- Biomedical Research (2013) Volume 24, Issue 3
The VKORC1 gene homozygous polymorphism is markedly higher in Crimean Congo Hemorrhagic Fever patients.
Kenan Ahmet Turkdogan1*, Mustafa Karabacak2, Orhan Akpinar1, Oguz Karahan3, Fatma Mutlu Kukul Güven4, Aynur Engin5, Figen Tunali Turkdogan61Departments of Emergency Isparta State Hospital, Isparta, Turkey
2Departments of Cardiology Isparta State Hospital, Isparta, Turkey
3Department of Cardiovascular Surgery, Dicle University Medical School, Diyarbakır, Turkey
4Departments of Emergency, Cumhuriyet University Medical School, Sivas, Turkey
5Departments of Infectious Diseases and Clinical Microbiology, Cumhuriyet University Medical School, Sivas, Turkey
6Departments of Radiology, Isparta State Hospital, Isparta, Turkey
- *Corresponding Author:
- Kenan Ahmet Turkdogan
Departments of Emergeny
Isparta State Hospital 32040 Isparta, Turkey
Tel: +90 246 2115566
Fax: +90 246 2237831
E-mail: drturkdogan@gmail.com,rafieian@yahoo.com
Accepted April 18 2013
Abstract
In Crimean-Congo Hemorrhagic Fever (CCHF), the main target of the virus is endothelial cells, monocytes and hepatocytes. The virus in these cells leads to the development of capillary vessels dysfunction, which induces clinical and pathological changes during the disease. Increase in capillary permeability and coagulation dysfunction constitute a tendency to bleed. In the current study, we aimed to investigate the relationship between VKORC1 and bleeding tendency in CCHF. Forty-eight consecutive patients with a laboratory-confirmed diagnosis of CCHF were treated with blood products, and 37 healthy volunteers as the control group were prospectively enrolled into the study. The DNA was obtained from each sample using PCR amplification method, and VKORC1 1639 G>A gene polymorphisms were scanned in the DNA samples. In CCHF group of patients with bleeding VKORC1 gene were analyzed. Normal genotype was detected in 5 (22.7%) patients, homozygote mutation was detected in 2 (9.1%) patients and heterozygote mutation was detected in 15 (68.2%) patients, respectively. Furthermore, the G allele frequency was statistically higher in study group (51 [53%] vs. 27 [36%]) (p<0.005). It seems to be that VKORC1 gene A allele frequencies saliently higher in CCHF. This might be associated with increased bleeding risk in CCHF. Analyzing of VKORC1 gene polymorphisms could help in the risk stratification of patients with CCHF.
Keywords
Crimean-Congo Hemorrhagic Fever; VKORC1 gene; Bleeding; Risk Stratification
Introduction
Crimean-Congo hemorrhagic fever (CCHF) is a zoonotic viral disease caused by a tick-borne virus of the genus Nairovirus [1]. CCHF has a fatality rate of 3–30% of cases [2], and the pathogenesis of the disease is not well understood. The most common cause of death in patients with CCHF are multiple organ failure (cardiac, cerebral, hepatic, renal and/or pulmonary), intracranial hemorrhage and other serious bleeding from internal organs [3,4]. In severe cases, death occurs as a result of disseminated intravascular coagulation and circulatory shock. Systemic inflammatory reactions also occur during hemorrhagic manifestations [5,6].
The enzyme vitamin K epoxide reductase (VKORC1) reduces vitamin K 2,3-epoxide to the biologically active vitamin K hydroquinone, which catalyzes the production of the blood-clotting proteins II, VII, IX, and X by carboxylation of glutamic acid residues [7,8]. In patients with CCHF, VKORC1 gene mutations inhibited the formation of coagulation by preventing activation of the Kvitamin.
This study was designed to determine whether VKORC1 gene polymorphism could be associated with CCHF and/or its complications such as bleeding.
