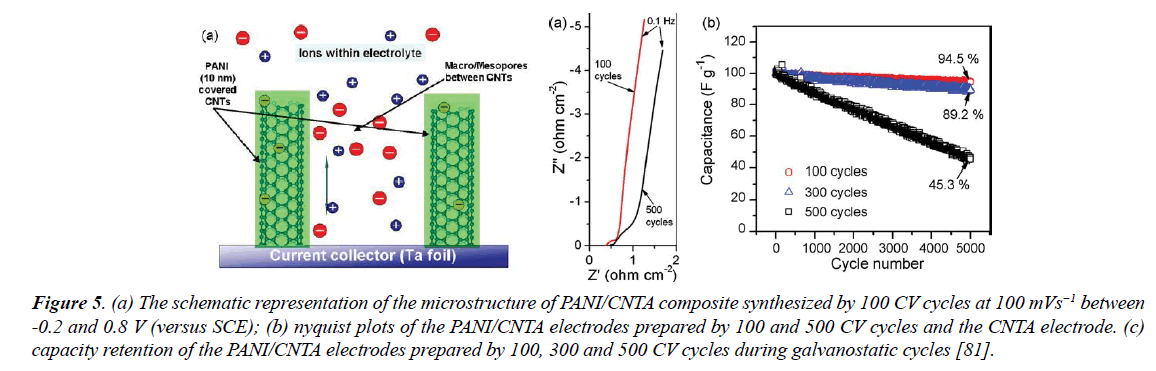
Chemical vapor deposition (CVD) technique: The composites formed through chemical vapors deposition (CVD) method showed lightweight, flexible features and ideal mechanical property. With this respect, Meng et al. [79] presented high synthesis of CNT/PANI composite electrode in thin films without using a binding material. Electrochemical measurements including CV measurements and galvanostatic charge-discharge measurements showed much higher electrochemical performance such as higher specific capacitance, more stability and lower internal resistivity under different current loads [79]. Benson et al. [80] presented the PANI-coated CNT fabric composite electrode synthesized by a commercial-scale continuous chemical vapor deposition (CVD) process. The composite exhibited a specific capacitance of 200 F/g in 1M NaCl electrolyte and retaining more than 80% of such a high capacitance at a current density more than 20 A/g. In addition, CV, EIS and galvanostatic charge-discharge have been used to study the electrochemical properties of the composite [80]. Zhang [81] investigated the effect of microstructure on the capacitive performance of PANI/CNTA composites after synthesized these composite electrodes by electrodeposition of PANI onto CNTA electrodes with a range of 100 up to 500 cycles using CV. The highest specific capacitance of 1030 F/g at a current density of 5.9A/g, longest cycle life and best rate performance, around 77% of the initial capacitance was retained after 100 CV cycles capacity at a current density of as high as 294 A/g, were obtained for the PANI/CNTA composite prepared, which are greater than that of activated carbon fiber cloth or PANI electrodeposited on stainless steel substrate. This high performance caused due to high ionic and electronic conductivity, high employment of electrode materials and best accommodation of the volume changes during charge-discharge [81] (Figure 5).
Figure 5. (a) The schematic representation of the microstructure of PANI/CNTA composite synthesized by 100 CV cycles at 100 mVs-1 between -0.2 and 0.8 V (versus SCE); (b) nyquist plots of the PANI/CNTA electrodes prepared by 100 and 500 CV cycles and the CNTA electrode. (c) capacity retention of the PANI/CNTA electrodes prepared by 100, 300 and 500 CV cycles during galvanostatic cycles [81].
Chemical oxidative polymerization: This approach present a new field designing high-performance carbon nanomaterials for super capacitors applications. It is worth addressing that the chemical oxidative polymerization process has led to the selfassembly of PANI/CNT core-shell morphologies. One instance of this, chemical oxidative polymerization of aniline in the presence of CNTs studied by Ramana et al. [82], which led to self-assembly of CNT–PANi nanotube core-shell structures. The core-shell structures are electrochemically active and exhibited a high specific capacitance of 368.4 F/g at different current densities. This work present a new field designing highperformance carbon nanomaterials based core-shell structures for energy storage applications. CV measurements were used to study the electrochemical properties of the composites [82].
