Research Article - Journal of Veterinary Medicine and Allied Science (2018) Volume 2, Issue 2
Use of a selective type V phosphodiesterase inhibitor, PAPiSOL (E4021), in the treatment of pulmonary hypertension in the canine.
Daniel Swartz 1,* Sandra Sexton2, Leslie Curtin2, Bryan Perry31Angiograft LLC, 1576 Sweet Home Road, Amherst, New York, USA
2Roswell Park Cancer Institute, Elm and Carlton Streets, ILAR, Buffalo, New York, USA
3VetNOx Inc, 7954 Transit Rd STE 153, Williamsville, New York, USA
- *Corresponding Author:
- Daniel D. Swartz
Angiograft LLC
1576 Sweet Home Road
Amherst
New York 14228
USA
Tel: 90 272 228 13 50
E-mail: dswartz@angio-graft.com
Accepted Date: 30 October, 2018
Citation: Swartz DD, Sandra S, Leslie C, et al . Use of a selective type V phosphodiesterase inhibitor, PAPiSOL (E4021), in the treatment of pulmonary hypertension in the canine. J Vet Med Allied Sci 2018;2(2):1-4.
Abstract
Pulmonary hypertension (PH) is a progressive disease characterized by chronic elevation in pulmonary arterial pressure (PAP). This disease is poorly recognized in dogs with limited economical and effective treatment available. The secondary messenger cyclic guanosine monophosphate (cGMP), which regulates pulmonary and systemic pressures, is itself regulated by type 5 Phosphodiesterase (PDE-5). In dogs, an intravenous (IV) infusion of U46619 induces pulmonary arterial constriction. Using a selective phosphodiesterase inhibitor (PDI) PAPiSOL (E4021) orally is an effective treatment for decreasing an induced elevated PAP in the dog. Animals:This study was approved by the Institutional Animal Care and Use Committee. We performed a total of 15 studies in 5 Class A, purpose bred, SPF Beagles, male and female. Methods:A dose of 2.5 mg was administered as a dissolved tablet through an oral gastric tube (Tablet) or as a dissolvable strip placed in the buccal cavity (Strip). An acute model of PH was created using the infusion of thromboxane A2 mimetic, U46619. Results:The IV infusion of U46619 caused a dose dependent increase in PAP and a concomitant increase in pulmonary vascular resistance (PVR). Following the stabilization of an elevated PAP, the simultaneous oral treatment with PAPiSOL (E4021) attenuated the level of PAP and PVR. The resultant decrease in PAP with oral treatment with PAPiSOL (E4021) was small in relation to the modest decreases in PVR. Conclusion:These results demonstrated an effective treatment of PH with oral PAPiSOL (E4021) as well, identified PVR as the responsive pulmonary vascular variable.
Keywords
Heart failure, right ventricular hypertrophy, pulmonary arterial hypertension, pulmonary vascular resistance.
Introduction
Pulmonary arterial hypertension (PAH) is the presence of elevated pulmonary arterial pressures (PAP), caused by either an increased pulmonary blood flow or an increase in pulmonary vascular resistance (PVR). PAH is a progressive disease characterized by chronic elevation of PAP in dogs and is defined by a PAP systolic/mean ratio >30/20 mmHg at rest in un-anaesthetized dogs [1,2]. In the absence of significant left to right cardiac shunting, increased resistance to pulmonary blood flow is the primary mechanism of PAH in dogs. Increased PVR is mediated by either an obstruction to blood flow, or pulmonary vasoconstriction. Intraluminal obstruction is seen in cases of Pulmonary Thromboembolism (PTE). PTE is generally a common complication of heartworm disease [3]. In most cases, PAH is associated with vasoconstriction of the pulmonary arteries. Pulmonary arterial vasoconstriction can be caused by primary pulmonary disease, chronically reduced pulmonary arterial oxygen tension, pulmonary arterial constriction due to elevated pulmonary venous pressures attributed to left-sided heart disease, or may be idiopathic. In severe cases, PAH can lead to right-sided heart failure and death [1,2,4].Thromboxane A2, U46619, is a potent mimetic that induces platelet aggregation and selective pulmonary vasoconstriction at low doses, and its plasma levels are increased in animals and humans with PAH [5,6]. In dogs, an intravenous (IV) infusion of U46619 induces pulmonary arterial constriction [7,8].