Method
Study Design
Forty-eight consecutive patients with a laboratoryconfirmed diagnosis of CCHF and 37 healthy volunteers as the control group were prospectively enrolled in the study between January 2011-October 2011. Informed consent was obtained from all participants. Patients who are using anticoagulant drugs and increases the liver enzymes were excluded. Nobody were not died in the CCHF group. This study was performed C type natriuretic peptide studied samples of patients in different laboratories. The diagnosis of CCHF was confirmed by positive qualitative polymerase chain reaction (PCR) test in the Virology Laboratory of the Refik Saydam National Hygiene Center of the Turkish Ministry of Health. The TaqMan-based one-step reverse transcriptase-PCR assay was used to detect CCHF viral RNA.
The activity of ALT (alanine aminotransferase), AST (aspartate aminotransferase) and LDH (lactate dehydrogenase) were measured by using a Beckman Coulter Synchron LX20 (Brea, CA), autoanalyzer with original kits
Study protocol
Total genomic DNA was extracted from the 200 μl of peripheral blood samples using the Nucleospin blood (250) DNA isolation procedure (Macherey - Nagel, Germany). Regions encompassing VKORC1 -1639G>A mutation was simultaneously in vitro amplified and biotinlabeled in a single (multiplex) amplification reaction (Vienna Lab, PGX-Trombo StripAssay, Austria). PCR was performed in a Perkin Elmer 9600 and the profile consisted ofan initial melting step of 2 minutes at 94°C; followed by 35 cycles of 15 seconds at 94°C, 30 seconds at 58°C and 30 seconds at 72°C; and a final elongation step of 3 minutes at 72°C. The mutation analysis was performed by StripAssay technique (Vienna Lab, PGXTrombo StripAssay GmbH, Austria) which is based on the reverse-hybridization principle automatically. The normal, heterozygous and homozygous mutant/nonmutant genotype profiles of each gene were determined using the enclosed CollectorTM sheet.
Statistical analysis
The SPSS 15.0 (SPSS Inc., Chicago, Illinois, USA) package was used for statistical analyses. Results are presented as mean±SD and interquartile ranges, or as percentages and numbers, for categorical data. Continuous variables that were normally distributed were analyzed with the Student’s two-tailed t-test, and unequally distributed variables were analyzed with the Mann–Whitney U-test. Categorical data and proportions were analyzed using the x2- test. A p-value less than 0.05 was considered statistically significant.
Results
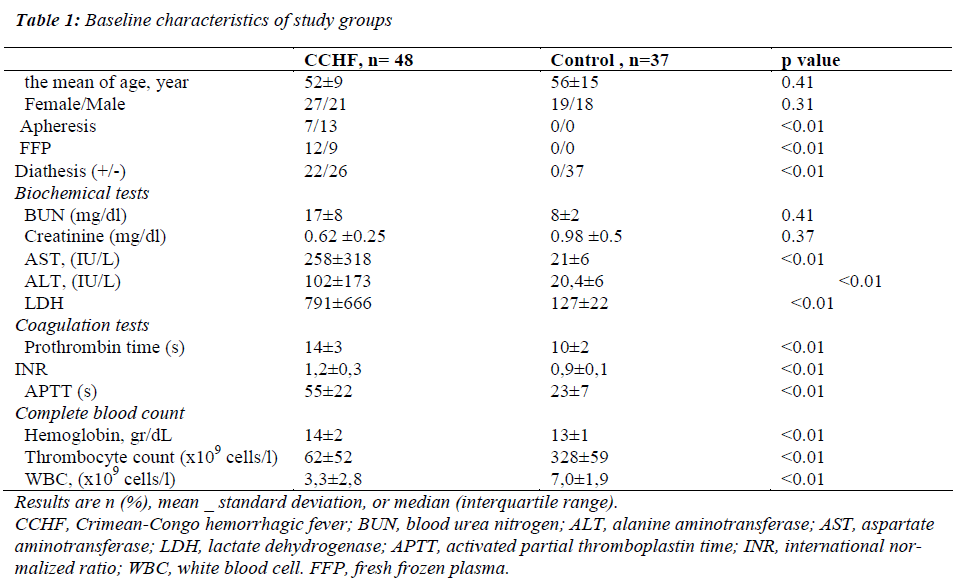