Dip-coating process: The super capacitors fabricated by this technique offered a fast and reversible chromatic transition between working stages and its stored capacity can be easily measured by naked eye in noticeable approach. For instance, Xiang et al. [83] represented an efficient and straightforward strategy based on the direct and convenient immobilization of a polyaniline hydrogel onto a porous CNT sheet via by a dipcoating process. The results showed excellent flexibility and electrochemical performance, a specific capacitance have been achieved of 315 F/g and maintained a high cycling stability by almost 92% after 1500 cycles of charge and discharge and 1% more (93%) after bending for 150 cycles. Furthermore, CV, Galvanostatic charge-discharge (GCD) and EIS measurements used to evaluate the electrochemical performances of the asfabricated super capacitor [83].
Self-organization method: This simple technique provokes the preparation of novel PANI/CNT morphologies where the total volume and mass are included in the high charge storage and SC values. Besides, Bavio et al. [84] synthesized CNT/PANI chemically using self-organization method and the nanoparticles and nanotubes were developed at room temperature. PANI/ CNT nanocomposites showed improved capacitive properties in the acid solution of specific capacitance 1744 F/g at 2 A/g current density and the stability of the electrodes after 1000 cycles with loss of capacitance less than 21%. Moreover, CV and galvanostatic charge/discharge measurements were used to study the capacitive behavior of the PANI/CNT nanocomposite materials. These features proved to their feasible application as super capacitors materials [84].
Capacitance characterization and performance of CNT/ PANI composites
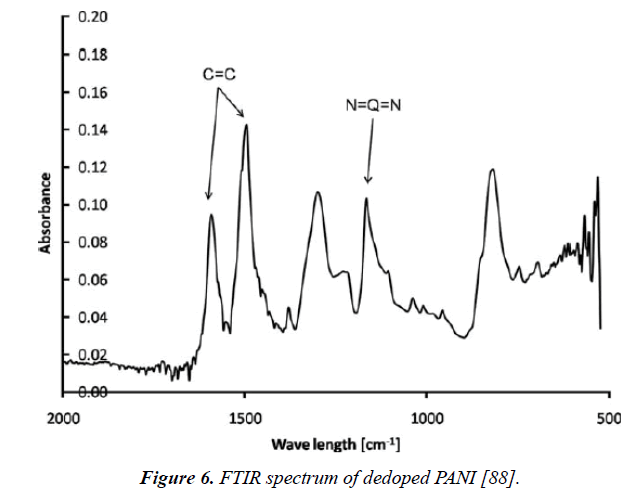
Composite materials were synthesized with CNT and polyaniline to form functional composites. This composite electrode can improve the processability of the materials, and also possibly provide them with new functions such as pseudocapacitances or electrocatalytic activities. Because of that, researchers studied the effect of different type of materials with CNT/PANI composites to improve the performance of the electrode for super capacitors. Yu et al. [85] presented a stretchable isotropic buckled carbon nanotube (CNT) sheet with high omnidirectional stretchability, electro-mechanical stability and low fabric resistance that used us super capacitor electrode, it’s followed by electrochemical deposition of PANI film. This combination reveals high specific capacitance of 1147.12 mF.cm-2 at the scan rate of 10 mV/s, while CNT film possesses 9.52 mF cm-2 at the scan rate of 50 mV/s. The energy density of CNT/PANI exhibits between 30 to 51 Wh.cm-2. Also, the results showed similar power density changing from 2.294 to 28.404 mW cm-2 at the scan rate from 10 to 200 mV/s which all that will refer to the great potential for super capacitor [85]. On the other hand, Oraon et al. [86] exploited the demand for environment-friendly and low-cost materials like nano clay (platelike montmorillonite) with 1 nm aluminosilicate film that known as eco-friendly materials and CNTs. Cloisite 30B and polyaniline (PANI) with CNTs electrodes have been synthesized usingIn situ and ex situ methods, and the electrochemical properties with the morphological study. They found that the specific capacitance increased 28% with the ex situ addition of Cloisite 30B concerning the CNT/PANI nanocomposite, which improved to 110% for theIn situ product. While the sample conserved around 92% of its initial specific capacitance after 2000 cycles. Also, the galvanostatic charging-discharging analysis used to examine the capacitance performance results [86]. Park et al. [87] reviewed the effects of surface treatment on the properties of PANI deposited on CNT/epoxy composites, such as the electrical conductivity and hydrophilicity of the epoxy composites, the surface motivated by acid treatment, potassium persulfate (KPS) and sodium dodecyl sulfate (SDS) to enhance their dispersion stability and reactivity with PANI which produced different amount of the polymer in the CNTs/ PANI. FT-IR and TGA carried out to characterize the surface and the structural changes of CNTs and CNTs/PANI [87]. Suckeveriene et al. [88] studied the ultrasonically assistedIn situ dynamic inverse emulsion polymerization of MWNT/PANI in chloroform. Comparing betweenIn situ and ex situ preparations, it’s found that, In situ method shows the better smooth coat of single SWNT with PANI, unlike the ex situ method where the coat of SWNT in PANI was improper dispersion. In addition, it has also been found that this composite presents a significant enhancement in separation and dispersion in toluene which otherwise would quickly coagulate and settle. FTIR and surface resistivity proved that the mechanical properties and electrical conductivity of PANI were improved after using ultrasound irradiation method [88] (Figure 6).