Methods
The Institutional Animal Care and Use Committee for Roswell Park Cancer Institute approved this study. Beagles used were Class A, purpose bred, SPF animals. Five beagles, (Marshal BioResources, North Rose, NY) male and female, were each used for three studies with one-week recovery between studies. Dogs weighed 6.2-14.2 kg. Dogs received a combined SQ injection of Glycopyrrolate (0.01 mg/kg), Butorphanol (0.2 mg/kg) and Acepromazine (0.02–0.05 mg/kg) for premedication/sedation in the lateral flank in their housing cage. Dogs then received an IV injection of telazol (tiletamine/ zolazepam mixture of 50 mg/mL each of tiletamine and zolazepam; 0.2 mL/4.5kg) to induce general anesthesia. Paralube ophthalmic ointment was applied to the dog’s corneas. The vocal cords were sprayed with Cetacaine spray to prevent laryngospasm. The dog was intubated with a size 7-10 mm cuffed endotracheal tube, and attached to an isoflurane anesthesia machine (2-5% isoflurane, titrated to effect) with a ventilator. The dog was ventilated at a rate of 7 breaths per minute with a tidal volume of 10-20 mL/kg. A 22 gauge IV catheter was placed in the cephalic vein and LRS was administered at a rate of 5-10 mL/kg/hr during the procedure. A second IV catheter was placed in the external jugular vein for IV administration of U46619 (thrombaxane A2 mimetic, 1.35-3.60 μg/kg/min) via syringe pump. The surgical area was shaved and prepared with chlorhexidine scrub, followed by betadine and alcohol. The dog was positioned in dorsal recumbency on the surgical table on a warm water recirculating heating pad or other heating device (forced air warmer, etc). A capnograph was used to monitor oxygen/CO2 during anesthesia. Reflexes were checked to ensure that the dog was in an adequate surgical plane prior to the start of surgery. Dogs were monitored continuously during surgery. Parameters that were monitored included: heart rate, EKG, blood pressure, respiratory rate, blood oxygen saturation, CO2 level, mucous membrane color, capillary refill time, body temperature, and anesthetic plane. A one inch incision was made in the skin and a 3-Fr fluidfilled catheter was placed via the left femoral artery into the descending aorta to measure systolic, mean, and diastolic systemic arterial pressure (SAP) The right and left groin were alternated for each consecutive procedure. A Swan– Ganz catheter (5 Fr thermodilution catheter, TC-504, Nihon Kohden) was inserted through the jugular vein and advanced into the pulmonary artery to measure the systolic, mean, and diastolic PAP. Catheter placement had taken take less than 2 Hrs. PAPiSOL was administered PO via gavage, (tablet 2.5 mg was dissolved in 2 mL saline) or buccal strip (Cure Pharmaceuticals, Oxnard, CA) placed in oral buccal cavity. Cardiac output (CO) was calculated as the average of three measurements obtained by thermodilution (CCOM monitor, CO-203, Terumo) with an injection of 5 mL of normal saline kept at 4°C [8,11]. Pulmonary vascular resistance (PVR), systemic vascular resistance (SVR) and PVR to SVR ratio (Rp/Rs ratio) were calculated [11]. At the conclusion of the monitoring period, the catheters were withdrawn and the vessel ligated with 2-0 silk suture. The incision in the groin was then sutured with 3-0 ethilon on a cutting needle. Buprenorphine (0.02 mg/kg) was given prior to recovering the dog from anesthesia. The dog was monitored until it recovered from anesthesia, the endotracheal tube cuff was deflated, and the endotracheal tube removed. The surgical procedure detailed here was repeated 3 times on each dog with the final procedure being terminal, the dogs were then euthanized (Fatal-Plus Solution, 1mL/4.5kg, IV).