The baseline characteristics of the CCHF group and control group are presented in the Table 1. Serum levels of prothrombin time (s), INR (international normalized ratio) , APTT (activated partial thromboplastin time), hemoglobin, thrombocyte count, WBC (white blood cell), ALT, AST and LDH were significantly higher in the CCHF group than in the control group (p< 0.001). There was no significant difference between two groups in terms of other clinic and laboratory parameters (Table 1) The ALT levels were detected to be 102±173 IU/L and 20,4±6 IU/L in the CCHF group and the control group, respectively. The AST levels were detected to be 258±318 IU/L and 21±6 IU/L in the CCHF group and the control group, respectively. The LDH levels were detected to be 791±666 U/L and 127±22 U/L in the CCHF group and the control group, respectively. The prothrombin time (s) levels were detected to be 14±3 and 10±2 in the CCHF group and the control group, respectively. The INR levels were detected to be 1,2±0,3 and 0,9±0,1 in the CCHF group and the control group, respectively. The APTT (s) levels were detected to be 55±22 and 23±7 in the CCHF group and the control group, respectively. The hemoglobin levels were detected to be 14±2 gr/dL and13±1 gr/dL in the CCHF group and the control group, respectively. The thrombocyte count were detected to be 62±52 and 328±59 in the CCHF group and the control group, respectively. The WBC were detected to be 3,3±2,8 and 7,0±1,9 in the CCHF group and the control group, respectively.
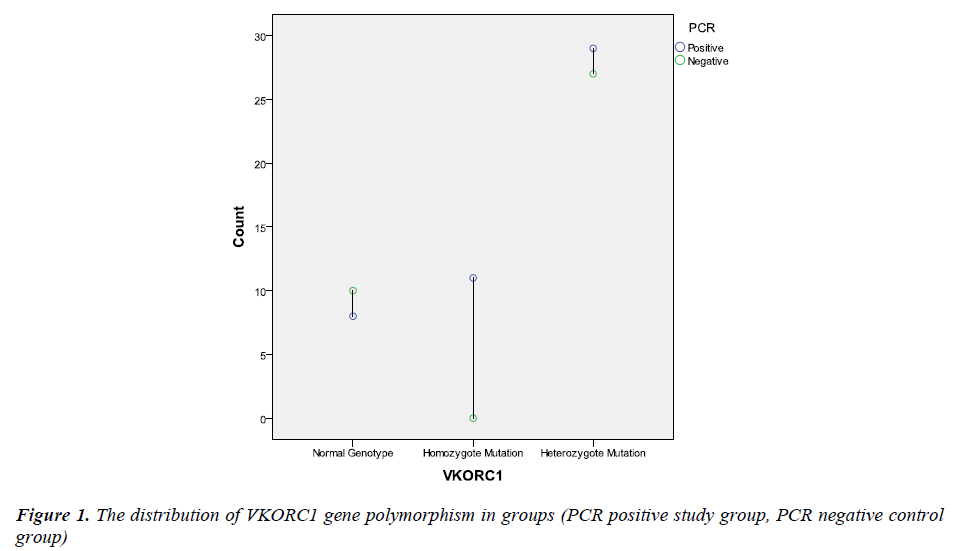
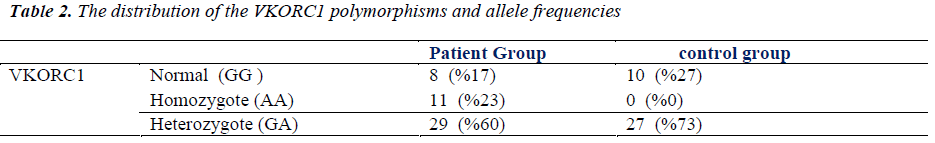
PCR amplification was investigated in all subjects both in control and study groups. All patients were PCR positive in study group, while control subjects were PCR negative. The groups were compared according to VKORC1 gene polymorphism. The distribution of the VKORC1 1639 G>A allelic variants as follows; wild-type normal allele (GG is the wild type) was detected at 8 (16.7%) subject, heterozygote polymorphism (GA is the heterozygous type) was detected at 29 (60.4%) subject and homozygote polymorphism (AA is the homozygous type) was detected at 11 (22.9%) subjects in study group; wild-type normal allele (GG is the wild type) was detected at 10 (27%) subject, heterozygote polymorphism (GA is the heterozygous type) was detected at 27 (73%) subject and homozygote polymorphism (AA is the homozygous type) was not detected at any subjects in control group (Figure 1). Homozygote VKORC1 1639 G>A polymorphism was determined as markedly higher in study group (29 vs. 0). Therefore, the groups were compared according to allele frequencies. The G allele frequency was 45 (47%) and the A allele frequency was 51 (53%) in study group. Moreover, the G allele frequency was 47 (64%) and the A allele frequency was 27 (36%) in control group. The A allele was significantly higher in study group (p<0.05). The distribution of the VKORC1 polymorphisms and allele frequencies were summarized in Table 2.