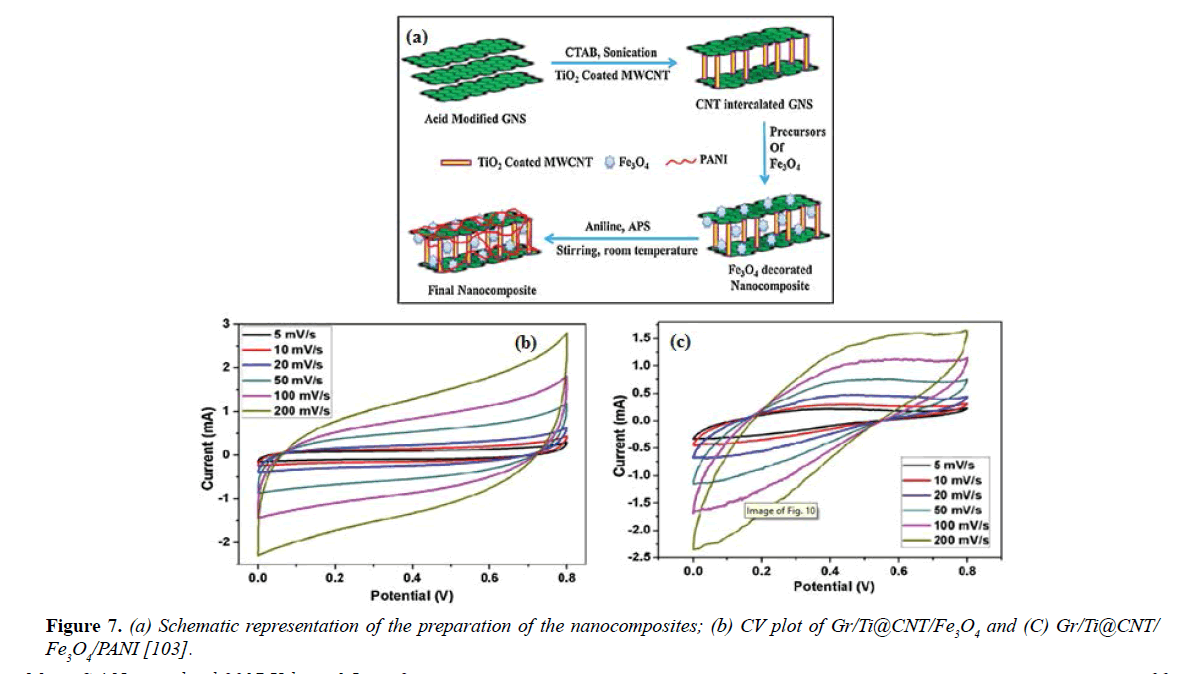
Also, Suckeveriene et al. [89] applied In situ interfacial dynamic inverse emulsion polymerization process to prepare hybrid CNT/PANI nanocomposites film forming a core-shell structure of nanowires, the composite materials cast into thin films having relatively low resistivity. In situ chemical oxidative polymerization method also used to prepare a nanocomposite of [poly(An-co-Py) Cu CNT] in present of ammonium persulfate (APS) as oxidant and hydrochloric acid (HCl) as dopant, Dhibar et al. [90] investigated these composites as high-performance super capacitor electrode materials using different electrochemical techniques. The [poly(An-co-Py) Cu CNT] nanocomposite electrode reached highest specific capacitance value of 383 F/g at scan rate equal to 0.5 A/g and nonlinear current-voltage characteristic, with presented an excellent electrical conductivity at room temperature [90]. The dropcasting technique used by Han et al. [91] to prepare a nanocomposite thin film of MWCNT/PANI for electrochemical super capacitor electrode applications and formed on flexible graphene-coated indium-tin-oxide (ITO) substrate as an adhesion layer. CV results showed a specific capacitance of the CNT/PANI nanocomposite film about -130 F/g and the nominal capacitance loss (-1.2%) which is higher than the pure PANI film with (-120 F/g) and the nominal capacitance loss (-18.1%) after 100 charge-discharge cycles. This technique advantage of being an easy and inexpensive route for improving conducting polymer-based flexible electrochemical super capacitors [91]. A scalable and simple method presented by Yang et al. [92] for fabricating hybrids graphene/pyrrole/CNT/PANI (GPCP), using graphene foam as the supporting template. The graphenepyrrole (G-Py) aerogels are synthesized via a green hydrothermal route from two-dimensional materials such as graphene sheets, whereas a (CNT/PANI) composite scuttle is achieved via the In situ polymerization method. The measurements revealed that the CNT/PANI was uniformly deposited onto the surfaces of the graphene. Also, its exhibited significant specific capacitance up to 350 F/g, making them promising in energy storage device applications [92]. Another nanocomposite of binder-free PANIg- MWCNT/TiO2NTs/Ti study done by Faraji et al. [93] and synthesized by using chemical and electrochemical methods. The storage energy performance was examined by CV, EIS and galvanostatic charge-discharge techniques in 1.0 M H2SO4 and exhibited Aa specific capacitance that reached 708 F/g at an ultra-high current density of 5 A/g. Furthermore, a high capacity retention rate of 88% achieved after 1000 cycles. The results confirmed that the thin film of PANI nanoparticles has been uniformly coated on the surface of the MWCNTs [93,94]. Two studies conducted in 2008 presented the preparation and the characterization of nanocomposites consisting of CNT/PANI, Zelikman et al. [95] deposited PANI with dodecyl benzene sulfonic acid (DBSA) as a matrix with MWCNT using polymerization method for the dispersion. Gajendran and Saraswathi [94] were also development a PANI/CNT composites using In situ chemical polymerization and electrochemical deposition. The structure and properties studied using absorption, X-ray photoelectron spectroscopy, infrared (IR), Raman, scanning probe microscopy and scanning electron techniques, cyclic voltammetry and thermogravimetry. Both studies showed great results indicate a favorable interaction between PANI and CNTs and good conductive, thermal, mechanical properties. Miao et al. [96] fabricated hybrid composites of carbon nanofibers/carbon nanotubes/ polyaniline (CNFs/CNTs/PANI), used as scaffolds for supporting pseudocapacitive materials and synthesized by In situ chemical polymerization of aniline monomers. The assembled super capacitors showed the maximum capacitance 315 F/g at 1 A/g, an energy density of 5.1 Wh/kg at 10.1 kW/kg, dramatic rate capability 