Results
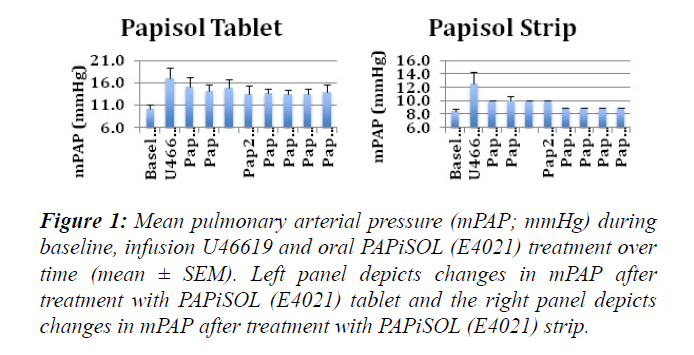
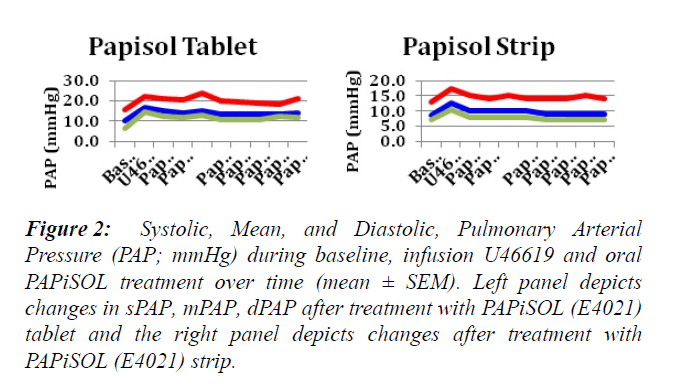
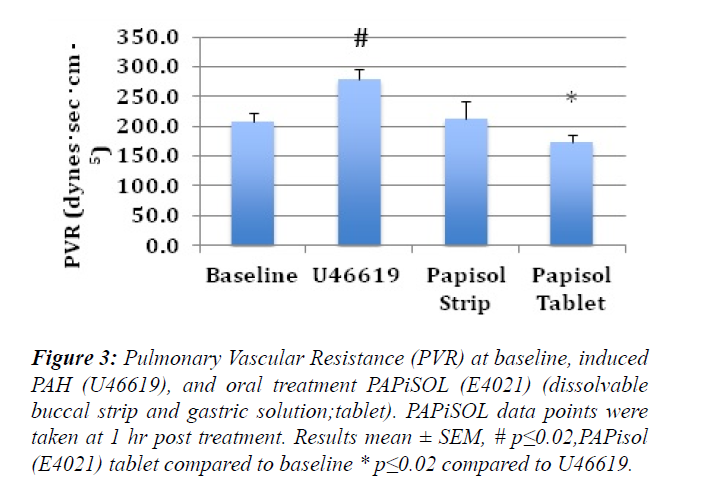
The induction of PAH with U46619 significantly increased the mPAP, sPAP, dPAP and PVR (Figures 1-3). The treatment of induced PAH with oral PAPiSOL (E4021), tablet and strip, decreased mean, systolic and diastolic PAP after 20 minutes and maintained the decrease 4 hours (Figures 1 and 2). The PAPiSOL (E4021) strip (15 min) had a tendency to have a more rapid onset of therapeutic effect in decreasing PAP than PAPiSOL (E4021) tablet (30 min), data not shown.The induction of PAH with U46619 did not significantly change the HR, SpO2, mSAP, sSAP, and dSAP. Also, the treatment with oral PAPiSOL (E4021) did not significantly decrease mSAP, sSAP, dSAP or alter HR and SpO2. (Table 1).The treatment of induced PAH with oral PAPiSOL (E4021), tablet and strip, decreased mean, systolic and diastolic PAP after 20 minutes and maintained the decrease 4 hours (Figure 2).Pulmonary vascular resistance (PVR) measurements:The pulmonary vascular resistance can be calculated in units of dyn·s·cm−5 as 80 (mean pulmonary arterial pressure – mean pulmonary arterial wedge pressure)cardiac output. Pulmonary vascular resistance (PVR) is significantly increased with the induction of PAH with constant infusion U46619. The treatment of PAH with oral PAPiSOL (E4021) (E4021) greatly decreased PVR utilizing the PAPiSOL strip and significantly decreased PVR with the PAPiSOL (E4021) tablet (Figure 3).
Figure 1: Mean pulmonary arterial pressure (mPAP; mmHg) during baseline, infusion U46619 and oral PAPiSOL (E4021) treatment over time (mean ± SEM). Left panel depicts changes in mPAP after treatment with PAPiSOL (E4021) tablet and the right panel depicts changes in mPAP after treatment with PAPiSOL (E4021) strip.
Figure 2: Systolic, Mean, and Diastolic, Pulmonary Arterial Pressure (PAP; mmHg) during baseline, infusion U46619 and oral PAPiSOL treatment over time (mean ± SEM). Left panel depicts changes in sPAP, mPAP, dPAP after treatment with PAPiSOL (E4021) tablet and the right panel depicts changes after treatment with PAPiSOL (E4021) strip.
Figure 3: Pulmonary Vascular Resistance (PVR) at baseline, induced PAH (U46619), and oral treatment PAPiSOL (E4021) (dissolvable buccal strip and gastric solution;tablet). PAPiSOL data points were taken at 1 hr post treatment. Results mean ± SEM, # p?0.02,PAPisol (E4021) tablet compared to baseline * p?0.02 compared to U46619.
