Research Article - Biomedical Research (2017) Volume 28, Issue 1
Thrombolysis for intra-hospital cardiac arrest related to pulmonary embolism-study design
Antoniu Octavian Petriş1*, Gabriel Tatu-Chiţoiu2, Diana Cimpoeşu3, Irina Costache1 and Diana Ţînţ41Cardiology Clinic, “St Spiridon” County Emergency Hospital, University of Medicine and Pharmacy “Grigore T. Popa”, Iasi, România
2Cardiology Clinic, Floreasca Emergency Hospital, Bucureşti, România
3Discipline Emergency Medicine, “St. Spiridon” County Emergency Hospital, University of Medicine and Pharmacy “Grigore T. Popa”, Iasi, România
4Faculty of Medicine, “Transilvania” University Braşov, ICCO Clinics Braşov, România
- *Corresponding Author:
- Antoniu Octavian Petriş
University of Medicine and Pharmacy “Grigore T. Popa” Iaşi
“St. Spiridon” County Emergency Hospital
Cardiology Clinic Iaşi
România
Accepted on April 28, 2016
Abstract
Background: Acute pulmonary embolism (PE) is a major cause of cardiac arrest with a high in-hospital mortality rate. If a fibrinolytic drug is given in these circumstances, survival and good neurological outcome have been reported in some cases although a study powered to reach significance has not yet been conducted.
Methods: We are planning a prospective, multicenter, double-blind, randomized study carried out in Emergency Department, Intensive Care Unit or hospital wards on assigned adult patients with witnessed in-hospital cardiac arrest induced by high risk pulmonary embolism (diagnosis by right heart dysfunction on Focused Echocardiographic in Emergency Life support - FEEL). Six centers will randomize 170 patients to receive either streptokinase or alteplase (1:1 ratio) during cardiopulmonary resuscitation (CPR).
Results: Primary endpoint of the study is the hospital discharge survival rate. Secondary endpoints are the return of spontaneous circulation, survival after 24 h, survival at 30 days, neurological performance (Cerebral Performance Categories), safety endpoints include major bleeding complications and intracranial hemorrhage.
Conclusions: Administration of the fibrinolytic drug immediately after CPR initiation in patients with cardiac arrest related to acute PE readily diagnosed using FEEL assessment and ensuring the continuation of resuscitative efforts for at least 60 min will provide statistically valid information about a topic which still relies on the experts' consensus.
Keywords
Thrombolysis, Intra-hospital cardiac arrest, Pulmonary embolism.
Introduction
Current guidelines recommend administration of fibrinolytic drugs when cardiac arrest is caused by proven or suspected acute pulmonary embolism (PE), although no prospective or retrospective studies have provided significant power sufficient for a sharp statistical analysis on a subgroup of patients with underlying PE [1]. If a fibrinolytic drug is given in these circumstances, an improvement in survival with good neurological outcome have been reported in cases without delay in thrombolysis administration (not a “rescue solution” in the case of unsuccessful resuscitation or the ‘‘last chance’’ of the patient!) requiring in excess for at least 60-90 min before termination of resuscitation attempts [2]. No substantial difference in overall bleeding risk has been revealed between these drugs, although some of them (streptokinase) are less fibrin-specific than others (rt-PA, alteplase) [3].
Acute PE causes up to 15% of hospital deaths [4]. For inhospital cardio-pulmonary resuscitation (CPR) the overall survival rate remained at approximately 17%, but neurologic recovery in survivors is reasonably good (almost 60% have good cerebral performance at the time of hospital discharge) [5]. Current recommendations based more on consensus than on data from large studies suggest that we may administered a fibrinolytic drug immediately when cardiac arrest is caused by proven or suspected acute pulmonary embolism [1,6,7]. All major Guidelines published recently agree that most contraindications to thrombolysis should be considered relative in patients with life-threatening, high-risk PE [8].
Two major problems are considered as limitations of studies on this topic: acute PE as cause of cardiac arrest needs a faster diagnosis and once initiated (as early as possible) this fibrinolytic therapy should be continued for long enough (60-90 minutes) to show its effect. The ability to determine the cause of arrest is challenging in this emergency situation and PE may be underdiagnosed as a cause of pulseless electrical activity (PEA) [9]. Nowadays it is well proved that echocardiography has the potential to detect reversible causes of PEA (e.g., cardiac tamponade, pulmonary embolism, regional wall motion abnormality, aortic dissection, hypovolaemia, and pneumothorax). The Focused Echocardiography in Emergency Life support (FEEL) protocol usually emphasizes abnormal findings in massive pulmonary embolism: right ventricular enlargement, a consequent increase in right ventricular to left ventricular diastolic diameter and paradoxical septal motion, both in systole and diastole [10]. It also allows the simultaneous exclusion of important left heart or aortic abnormalities [11].