75% at 32 A/g and notable cycling stability of 92% capacitance retention after 1000 cycles at 2 A/g [96]. Hybrid composites of CNT as high toughness sample with PANI nanowire arrays deposited on the CNT to increase the energy storage capacity have been developed for super capacitor. In situ polymerization process was used to syntheses a composite yarn of CNT/PANI yarn as a first step and a thin layer of polyvinyl alcohol (PVA)-H2SO4 gel electrolyte was coated on the surface of the CNT/PANI yarn to produce a CNT/PANI/PVA yarn as a second step. CV, EIS and galvanostatic chargedischarge were utilized for the electrochemical properties. The CNT/PANI composite yarn a capacitance of 38 mF.cm−2 whereas the areal capacitance of the pure CNT yarn-based super capacitor was 2.3 mF.cm−2 and also, showed excellent cyclic stability with its capacitance maintained at 91% of its original value after 800 charge-discharge cycles whereas, the pure CNT yarn super capacitor did not show any reduction in capacitance after the same number of cycles [97]. Peng et al. [98] fabricated a composite film of CNTs with PANI, polypyrrole (PPY) or poly[3,4-ethylenedioxythiophene] (PEDOT) using electrochemical co-deposition technique. The electrochemical properties showed that the films have great mechanical integrity, larger electrode specific capacitance, higher ionic and electronic conductivity and fast charge-discharge switching compared with the polymer alone. CNT/PANI composite electrode was the highest electrode specific capacitance and largest conductivity between the three composites but the smallest potential window [98]. Zhang et al. [99] reported the usage of CNTs to prepare PANI–graphene–CNT composite electrodes for super capacitors and fabricated by the In situ polymerization. The film exhibits a large reversible capacity around 430 F/g at a current 0.5 A/g comparing with G–CNT composite paper about 172 F/g which was much less than the composite paper. After 600 charge-discharge cycles, the composite paper improved of its cyclic performance to reach more than 96% of its original capacitance. CV and galvanostatic charge-discharge measurements used to test all electrochemical properties [99]. Zhou et al [100] studied the synthesis and electrochemical performance of PANI/CNT/CNF composite film electrode via electrospinning, CNT growth and In situ polymerization for pseudo supercapacitors. The electrochemical properties were characterized by EIS, CV and galvanostatic charge/discharge (GCD) in H2SO4 solution as an electrolyte. The results showed a high specific capacitance of 503 F/g at a current density of 0.3 A/g and decreased only 6% at 3 A/g to exhibit 471 F/g. Also, at a current density of 15 A/g, around 92% of the initial capacitance was retained after 1000 charge/discharge cycles. The power densities reached 15 kW/kg and the maximum energy was 70 W h/kg [100]. Huang et al. [101] deposited PANI layers at reduced graphene oxide/carbon nanotube (RGO/CNT) papers via In situ anodic electro-polymerization (AEP) technique. The composite was examined to evaluate its potential as an electrode for super capacitors using CV, EIS and the charge/discharge cyclic. The highest capacitance was 229 F/g (at 0.2 A/g) [101]. Lu et al. [102] synthesized a composite film of graphene/CNT /PANI by flow-directed assembly from a complex dispersion of GO and PANI/CNT and then re-oxidation and redoping of the reduced PANI in the composite to restore the conducting PANI structure. The composite produced high electrochemical capacitance 569 F/g, good rate capability 60% capacity retention at 10 A/g and excellent electrochemical stability with only 4% capacity loss after 5000 cycles [102]. Bhattacharya et al. [103] used the advantages of developing the multifunctional nanocomposites in his research as aim for improving the properties and the efficiency of electrochemical devices and microwave absorption. They have prepared a novel hybrid networking nanostructure with bi-functionality based on graphene (Gr), Ti coated CNT, Fe3O4 and PANI. CV and galvanostatic charge-discharge analysis in 1M aqueous KCl electrolyte have been used to test the electrochemical performances of the prepared nanocomposites. The highest specific capacitance of Gr/Ti at CNT/Fe3O4 /PANI displayed 464.7 F/g at 5 mV/s in contrast with Gr/Ti at CNT/Fe3O4 which exhibited 365.2 F/g at same scan rate [103] (Figure 7).