Table 1: Systemic and physiological parameters. Data are shown as means  ±  standard deviation (SD). Bpm, beats/min. Papisol(E4021) values represent measurements taken at 1 hr. Comparing to baseline or U46619, no values have P<0.05.
| Papisol Tablet | Papisol Strip | |||||
|---|---|---|---|---|---|---|
| Â Â Baseline | Â U46619 | Â Papisol | Â Baseline | Â Â U46619 | Â Papisol | |
| Heart rate (bpm) | 109 ± 14 | 121 ± 9 | 131 ± 14 | 120 ± 15 | 115 ± 15 | 122 ± 15 |
| Pulse oximetry (%O2) | 95 ±2 | 96±1 | 97 ± 2 | 94 ± 2 | 95 ± 1 | 94 ± 1 |
| Systolic SAP (mmHg) | 108.8 ± 14.5 | 121.0 ± 9.5 | 131.5 ± 14.0 | 115.3 ± 5.7 | 124.0 ± 11.6 | 122.7 ± 5.5 |
| Mean SAP (mmHg) | 89.3 ± 11.5 | 92.3 ± 5.9 | 95.5 ± 8.3 | 92.0 ± 7.4 | 97.5 ± 8.5 | 91.7 ± 6.7 |
| Diastolic SAP (mmHg) | 75.0 ± 13.7 | 81.5 ± 8.7 | 81.5 ± 9.0 | 81.3 ± 9.2 | 84.8 ± 8.2 | 76.0 ± 6.9 |
Discussion
The present summary relates to the pulmonary vascular hemodynamics of the Canine and a therapeutic platform employing the Type 5 Phosphodiesterase Inhibitor (PDI) PAPiSOL (E4021) to treat pulmonary hypertension (PH). It also serves to call attention to the untoward effects of sustained pulmonary hypertension, which often results in irreversible vascular pathology leading to narrowing and/or occluding of the pulmonary arteries and arterioles [12]. The drug PAPiSOL (E4021) is selective for the lung based on its high potency as evidenced from the low dosage regimen required for effect (i.e 100 μg/kg versus Sildenafil 1-2 mg/kg). This is a unique characteristic found exclusive to PAPiSOL (E4021) and not available among all the other PDIs currently employed to manage this condition. It is generally accepted by the scientific community that sustained pulmonary hypertension from an assorted number of etiologies can result in clinically evident pulmonary vascular pathophysiologic events [13] with a deteriorating condition resulting in eventual right-sided heart failure and death. Pulmonary hypertension is defined as elevated blood pressure in the pulmonary vascular system. In the canine, acute Pulmonary Arterial Hypertension is most commonly recognized in association with heartworm infection. Notwithstanding PH has numerous etiologies related to respiratory disease and / or cardiovascular disorders and can be accompanied by a poor prognosis [13,14]. The normal pulmonary vasculature is a low pressure, low resistance, high capacitance system. Basal physiologic values for pulmonary arterial (PA) pressures in dogs are approximately 25 mm Hg systolic, 8 mm Hg diastolic, and 12-15 mm Hg mean. Significant increases in pulmonary blood flow are normally well tolerated with minimal increase in PA pressure because of the highly distensible pulmonary vasculature in conjunction with the large pulmonary capillary surface area. Pulmonary hypertension is a life threatening disease that was until recently under diagnosed in the canine. It is defined as an increase in PA systolic pressures greater than 35 mm Hg or a mean PA pressure greater than 25 mm Hg. A number of pathophysiologic events are responsible for PH in dogs. They are divided into primary PH and PH secondary to an underlying disorder. Diseases, which are commonly seen to result in secondary PH, include chronic respiratory disease (chronic bronchitis, interstitial lung disease, pulmonary thromboembolism) and cardiovascular disorders resulting in elevated left atrial or left ventricular end diastolic pressures (degenerative mitral valve disease), cardiomyopathies, and congenital heart disease. Acute pulmonary hypertension can be a life-ending event. Whether as acute or chronic presentation an increase in pulmonary vascular resistance (PVR) is responsible for PH. A now common treatment suggests the use of the type V PDI Sildenafil Citrate (Viagra) and Tadalafil (Cialis) as agents for managing PH in the Canine. Though they have shown some utility in this regard, it is not without a number of less than optimum side effects related to their lack of comparable selectivity for the pulmonary vasculature. The PDI PAPiSOL (E4021) has shown to be highly selective for the lung vasculature without any untoward side effects. The type V PDI PAPiSOL (E4021) has shown, when given as a single agent in well-defined concentrations in the Canine to sustain a reduction in pulmonary vascular resistance for several hours. The subjects did not experience any untoward effects. Systemic hemodynamics (eg. cardiac output, blood pressure) remained unchanged from controls and was within normal limits.