In patient group bleeding was observed in 22 (45.8%) patient, but 26 (%54.2) patient was not observed. The
localization of bleeding observed in this 22 patients,8 patients had upper gastrointestinal bleeding, 6 patients had lower gastrointestinal bleeding, 5 patients had hemoptysis and 3 patients had microvascular bleeding. There was no statistically significant difference between the localization of bleeding and VKORC1 polymorphism. CCHF patients with a bleeding diathesis, no significant relationship between homozygote polymorphism (p: 0167), the relationship between CCHF patients with bleeding diathesis heterozygote polymorphism was statistically significant (p< 0.001).
Homozygote polymorphism was identified more effective on bleeding in the multivariate logistic regression analysis . But it was not statistically significant (p> 0.05).
Discussion
To the best of our knowledge, this study is the first in the published literature to investigate the VKORC1 gene polymorphisms on the CCHF. We found that VKORC1 gene mutation was significantly increased in CCHF patients. Moreover, A allele frequencies were higher in CCHF patients. Furthermore, even after controlling for confounding parameters, we found that VKORC1 gene polymorphisms were strongly associated with bleeding tendency in patients with CCFH. Previous studies have reported that decreased levels of thrombocytes and fibrinogen, and increased levels of white blood cells, AST, alanine aminotransferase (ALT), creatine kinase, LDH, and soluble urokinase plasminogen activator receptor, as well as a prolonged APTT, are associated with a poor outcome in CCHF patients [10-13]. Our findings of higher ALT, AST, and LDH levels in patients with more severe disease are in accordance with those of previous studies [10-12].
Ozturk et al. and Bodur et al. have shown that the level of hyaluronic acid, sICAM-1 (soluble intercellular adhesion molecule 1), sVCAM-1 (soluble vascular cell adhesion molecule 1), and VEGF-A (vascular endothelial growth factor A) can be used as a prognostic marker in CCHF [14,15]. Furthermore, the severe form of CCHF is characterized by hemorrhage, disseminated intravascular coagulation, vascular dysfunction, and shock [12,16].
Vitamin K is fat soluble vitamin that plays a pivotal role in homeostasis. Some of the haemostatic factor activities are dependent for K vitamin (Factor II, VII, IX and X and also protein C,S) [17,18]. Vitamin K deficiency can cause bleeding in an infancy or geriatric population. Nevertheless, it is not neglected in clotting disorders. Vitamin K epoxide reductase (VKOR) is an integral membrane protein that is an important regulatory of K vitamin metabolism. VKORC1 gene regulates these mechanisms and might be lead to clotting disorders [19,20]. Major bleeding cases were also reported with vitamin K antagonism in previous reports [21]. So, CCHF might have a significant effect on vitamin K. Nabeth and colleagues reported that bleeding was stop after K vitamin supplementation in their case [22]. There was not any definitive report about vitamin K replacement in CCHF. Nevertheless, it was mentioned some vector base reports [23]. The obtained results could be noted as VKORC1 gene mutation, K vitamin and CCHF might have been related with each other.