Shi et al. [104] fabricated a film of cellulose/carbon nanotube (CNT) as a template for In situ synthesis of PANI for flexible super capacitors with improved cyclic stability. CV, EIS and galvanostatic charge-discharge analysis used to evaluate the potential of the film. The specific capacitances of 495.7 and 408.8 F/g were obtained at the scan rates of 0.2 and 5 mA cm-2, respectively. However, the limited mass loading of PANI on cellulose/CNT films (1.3 mg/cm2 in this case) could limit the increasing of high energy and power requirements for most practical applications in wearable electronics [104]. Graphene/CNT and graphene/CNT/PANI as an electrode film have been fabricated and reported for asymmetric super capacitors by Cheng et al. [105]. The composites electrode exhibited a specific capacitance of 134.5 F g-1 at large current density of 3.1 A/g, also highest energy and power densities of 188 Wh/kg and 200 kW/kg respectively. The capacitance decreased around 18% after 1000 cycles, representing excellent cyclability of the electrodes. The capacitance of the super capacitor electrodes was studied by CV, EIS and galvanostatic charge and discharge techniques [105]. Xie et al. [106] prepared CNT/PANI cotton-shaped fiber structure composite by In situ chemical polymerization and used four-iron oxide (Fe3O4) as the template. CV, galvanostatic charging/discharging and cycle life tests were used to tested electrochemical performances. The results displayed that the gained CNT/PANI composite had the higher specific capacitance of 144 F/g (inorganic electrolyte), comparing to the pure PANI around 71 F/g which promising for good electrochemical performances [106]. Yan et al. [107] synthesized the composite of graphene nanosheet (GNS) with CNT/PANI via In situ polymerization. The composite exhibited the specific capacitance of 1035 F/g at 1 mV/s scan rate that was much higher than pure CNT/PANI composite 780 F/g and PANI 115 F/g. The capacitance dropped about 6% of initial capacitance compared to 52% and 67% for GNS/PANI and CNT/PANI composites after 1000 cycles. The electrochemical performances were investigated by CV, EIS and galvanostatic charge/discharge techniques [107]. Dhibar and Das [108] fabricated a silver/polyaniline/multi-walled carbon nanotubes ((Ag-PANI)/MWCNTs) nanocomposite electrodes for super capacitor via In situ polymerization method. The electrochemical properties of the composites were investigated by CV, galvanostatic charge-discharge (GCD) and EIS analysis with a three-electrode system. The nanocomposite exhibited a better electrical conductivity of 4.24 S/cm at room temperature and attained nonlinear current-voltage characteristics. Moreover, it’s obtained the highest specific capacitance of 528 F/g at 5 mV/s scan rate. The nanocomposite furthermore showed great power density and energy storage [108]. Zhou et al. [109] studied the effect and electrochemical performances of the morphologies of different nano carbons; carbon blacks (CB), CNTs and graphene nanosheets (GNSs) with deposit PANI. These composites were prepared via In situ polymerization method as electrodes of super capacitors. Comparing those three composites, it’s found that the GNS/PANI composite has the highest PANI loading with high specific capacitance 450 F/g at 5 mV/s, high energy and power densities 15.6 Wh/kg and 1125 W/kg, respectively, low internal resistance as well as enhanced cycling stability around 90% after 1000 cycles and rate capability than PANI, CB/PANI and CNT/PANI [109].
