Research Article - Biomedical Research (2017) Volume 28, Issue 16
The use of adipose-derived stem cells for the fabrication of three-dimensional spheroids for the osteogenic differentiation
Jae-Yong Tae#, Sung-Il Lee#, Youngkyung Ko and Jun-Beom Park*
Department of Periodontics, College of Medicine, The Catholic University of Korea, Seoul, 06591, Republic of Korea
#These authors contributed equally
- *Corresponding Author:
- Jun-Beom Park
Department of Periodontics, Seoul St Mary's Hospital
College of Medicinem, The Catholic University of Korea
Republic of Korea
Accepted date: July 18, 2017
Abstract
Adipose-derived stem cells have been applied for the treatment of various diseases. This study was performed to fabricate the three-dimensional stem cell spheroids with adipose-derived stem cells and to evaluate the effects of cell number and culture duration on the viability and the osteogenic differentiation of three-dimensional cultures. Adipose-derived stem cells were purchased, and stem cell spheroids were formed using the silicon elastomer-based concave microwells in the amount of 1 × 105, 3 × 105, and 6 × 105. The morphology, the cell viability, and the osteogenic differentiation of stem cell spheroids were evaluated. Adipose-derived stem cells formed spheroids in the silicon elastomer-based concave microwells. A higher stem cell number led to spheroids with larger diameters. Most of the cells in the cell spheroids emitted green fluorescence. The relative Cell Counting Kit-8 showed that higher values were seen with higher stem cell numbers. The increase of alkaline phosphatase activity assays with longer incubation time in low dosage group and mineralized extracellular deposits were evenly noted in each group. This study clearly showed that adipose-derived stem cells formed spheroids in the silicon elastomer-based concave microwells and that osteogenic differentiation was achieved with higher activity and longer incubation time. Spheroid-based cell delivery could be used as a simple and effective strategy for stem cell therapy.
Keywords
Adipose tissue, Cell differentiation, Cellular spheroids, Cell survival, Stem cells
Introduction
During the last few decades, stem cell therapies have been highlighted for their potential to heal damaged tissue and to aid in tissue reconstruction [1,2]. Mesenchymal stem cell transplantation has been tested in animal and clinical fracture studies [3]. Bone marrow stem cells have been used most widely, and bone marrow mesenchymal stem cells are a good candidate for tissue engineering and clinical application [2,4]. Mesenchymal stem cells can be derived from various adult tissues, including adipose tissues, skeletal muscle, synovium, dental follicles of wisdom teeth, dental pulp, and gingiva [5,6]. It was suggested that mesenchymal stem cells derived from adipose tissue could be a good alternative to mesenchymal stem cells derived from bone marrow for use in regenerative treatments [7]. Adipose-derived mesenchymal stem cells are multipotent and can differentiate into various cell types, including osteocytes, adipocytes, neural cells, vascular endothelial cells, cardiomyocytes, pancreatic β-cells, and hepatocytes [8]. Adipose-derived mesenchymal stem cells have a competitive advantage over other types of stem cells, including immunomodulatory properties, low immunogenicity, and safety [9]. Moreover, their secretion of trophic factors renders the therapeutic and regenerative outcome in a wide range of applications [10].
Adipose-derived stem cells have been applied for the treatment of various diseases [7,11,12]. The assessment of meniscal lesion regeneration by use of mesenchymal stem cells is derived from adipose tissue [7]. Patients treated with low-dose adipose mesenchymal stem cells experienced significant improvements in knee pain levels and function compared with baseline [11]. Adipose-derived mesenchymal stem cells are reported to be associated with increased cardioprotective and reparative mechanisms and with better cardiac magnetic resonance-measured perfusion [12]. It should be noted that monolayer cell cultures cannot completely represent three-dimensional tissue characteristics [13]. A three-dimensional culture system can be made with multi-cells, and this may be used for the evaluation of cell-to-cell interaction [14]. This study was performed to fabricate the three-dimensional stem cell spheroids with adipose-derived stem cells and to evaluate the effects of cell numbers and the culture duration on the viability and the osteogenic differentiation of three-dimensional cultures.
Material and Methods
Formation of cell spheroids with human adiposederived stem cells
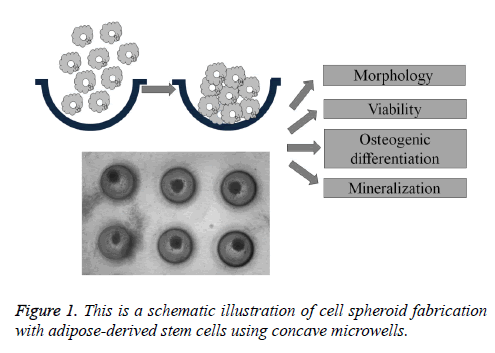
Adipose-derived stem cells derived from human lipoaspirate tissue were purchased (R7788-115, StemPro® human adiposederived stem cells, Invitrogen Corporation, Carlsbad, CA, USA). Stem cell spheroids were formed using the silicon elastomer-based concave microwells (H389600, StemFIT 3D; MicroFIT, Seongnam, Korea) with 600 μm diameters. Stem cells were loaded in the amount of 1 × 105, 3 × 105, and 6 × 105 and grown in an alpha-modified, minimal essential medium (α-MEM, Gibco, Grand Island, NY, USA) at 37°C with 5% CO2 and 95% O2 (Figure 1). An inverted microscope (Leica DM IRM, Leica Microsystems, Wetzlar, Germany) was used to evaluate the stem cell spheroid formation, and diameter and shape evaluation were done on Day 1 and Day 4.