The specific mechanisms underlying the pathogenesis of CCHF infection have not been clearly explained. Endothelial cells and mononuclear phagocytes are major targets for the CCHF virus [24]. In particular, infection of the endothelium has an important role in the pathogenesis of CCHF. Endothelial damage contributes to hemostatic failure by stimulating platelet aggregation and degranulation, with consequent activation of the intrinsic coagulation cascade [25,26]. Furthermore, it is probable that the endothelium plays a role in the pathogenesis of CCHF through the secretion of cytokines and other inflammatory mediators [24]. Recent studies have demonstrated that serum levels of tumor necrosis factor alpha, interleukin-1, and interleukin-6 are higher in fatal than nonfatal CCHF cases [27,28]. On the other hand, in CCHF patients, tolllike receptors can potentially be related to the natural course and/or severity of the disease, as described in a recent paper [29].
There are some limitations of the current study. First this was a single-center study and it represents data from a tertiary care center. Furthermore, viral load measurements would have provided more insight with regard to the VKORC1 mutation. However, the central laboratory does not provide quantitative results with the confirmation of CCHF in our country.
Within the light of our study, we think that normal allele of VKORC1 could potentially identify those with mild progression of the disease. In addition, we believe that the polymorphism of VKORC1 could be helpful for the selection of patients for aggressive treatment and intensive care unit admission. At the other hand vitamin K concomitant treatment strategies might be beneficial for CCHF. Our results should be supported with further large cohort studies.
Acknowledgements
The authors thank the Refik Saydam Hygiene Center of Ankara, Turkey for testing the serum samples, and our colleagues from the Turkish Ministry of Health for their contributions.
Conflict of interest
No conflict of interest and no funding source to declare.
Ethical approval
The study was performed in accordance with the Declaration of Helsinki for Human Research, and was approved by the institutional ethics committee
References
- Whitehouse CA. Crimean-Congo hemorrhagic fever.Antiviral Res 2004; 64: 145-160.
- Ergonul O, Celikbas A, Dokuzoguz B, Eren S, Baykam N, Esener H. The characteristics of Crimean-Congo hemorrhagic fever in a recent outbreak in Turkey and the impact of oral ribavirin therapy. Clin Infect Dis 2004; 39: 285-289.
- Stickland H. Crimean-Congo hemorrhagic fever. Đn tropical medicine and emerging infectious diseases, Philadelphia, 284-287 p, 2000.
- Mayers D. Tickborne hemorhhagic fever, in Tickborn Infectious Diseases, Diagnosis and Management. Mar- cel Dekker Inc, New. York, 215-31 p, 2000.
- Schnittler HJ, Feldman H. Viral hemorrhagic fever a vascular disease? Thromb Haemost 2003;89:967–72.
- Sannikova IV, Pacechnikov VD, Maleev VV. Respira- tory lesions in Crimean-Congo hemorrhagic fever. Ter Arkh 2007; 79: 20-23.
- Doganci L, Ceyhan M, Tasdeler NF, Sarikayalar H, Tulek N. Crimean-Congo hemorrhagic fever and dif- fuse alveolar haemorrhage. Trop Doct 2008; 38: 252-254.
- Li, T., Chang, C.Y., Jin, D.Y., Lin, P.J., Khvorova, A. & Stafford, D.W. Identification of the gene for vitamin K epoxide reductase. Nature 2004; 427, 541-544 (2004).
- Rost, S., Fregin, A., Ivaskevicius, V., Conzelmann, E., Hortnagel, K. & Pelz, H.J. et al. Mutations in VKORC1 cause warfarin resistance and multiple coagulation fac- tor deficiency type 2. Nature 2004; 427, 537-541..
- Schnittler HJ, Feldman H. Viral hemorrhagic fever a vascular disease? Thromb Haemost 2003; 89: 967-672.
- Swanepoel R, Gill DE, Shepherd AJ, Leman PA, Myn- hardt JH, Harvey S. The clinical pathology of Crimean-Congo hemorrhagic fever. Rev Infect Dis 1989; 11: 794-800.