Materials and Methods
In a double-blind, multicenter trial (six centres will randomize 170 patients), we intend for randomly assigned adult patients with witnessed cardiac arrest in Emergency Department, Intensive Care Unit or hospital wards to receive either SK or rt-PA (1:1 ratio) during CPR for a PE-induced cardiac arrest (excluding the enrollment of asystolic patients, amended also in TROICA study because of low survival) [12]. We will compare rt-PA vs streptokinase because nowadays, for all other drugs (reteplase, desmoteplase, and tenecteplase), their use in PE is still off-label [8].
Inclusion criteria:
Patients with IHCA above 18 years and less 75 years old, presenting with/ developing a witnessed IHCA of presumed cardiac origin in Emergency Department, in Intensive Care Unit or in hospital wards, either basic life support had started within 10 min of onset or advanced life support (ALS) is started within 10 min of onset of IHCA, FEEL had been performed within 10 min of onset of IHCA showing sign of right ventricle dysfunction.
Exclusion criteria:
Suspected non-cardiac cause of the IHCA, asystolic patient, known internal bleeding, coagulation disorders, pregnancy, participation in other clinical study, hypersensitivity to the study medication, any other condition that the investigator believed would place the patient at increased risk if included in the trial.
FEEL assessment will be performed using a pocket-sized portable transthoracic echocardiographic imaging device (Acuson P10, Siemens Medical Solutions®), which has a 64- element phased array transducer with a frequency range of 2-4 MHz, with digital storage capabilities and with a set-up time for the image acquisition less than 10 s [13].
When a patient underwent randomization, either streptokinase (accelerated regimen 1.5 M UI in 60 min) or matching alteplase (100 mg in 60 minutes) was injected intravenously during ongoing CPR. Resuscitation according to international guidelines was continued for 60 after administration of the study drug, using a chest compression mechanical device (Lucas-2), which will reduce the effect of rescuer fatigue [14] on the quality of chest compressions [15]. Thrombolitic therapy will be administered as early as possible, with delivery after the first cycle of ALS. Adjunctive heparin can be used. Unfractionated heparin infusion should be stopped during administration of streptokinase or urokinase; it can be continued during rtPA infusion [8]. Based on pulmonary embolism Guidelines recommandation, after this first hour, unfractioned heparin can be added with 1,000 UI/h continuous infusion [8]. The most recent CPR guidelines will be also followed [1].
For ventricular fibrillation/pulseless ventricular tachycardia (VF/VT): three shocks are delivered immediately and CPR is performed during 1 min, during which an endotracheal intubation attempt is performed and if possible mechanical ventilation is initiated.
For non-ventricular fibrillation/non-ventricular tachycardia (non-VF/VT): a 3-min CPR sequence is performed, during which endotracheal intubation is performed and if possible mechanical ventilation is initiated.
• An IV line is inserted;
• The criteria for inclusion/exclusion are checked;
• If they are eligible, the patient is randomized by opening the study drug kit;
• The first injection of adrenaline is performed and the study drug is prepared;
• The IV line is flushed;
• The study drug is injected over 60 min. The time of injection is precisely recorded;
• Further resuscitation does not differ with standard ALS as described in international recommendations:
• In VF/VT sequences of three shocks are delivered every loop and an amiodarone injection is given if defibrillation is unsuccessful;
• Adrenaline boluses are injected every 3 min;
• Sodium bicarbonate may be considered after 10 min of unsuccessful resuscitation.
• Successfully resuscitated patients can be treated by lowering the body temperature (hypothermia) according to local conditions.
Subjects with clinically suspected stroke at any time after randomization should be taken for immediate (within 24 h) computed tomographic scanning (CT) to determine whether an intracerebral hemorrhage (ICH) has occurred. In addition, all such patients should also be seen by a neurologist who could assist with the completion of the necessary documentation. In case of fatal stroke, if no imaging was performed, an autopsy should be performed whenever possible. Neurological and overall outcome will be evaluated on the day of discharge according to assessed by Cerebral Performance Categories (CPC), where CPC 1 indicates a good cerebral performance, CPC 2 moderate cerebral disability, CPC 3 severe neurological disability, CPC 4 coma or vegetative state and CPC 5 dead [16].