Cell viability determination
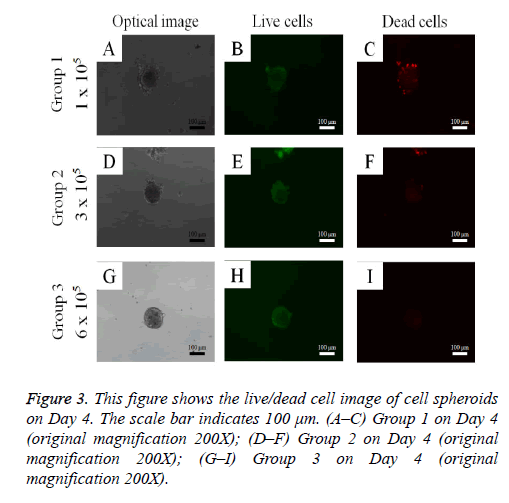
Cell spheroid viability was qualitatively analyzed using a commercially available kit (Live/Dead Kit assay; Molecular Probes, Eugene, OR, USA) on Day 4. Cell spheroids were washed twice with phosphate-buffered saline (PBS; Welgene, Daegu, South Korea), followed by suspension in 1 ml of α- MEM, containing 2 μl of 50 mM calcein acetoxymethyl ester working solution and 4 μl of the 2 mM ethidium homodimer-1 for 15 min at room temperature. The cell spheroids stained with calcein acetoxymethyl ester and ethidium homodimer-1 were observed under a fluorescence microscope (Axiovert 200; Zeiss, Germany). The viable cells produced an intense, uniform, green fluorescence, and dead cells showed a red fluorescence with this assay.
Quantitative cell-viability analysis was performed using a commercially available kit (Cell Counting Kit-8; Dojindo, Tokyo, Japan) on Day 4. WST-8 [2-(2-methoxy-4- nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H tetrazolium, monosodium salt] was added, and the spheroids were incubated for 1 h at 37°C. The spectrophotometric absorbance of the samples was measured at 450 nm using a microplate reader (BioTek, Winooski, VT, USA). This assay relies on mitochondrial dehydrogenases’ ability to oxidize WST-8 into a formazan product.
Alkaline phosphatase activity assays
Stem cell spheroids grown with osteogenic induction media (STEMPRO® Osteogenesis Differentiation Kit; Gibco) were obtained on Day 4. Alkaline phosphatase activity assays were performed using a commercially available kit (K412-500, BioVision, Inc., Milpitas, CA, USA). The cells were resuspended with an assay buffer, sonicated, and then centrifuged to remove insoluble material. Supernatant was mixed with p-nitrophenylphosphate substrate and incubated at 25°C for 60 min. The optical density was determined spectrophotometrically at 405 nm.
Mineralization assay with Alizarin Red S staining
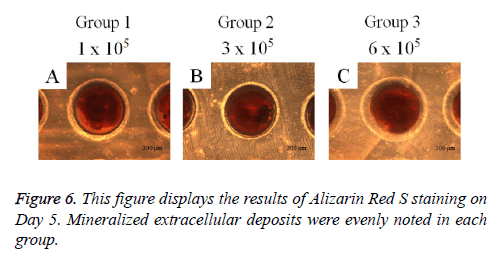
Cell spheroids were grown with osteogenic induction media (STEMPRO® Osteogenesis Differentiation Kit; Gibco). On Day 5, cell spheroids were washed twice with PBS (Welgene), fixed with 70% ethanol, and rinsed twice with deionized water. Cell spheroids were stained with Alizarin Red S for 30 min at room temperature. Cell spheroids were then observed under an inverted microscope (Leica DM IRM), and the images were saved as JPEGs.
Statistical analysis
The data are represented as means ± the experiments’ standard deviations. A Shapiro-Wilk normality test was performed, and a one-way analysis of variance using Tukey’s post hoc test was performed to determine the differences between the groups using a commercially available program (SPSS 12 for Windows, SPSS Inc., Chicago, IL, USA). The significance level was determined at 0.05.