Summary of the Recent Trends in the Synthesis and Performance Properties of CNT/PANI Composites
These composites are a rapidly growing advance of the nanotechnology research where the characteristics of the composites are being enhanced as fast as applications can be recognized. The following table will summarize the main concepts addressed in this review (Table 1).
| Materials | References | Synthesis method | Specific capacitance (F/g) | Number of cycles: capacity loss |
|---|---|---|---|---|
| PANI/MWCNTs | Wang et al. [66] | In situ electropolymerization | 296 | 2000: 5% |
| PANI/CNT | Zeng et al. [67] | Floating catalyst chemical vapor deposition | 680 mF.cm-2 | 200, 300, 400 and 500 cycles: - |
| PANI/MWCNTs | Lin et al. [68] | Electrochemical deposition | 233 | 1000: - |
| PANI/MWCNTs | Taki et al. [69] | In situ electrochemical polymerization | 500 | 100 |
| PANI/CNT | Deng et al. [70] | In situ emulsion polymerization | - | - |
| PANI/CNT | Ramana et al. [82] | Chemical oxidative polymerization | 368.4 | - |
| PANI/CNT | Hui et al. [71] | In situ galvanostatic electropolymerization | - | 1000 |
| PANI/CNT | Park et al. [72] | Modified with acid mixtures, potassium persulfate (KPS) and sodium dodecylsulfate | - | - |
| PANI/MWCNTs | Guo and Li [73] | Electrochemical polymerization | - | 50; - |
| PANI/CNTA | Zhang et al. [74] | Electrodeposition technique | 1000 | 5000- 5% |
| unzipped carbon nanotube (UCNT)/PANI | Fathi et al. [75] | In situ chemical oxidative polymerization | 762 | 1000; 20% |
| PANI/CNT | Zhang et al. [76] | Single step electrospinning process | 385 | 1000; 20% |
| CNT/PANI | Meng et al. [79] | Chemical vapors deposition | 371 | 1000; 30% |
| CNT/PANI | Benson et al. [80] | Chemical vapor deposition | 200 | >30,000: 35% |
| CNT/PANI | Xiang et al. [83] | Dip-coating | 315 | 1500; 8% |
| CNT/PANI | Bavio et al. [84] | Self-organization | 1744 | 1000; <21% |
| Stretchable isotropic buckled carbon nanotube/PANI | Yu et al. [85] | Floating catalyst chemical vapor deposition method | 1147.12 | 10,000; 3.13% after 20 cycles |
| Nano clay/CNT/PANI | Oraon et al. [86] | Ex situ electropolymerization | 275 | 2000; 7% |
| CNT/epoxy/PANI | Park et al. [87] | In situ polymerization method | - | - |
| MWNT/PANI in chloroform | Suckeveriene et al. [88] | In situ dynamic inverse emulsion polymerization | - | - |
| poly(An-co-Py) Cu CNT | Dhibar et al. [108] | In-situ chemical oxidative polymerization | 383 | 28% and 33% |
| MWCNT/PANI/ graphene-coated indium-tin-oxide (ITO) | Han et al. [91] | Drop-casting | 130 | 100; 18.1% |
| graphene/pyrrole/CNT/PANI (GPCP) | Yang et al. [92] | In situ polymerization | 350 | 1000; |
| PANI-g-MWCNT/TiO2NTs/Ti | Faraji et al. [93] | Chemical and electrochemical methods | 708 | 1000; 12% |
| PANI with dodecyl benzene sulfonic acid (DBSA)/MWCNT | Zelikman et al. [95] | Polymerization method | - | - |
| PANI/CNT | Gajendran and Saraswathi [94] | In situ chemical polymerization and electrochemical deposition | - | - |
| CNFs/CNTs/PANI | Miao et al. [96] | In situ chemical polymerization | 315 | 1000; 8% |
| CNT/PANI | Wang et al. [97] | In situ polymerization | 38 mF cm-2 | 800; 9% |
| PANI/CNTA | Zhang et al. [81] | Electrodeposition method | 1030 | 100; 23% |
| PANI/CNT | Zhang et al. [76] | In situ chemical oxidative polymerization | 7926 | 1000; 4% |
| PANI/CNT/Ppy/PEDOT | Peng et al. [98] | Electrochemical co-deposition technique | ~200 | 5000; 25% |
| PANI/graphene/CNT | Zhang et al. [99] | In situ polymerization | 430 | 600; 4% |
| PANI/CNT/CNF | Zhou et al. [100] | In situ polymerization | 503 | 1000; 8% |
| Graphene oxide/CNT | Huang et al. [101] | In situ anodic electro-polymerization (AEP) | 229 | - |