Study design and power calculation
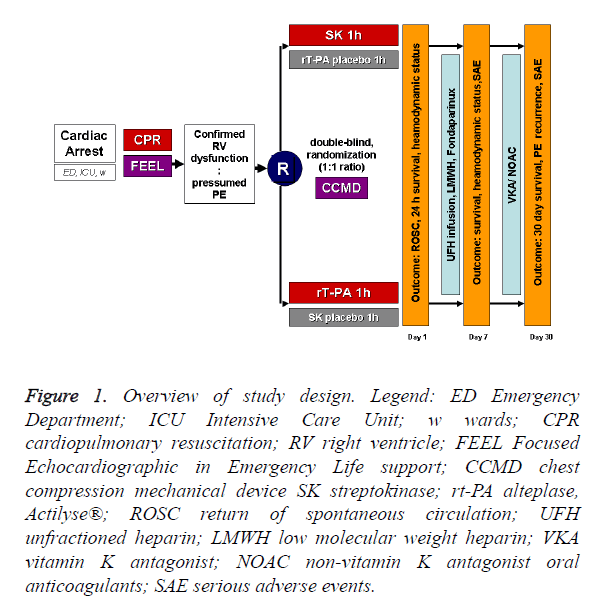
We are planning a prospective, multicenter, double-blind, randomized study (JADAD score=5) [17] carried out in the Emergency Department, Intensive Care Unit or hospital wards on assigned adult patients with witnessed IHCA induced by high risk PE (diagnosis by right heart dysfunction on FEEL) receiving streptokinase or alteplase (1:1 ratio) during cardiopulmonary resuscitation (CPR). For overview of study design see Figure 1. Similar to a pilot study [18], if an improvement in survival to discharge to 50% is considered to be highly significant clinically, 58 patients would be required in each treatment arm for the study to have a power of 80% (α=0.05) to show a significant difference.
Figure 1. Overview of study design. Legend: ED Emergency Department; ICU Intensive Care Unit; w wards; CPR cardiopulmonary resuscitation; RV right ventricle; FEEL Focused Echocardiographic in Emergency Life support; CCMD chest compression mechanical device SK streptokinase; rt-PA alteplase, Actilyse®; ROSC return of spontaneous circulation; UFH unfractioned heparin; LMWH low molecular weight heparin; VKA vitamin K antagonist; NOAC non-vitamin K antagonist oral anticoagulants; SAE serious adverse events.
In previous studies the response within each subject group was normally distributed with standard deviation 20 [12]. If the true difference in the experimental and control means is 10, we will need to study 85 patients in each treatment arm to be able to reject the null hypothesis that the population means of the experimental and control groups are equal with probability (power) 90% (http://biostat.mc.vanderbilt.edu/wiki/Main/ PowerSampleSize). The Type I error probability associated with this test of this null hypothesis is 0.05. We plan to randomly select 200 patients (in order to take into account possible protocol deviations) which will be analysed according to the intention-to-treat principle. The study will be concluded within 2 years after the first randomization.
Data will be stored electronically and analysed using IBM® SPSS® Statistics 20.0 software. All data analysis will be carried out according to a pre-established intention-to-treat analysis plan. Dichotomous data (including the primary outcome) will be compared by using a two-tailed λ2 test with the Yates correction or Fisher’s exact test when appropriate. Continuous measurements will be compared using the Mann- Whitney U test. Two-sided significance tests will be used throughout. Data will be presented as medians (25th and 75th percentiles) or as means (+ standard deviation -SD).
Expected Results
Primary endpoint of the study is the hospital discharge survival rate.
Secondary endpoints are:
• The return of spontaneous circulation (ROSC)
• Survival after 24 h,
• Survival at 30 days,
• Neurological performance: Cerebral Performance Categories (CPC).
• Safety endpoints include:
• Major bleeding complications and intracranial haemorrhage (ICH).
Ethical issues
This study will be conducted according to Good Clinical Practice. The study protocol will be approved by the local institutional review boards of all the participating centers. The informed consent before enrollment was waived: a deffered consent [19].