Conclusion
In summary the results of this study are presented to validate the treatment effectiveness of the PDI PAPiSOL (E4021) on pulmonary hypertension, without the side effect of systemic hypotension, as demonstrated from an experimental model of pulmonary hypertension in the Canine.
Discussion
An alternative mode of application is the administration of an oral preparation of the PAPiSOL (E4021) at (100μg – 200μg / kg BW; 1x to 2x every 24 hours) for the remaining life of the subject. Current veterinary medical practice and the veterinary scientific community proffer that the Canine is only to be treated for aberrant elevated PH when the systolic value is 75 mm hg or > [3]. Evidenced based medicine suggests an attempt should be made to manage the deleterious effects of elevated PH over time. The drug moiety PAPiSOL (E4021), with its high selectivity for the lung vasculature and little or no known untoward effects, offers a sustainable therapeutic platform to manage this condition in the Canine. It is critical that the dog with mild to moderate Pulmonary Hypertension (35 mmHg - <75 mmHg) be treated prophylactically on a daily basis in order to mute the chronic exposure of ever increasing detrimental sheer forces experienced by the pulmonary vasculature with elevated PA pressures. This represents a new paradigm using PAPiSOL (E4021) in the management of PH in the Canine.
Acknowledgment
VETNOx LLC, Williamsville, NY, supported this project. VETNOx LLC is headquartered in Williamsville, NY and National Cancer Institute (NCI) grant P30CA016056.
Conflict of Interest
Bryan J. Perry, CSO, VETNOx supervised the project independent of the data collection and manuscript preparation. All other authors have no conflict of interest to report. Project was supported by VETNOx LLC.
References
- Johnson L, Diagnosis of pulmonary hypertension. Clinical Techniques in Small Animal Practice. 1999;14:231-36.
- Johnson L, Boon J, Orton EC. Clinical characteristics of 53 dogs with Doppler-derived evidence of pulmonary hypertension: 1992–1996. J Vet Intern Med. 1999;13:440-47.
- Uchide T, Saida K. Elevated endothelin-1 expression in dogs with heartworm disease. J Vet Med Sci. 2005;67:1155-61.
- Zabka TS, Campbell FE, Wilson DW. Pulmonary arteriopathy and idiopathic pulmonary arterial hypertension in six dogs. Vet Pathol. 2006;43:510-22.
- Christman BW, McPherson CD, Newman JH, et al. An imbalance between the excretion of thromboxane and prostacyclin metabolites in pulmonary hypertension. N Engl J Med.1992;327:70–5.
- Adatia I, Barrow SE, Stratton PD, et al. Thromboxane A2 and prostacyclin biosynthesis in children and adolescents with pulmonary vascular disease. 1993;88:2117-122.
- Yamada T, Yukioka H, Hayashi M, et al. Effects of inhaled nitric oxide on platelet-activating factor-induced pulmonary hypertension in dogs. Acta Anaesthesiologica Scandinavica. 1998;42:358-68.
- Gong F, Shiraishi H, Kikuchi Y, et al. Inhalation of nebulized nitroglycerin in dogs with experimental pulmonary hypertension induced by U46619. Pediatrics International. 2000;42:255-58.
- Steinhorn BS, Loscalzo J, Michel T. Nitroglycerin and Nitric Oxide--A Rondo of Themes in Cardiovascular Therapeutics. N Engl J Med. 2015;373:277-80.
- Keravis T, Lugnier C. Cyclic nucleotide phosphodiesterase (PDE) isozymes as targets of the intracellular signalling network: benefits of PDE inhibitors in various diseases and perspectives for future therapeutic developments. Br J Pharmacol. 2012;165:1288-305.
- Tamura M, Kurumatani H, Matsushita T. Comparative effects of beraprost, a stable analogue of prostacyclin, with PGE(1), nitroglycerin and nifedipine on canine model of vasoconstrictive pulmonary hypertension. Prostaglandins Leukot Essent Fatty Acids. 2001;64:197-202.
- Firth AL, Mandel J, Yuan JX. Idiopathic pulmonary arterial hypertension. Dis Model Mech. 2010;3: 268-73. 1Division of Pulmonary and Critical Care Medicine, Department of Medicine, MC 0725, University of California, San Diego, 9100 Gilman Drive, La Jolla, CA.
- Fleming E, Ettinger SJ. Pulmonary Hypertension. Compendium: Cardiology. 2006;28:720-30.
- Hines R. Pulmonary Hypertension and Right-Side Heart Failure In Your Dog:
It’s Signs And Treatment. https://www.2ndchance.info/pulmonaryhypertension.htm.