| Graphene/CNT/PANI | Lu et al. [102] | Flow-directed assembly | 569 | 5000; 4% |
| Graphene (Gr), Ti coated CNT/Fe3O4/PANI | Bhattacharya et al. [103] | Sol-gel and acid mixture | 464.7 | 1000 |
| cellulose/CNT/PANI | Shi et al. [104] | In situ synthesis | 495.7 and 408.8 | 1000; 19% |
| Graphene/CNT and Graphene/CNT/PANI | Cheng et al. [105] | Electrodeposition | 134.5 | 1000;18% |
| CNT/PANI cotton-shaped fiber | Xie et al. [106] | In situ chemical polymerization | 144 | 200; 15.5% |
| Graphene nanosheet (GNS)/CNT/PANI | Yan et al. [107] | In situ polymerization | 1035 | 1000; 6% |
| (Ag-PANI)/MWCNTs | Dhibar and Das [108] | In situ polymerization | 528 | 1000; 16% for PANI 9% for Ag/PANI |
| CNT/CB/GNSs PANI | Zhou et al. [109] | In situ polymerization | 450 | 1000; 16% for PANI 9% for Ag/PANI |
Table 1. The following table will summarize the main concepts addressed in this review.
Conclusion
Nowadays, the development of carbon nanotube-based nanocomposite materials attracted a lot of attention. As a result, R&D teams in different areas and countries started the development of novel methodologies and nanocomposites to utilize the advantages of conductive nanoparticles like polyaniline and carbon nanotube to improve the electrochemical properties of super capacitors and energy applications. Meanwhile, for CNT/PANI electrodes and other materials deposited with CNT/PANI have been examined and showed a significant enhancement in the capacitance and energy. It is worth mentioning that PANI/CNT composites seem to achieve such high capacitance due to the combination of low electrochemical impedance and excellent intra and interstructure porosity, which facilitates ion diffusion on the PANI active sites. Of these, a maximum specific capacitance reported in literature of 1147 mF/cm2 at a scan rate of 10 mV/s for a stretchable isotropic buckled carbon nanotube (CNT) electrode could be easily achieved using electrochemical deposition of PANI film on CNT. Respectively, a maximum energy density was found to be 114 Wh/kg at a current density of 1 A/g for a hybrid CNT/PANI electrode. These are promising results for cheap and simple super capacitors and energy-storage devices with both high specific capacitance and energy density, using carbon material (carbon nanotube) with conductive polymer (polyaniline) for the electrodes with a very long lifetime of more than thousands of cycles. A large variety of flexible, stretchable, wearable and even transparent super capacitors based on CNT/ PANI electrodes of various shapes (e.g. planar, fiber-like) with controllable architectures (e.g. aligned, pillared, foam-like) have been developed. The integration of flexible super capacitors with other energy devices into planar and wire-shaped self-powered systems has also been demonstrated in some elecronic devices. In many cases, however, the material properties and/or device characteristics need to be optimized for further enhancement of the device performance.
Due to their tedious processability, these composites have very small commercial production. The most common shortcomings of PANI-CNT based electrodes are the high toxicity and high cost of production. Recent developments in the field have clearly indicated the versatility of CNT/PANI composites for making flexible super capacitors with novel features for medical applications. Because of the wide range of their applications, it is believed that shortly carbon-based conducting polymer composites-based technologies will be reliable, short and industrially available. Continued efforts in this promising area could make flexible super capacitors one of the most powerful and efficient energy storage technologies, which will affect every aspect of our modern society.