Discussion
After the first case report by Renkes-Hegendorfer et al. [20] or the first prospective trial by Padosch et al. [21] with streptokinase, two thrombolytic drugs have been studied in randomised studies [12,22]: alteplase (rt-PA) and tenecteplase (TNK). The main outcomes and safety endpoints for this thrombolitic drugs are (TNK vs rt-PA): ROSC 55% vs 21.4%, 24 h survival 30.6% vs 3.6%, survival to discharge 15.1% vs 15, major hemorages 8.9% vs 1.7% and ICH 2.7% vs not reported. Major bleeding complications appear to occur more frequently in patients with cardiac arrest (CA) due to massive PE (in a retrospective cohort study) treated with thrombolytics (9/36 (25%) vs. 3/30 (10%)) even though the difference was statistically not significant (p=0.15) [23].
In a double-blinded randomized controlled trial (low level of evidence; downgraded for imprecision) were 1050 patients were randomized to receive either thrombolytic treatment (tenecteplase) or placebo during CPR only 37 of these patients had confirmed pulmonary embolism as primary cause of cardiac arrest [12]. So, this study was not powered to reach significance in this small subgroup.
Our study advantages
This study will add prospective, multicenter, double-blind, randomized data to the currently limited informations on in-hospital cardiac arrest (IHCA) related to PE comparing the effectiveness of two thrombolytic drugs, in contrast to other studies who compare only thrombolytic vs placebo. Study does not evaluate off-label indications/use of a study drug(s): we compare streptokinase vs alteplase (both with on-label indication for the treatment of PE).
Focused Echocardiographic in Emergency Life support (FEEL) assessment offer in emergency time-sensitive conditions arguments for the diagnosis of PE. An optimal 60-min CPR effort is ensured by the use of a chest compression system. Evidence resulting from this study will be of primary importance in the management of high risk patients with cardiac arrest related to pulmonary embolism.
Conclusion
Using the FEEL assessment for a quick diagnosis of pulmonary embolism as cause of intra-hospital cardiac arrest, during a prospective randomized study in which the onset of thrombolysis therapy was shortly administered after CPR initiation and the chest compression mechanical device assure at least 60 min of CPR effort before discontinuing the resuscitation attempts will provide statistically valid information about a topic that is still based on the experts’ consensus.
Acknowledgements
Scientific research supported by a research grant from University of Medicine and Pharmacy “Grigore T. Popa” Iaşi, based on the contract no. 1851/05.02.2013.
References
- Soar J, Nolan JP, Böttiger BW, Perkins GD, Lott C, Carli P, Pellis T, Sandroni C, Skrifvars MB, Smith GB, Sunde K, Deakin CD. On behalf of the Adult advanced life support section Collaborators. European Resuscitation Council Guidelines for Resuscitation 2015 Section 3. Adult advanced life support. Resuscitation 2015; 95: 100-147.
- Pedley DK, Morrison WG. Role of thrombolytic agents in cardiac arrest. Emerg Med J 2006; 23: 747-752.
- Spöhr F, Böttiger BW. Safety of thrombolysis during cardiopulmonary resuscitation. Drug Saf 2003; 26: 367-379.
- Bailen MR, Cuadra JA, Aguayo De Hoyos E. Thrombolysis during cardiopulmonary resuscitation in fulminant pulmonary embolism: a review. Crit Care Med 2001; 29: 2211-2219.
- Peberdy MA, Kaye W, Ornato JP, Larkin GL, Nadkarni V, Mancini ME, Berg RA, Nichol G, Lane-Trultt T. Cardiopulmonary resuscitation of adults in the hospital: A report of 14720 cardiac arrests from the National Registry of Cardiopulmonary Resuscitation. Resuscitation 2003; 58: 297-308.
- Böttiger BW, Bode C, Kern S, Gries A, Gust R, Glätzer R, Bauer H, Motsch J, Martin E. Efficacy and safety of thrombolytic therapy after initially unsuccessful cardiopulmonary resuscitation: a prospective clinical trial. Lancet 2001; 357: 1583-5.
- Spöhr F, Arntz HR, Bluhmki E, Bode C, Carli P, Chamberlain D, Danays T, Poth J, Skamira C, Wenzel V, Böttiger BW. International multicentre trial protocol to assess the efficacy and safety of tenecteplase during cardiopulmonary resuscitation in patients with out-of-hospital cardiac arrest: The Thrombolysis in Cardiac Arrest (TROICA) Study. European Journal of Clinical Investigation 2005; 35: 315-323.