- Ergonul O, Celikbas A, Baykam N, Eren S, Dokuzoguz B. Analysis of risk-factors among patients with Cri- mean-Congo haemorrhagic fever virus infection: sever- ity criteria revisited. Clin Microbiol Infect 2006; 12: 551-554.
- Yilmaz G, Mentese A, Kaya S, Uzun A, Karahan SC, Koksal I. The diagnostic and prognostic significance of soluble urokinase plasminogen activator receptor in Crimean-Congo hemorrhagic fever. J Clin Virol 2011; 50: 209-211.
- Ozturk B, Kuscu F, Tutuncu E, Sencan I, Gurbuz Y, Tuzun H. Evaluation of the association of serum levels of hyaluronic acid, sICAM-1, sVCAM-1, and VEGF-A with mortality and prognosis in patients with Crimean- Congo hemorrhagic fever. J Clin Virol 2010; 47: 115-119.
- Bodur H, Akinci E, Onguru P, Uyar Y, Basturk B, Gozel MG, et al. Evidence of vascular endothelial damage in Crimean-Congo hemorrhagic fever. Int J In- fect Dis 2010; 14: 704-707.
- Joubert JR, King JB, Rossouw DJ, Cooper R. A noso- comial outbreak of Crimean- Congo haemorrhagic fe- ver at Tygerberg Hospital. Part III. Clinical pathology and pathogenesis. S Afr Med J 1985; 68: 722-728.
- Quick AJ. The role of vitamins in hemostasis. Thromb Diath Haemorrh. 1975; 33(2): 191-198.
- Weston BW, Monahan PE Familial deficiency of vita-min K-dependent clotting factors. Haemophilia. 2008; 14(6): 1209-1213
- Tie JK, Stafford DW. Structure and function of vitamin K epoxide reductase. Vitam Horm. 2008; 78: 103-130
- Garcia AA, Reitsma PH. VKORC1 and the vitamin K cycle. Vitam Horm. 2008; 78: 23-33.
- Henkens IR, Hazenoot T, Boonstra A, Huisman MV, Vonk-Noordegraaf A. Major bleeding with vitamin K antagonist anticoagulants in pulmonary hypertension. Eur Respir J. 2012 Aug 30. (Epub ahead of print) doi:10.1183/09031936.00039212
- Nabeth P, Thior M, Faye O, Simon F. Human Crimean- Congo hemorrhagic fever, Sénégal. Emerg Infect Dis. 2004; 10(10): 1881-1882
- Bausch DG: Marburg and Ebola viruses, “PIER: The Physicians’ Information and Education Resource American College of Physicians, electronic publication: http://pieracponlineorg/physicians/diseases/ d891/d891html (2006).
- Burt FJ, Swanepoel R, Shieh WJ, Smith JF, Leman PA, Greer PW, et al. Immunohistochemical and in situ lo- calization of Crimean-Congo hemorrhagic fever (CCHF) virus in human tissues and implications for CCHF pathogenesis. Arch Pathol Lab Med 1997; 121: 839-846.
- Ergonul O. Crimean-Congo haemorrhagic fever. Lan- cet Infect Dis 2006; 6: 203-214.
- Appannanavar SB, Mishra B. An update on Crimean Congo hemorrhagic fever. J Glob Infect Dis 2011; 3: 285-292.
- Ergonul O, Tuncbilek S, Baykam N, Celikbas A, Do- kuzoguz B. Evaluation of serum levels of interleukin (IL)-6, IL-10, and tumor necrosis factor-alpha in pa- tients with Crimean-Congo hemorrhagic fever. J Infect Dis 2006; 193: 941-944.
- Papa A, Bino S, Velo E, Harxhi A, Kota M, Antoniadis Cytokine levels in Crimean-Congo hemorrhagic fe- ver. J Clin Virol 2006; 36: 272-276.
- Engin A, Arslan S, Kizildag S, Ozturk H, Elaldi N, Dokmetas I, et al. Toll-like receptor 8 and 9 polymor- phisms in Crimean-Congo hemorrhagic fever. Mi- crobes Infect 2010; 12: 1071-1078.