Acknowledgment
Dr. Nasr Bensalah and Hanaa Dawood thank Qatar University for funding this work through an internal grant number QUUGCAS- DCES-14/15-11.
References
- Béguin F, Presser V, Balducci A, et al. Carbons and electrolytes for advanced super capacitors. Adv Mater. 2014;26(14):2219-51.
- Wang K, Wu H, Meng Y, et al. Conducting polymer nanowire arrays for high performance super capacitors. Small. 2014;10(1):14-31.
- Wang GP, Zhang L, Zhang JJ. A review of electrode materials for electrochemical super capacitors. Chem Soc Rev. 2012;41(2):797-828.
- Stoller MD, Park S, Yanwu Z, et al. Graphene-Based ultracapacitors. Nano Lett. 2008;8(10):3498-502.
- Yan J, Wang Q, Wei T, et al. Recent advances in design and fabrication of electrochemical super capacitors with high energy densities. Adv Energy Mater. 2014;4(4).
- Liu C, Yu Z, Neff D, et al. Graphene-based super capacitor with an ultrahigh energy density. Nano Lett. 2010;10(12):4863-8.
- Zheng X, Luo J, Lv W, et al. Two-dimensional porous carbon: Synthesis and ion-transport properties. Adv Mater. 2015;27(36):5388-95.
- Bai MHMH, Liu TYTY, Luan F, et al. Electrodeposition of vanadium oxide-polyaniline composite nanowire electrodes for high energy density super capacitors. J Mater Chem A. 2014;2(28):10882-8.
- Wang X, Deng J, Duan X, et al. Crosslinked polyaniline nanorods with improved electrochemical performance as electrode material for super capacitors. J Mater Chem A. 2014;2:12323.
- Kumar V, Lee PS. Redox active polyaniline-h-MoO3 hollow nanorods for improved pseudocapacitive performance. J Phys Chem C. 2015;119(17):9041-9.
- Cai S, Long X, Gan Z. A numerical study of the generation and propagation of internal solitary waves in the Luzon Strait. Oceanol Acta. 2002;25(2):51-60.
- Li J, Zhao X, Zhang Z, et al. Facile synthesis of hollow carbonized polyaniline spheres to encapsulate selenium for advanced rechargeable lithium-selenium batteries. J Alloys Comp. 2015;619:794-9.
- Kerdnawee K, Termvidchakorn C, Yaisanga P, et al. Present advancement in production of carbon nanotubes and their derivatives from industrial waste with promising applications. KONA Powder Part J. 2017;(January).
- Hamouda Z, Wojkiewicz J, Pud AA, et al. Polyaniline-carbon nanotubes composites ? based patch antenna. 2014;(EuCAP):2635-8.
- Zhou C. Carbon nanotube based electrochemical super capacitors. PHD thesis. 2006:1-212.
- Guittet E, Aria AI, Gharib PM, et al. Vertically-aligned carbon nanotubes for super capacitor and the effect of surface functionalization to its performance. (c):130.
- Baughman RH, Zakhidov A, Heer W a. Carbon nanotubes --- the route toward applications. Science (80-). 2002;297(5582):787-92.
- Science IS. Applications of carbon nanotubes. Seminar. 2005;425:391-425.
- Singh R, Dhand C, Sumana G, et al. Polyaniline/carbon nanotubes platform for sexually transmitted disease detection. J Mol Recognit. 2010;23(5):472-9.
- Odom TW, Huang JL, Kim P, et al. Atomic structure and electronic properties of single-walled carbon nanotubes. Nature. 1998;391(January):62-4.
- Shirakawa H. The discovery of polyacetylene film - The dawning of an era of conducting polymers. Curr Appl Phys. 2001;1(4-5):281-6.
- Heeger AJ. Semiconducting and metallic polymers: The fourth generation of polymeric materials. Curr Appl Phys. 2001;1(4-5):247-67.
- Oueiny C, Berlioz S, Perrin FX. Carbon nanotube-polyaniline composites. Prog Polym Sci. 2014;39(4):707-48.
- Snook GA, Kao P, Best AS. Conducting-polymer-based supercapacitor devices and electrodes. J Power Sources. 2011;196(1):1-2.
- Sabzi RE, Rezapour K, Samadi N. Polyaniline-multi-wall-carbon nanotube nanocomposit