- Konstantinides S, Torbicki A, Agnelli G, Danchin N, Fitzmaurice D, Galie N, Gibbs JSR, Huisman M, Humbert M, Kucher N, Lang I, Lankeit M, Lekakis J, MaackCh, Mayer E, Meneveau N, Perrier A, Pruszczyk P, Rasmussen LH, Schindler TH, Svitil P, Noordegraaf AV, Zamorano JL, Zompatori M. 2014 ESC Guidelines on the diagnosis and management of acute pulmonary embolism. The Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC). Endorsed by the European Respiratory Society (ERS). Eur Heart J 2014; 35: 3033-3073.
- Comess KA, DeRook FA, Russell ML, Tognazzi-Evans TA, Beach KW. The incidence of pulmonary embolism in unexplained sudden cardiac arrest with pulseless electrical activity. Am J Med 2000; 109: 351-356.
- Breitkreutz R, Walcher F, Seeger FH. Focused echocardiographic evaluation in resuscitation management: concept of an advanced life support-conformed algorithm. Crit Care Med 2007; 35: S150-61.
- Lancellotti P, Price S, Edvardsen T, Cosyns B, Neskovic AN, Dulgheru R, Flachskampf FA, Hassager C, Pasquet A, Gargani L, Galderisi M, Cardim N, Haugaa KH, Ancion A, Zamorano JL, Donal E, Bueno H, Habib G. The use of echocardiography in acute cardiovascular care: recommendations of the European Association of Cardiovascular Imaging and the Acute Cardiovascular Care Association. Eur Heart J Acute Cardiovasc Imaging 2015; 16: 119-146.
- Böttiger BW, Arntz HR, Chamberlain DA, Bluhmki E, Belmans A, Danays T, Carli PA, Adgey JA, Bode C, Wenzel V; TROICA Trial Investigators; European Resuscitation Council Study Group. Thrombolysis during resuscitation for out-of-hospital cardiac arrest. N Engl J Med 2008; 359: 2651-62.
- Fukuda S, Shimada K, Kawasaki T, Fujimoto H, Maeda K, Inanami H, Yoshida K, Jissho S, Taguchi H, Yoshiyama M, Yoshikawa J. Pocket-sized transthoracic echocardiography device for the measurement of cardiac chamber size and function. Circ J 2009; 73: 1092-6.
- Ochoa FJ, Ramalle-Gómara E, Lisa V, Saralegui I. The effect of rescuer fatigue on the quality of chest compressions. Resuscitation 1998; 37: 149-52.
- Tranberg T, Lassen JF, Kaltoft AK, Hansen TM, Stengaard C, Knudsen L, Trautner S, Terkelsen CJ. Quality of cardiopulmonary resuscitation in out-of-hospital cardiac arrest before and after introduction of a mechanical chest compression device, LUCAS-2; a prospective, observational study. Scand J Trauma ResuscEmerg Med 2015; 23: 37.
- Jennett B, Bond M. Assessment of outcome after severe brain damage. Lancet 1975; 1: 480-484.
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996; 17: 1-12.
- Fatovich DM, Dobb GJ, Clugston RA. A pilot randomised trial of thrombolysis in cardiac arrest (The TICA trial). Resuscitation. 2004; 61: 309-313.
- Petriş A, Cimpoeşu D, Ungureanu D. What’s new in ethics of cardio-pulmonary resuscitation research: too little time and too many rules? Intensive Care Med 2015; 41: 120-122.
- Renkes-Hegendörfer U, Hermann K. ErfolgreicheBehandlungeinermassivenfulminantenLungenemboliedurch Streptokinase. Anaesthesist 1974; 23: 500-501
- Padosch SA, Motsch J, Böttiger BW. Thrombolysewährend der kardiopulmonalen Reanimation. Anaesthesist 2002;51:516-532.
- Abu-Laban RB, Christenson JM, Innes GD, van Beek CA, Wanger KP, McKnight RD, MacPhail IA, Puskaric J, Sadowski RP, Singer J, Schechter MT, Wood VM. Tissue plasminogen activator in cardiac arrest with pulseless electrical activity. N Engl J Med 2002; 346: 1522-8. N Engl J Med 2003; 349: 1487.
- Janata K, Holzer M, Kürkciyan I, Losert H, Riedmüller E, Pikula B, Laggner AN, Laczika K. Major bleeding complications in cardiopulmonary resuscitation: the place of thrombolytic therapy in cardiac arrest due to massive pulmonary embolism.Resuscitation 2003; 57: 49-55.