Results
Cell morphology evaluation and cell spheroid diameter changes
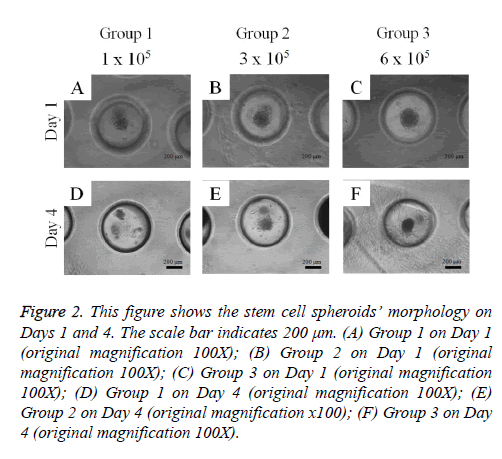
Adipose-derived stem cells formed spheroids in concave microwells (Figure 2). There was an increasing trend in the diameter of the spheroids with higher cell numbers. The spheroids’ morphology on Day 4 was similar to that on Day 1.
Figure 2: This figure shows the stem cell spheroids’ morphology on Days 1 and 4. The scale bar indicates 200 μm. (A) Group 1 on Day 1 (original magnification 100X); (B) Group 2 on Day 1 (original magnification 100X); (C) Group 3 on Day 1 (original magnification 100X); (D) Group 1 on Day 4 (original magnification 100X); (E) Group 2 on Day 4 (original magnification x100); (F) Group 3 on Day 4 (original magnification 100X).
Cell viability determination
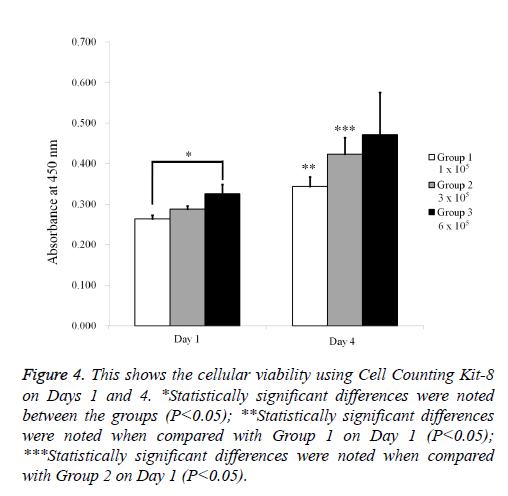
The qualitative cell spheroid viability results were analyzed using a Live/Dead Kit assay on Day 4 (Figure 3). In all cases, most of the cells in the cell spheroids emitted green fluorescence, and the morphology was well maintained. The relative Cell Counting Kit-8 assay values of Groups 1, 2, and 3 on Day 1 were 100.0 ± 3.0%, 109.0 ± 3.1%, and 123.3 ± 9.1%, respectively (P<0.05; Figure 4). The relative Cell Counting Kit-8 assay values of Groups 1, 2, and 3 on Day 4 were 130.5 ± 8.7%, 160.4 ± 15.6%, and 178.9 ± 39.6% when the value of Group 1 on Day 1 was considered 100%.
Figure 4: This shows the cellular viability using Cell Counting Kit-8 on Days 1 and 4. *Statistically significant differences were noted between the groups (P<0.05); **Statistically significant differences were noted when compared with Group 1 on Day 1 (P<0.05); ***Statistically significant differences were noted when compared with Group 2 on Day 1 (P<0.05).
Alkaline phosphatase activity assays and mineralization assay with Alizarin Red S staining
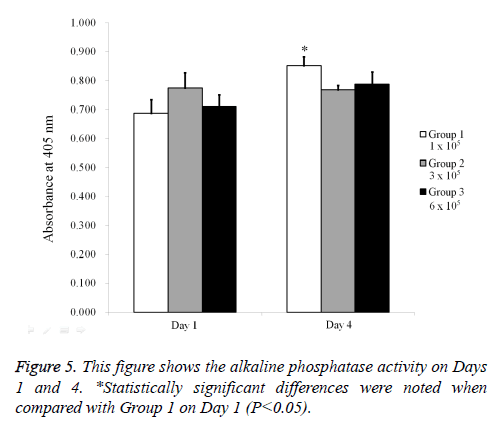
The results of the alkaline phosphatase activity assays are shown in Figure 5. The relative value on Day 1 for Groups 1, 2, and 3 were 100.0 ± 6.8%, 112.7 ± 7.7%, and 103.3 ± 5.9%, respectively. The relative value on Day 4 for Groups 1, 2, and 3 were 124.1 ± 4.5%, 111.7 ± 2.3%, and 114.6 ± 6.2% when the value of Group 1 on Day 1 was considered 100%. The mineralization assay’s results on Day 5 are shown in Figure 6. Mineralized extracellular deposits were noted in each group. The results showed that the calcium deposition was seen evenly seen from whole surface of the spheroids.
Discussion
This study was performed to fabricate stem cell spheroids with adipose-derived stem cells and to evaluate the effects of cell number and culture duration on the viability and the osteogenic differentiation of three-dimensional cultures. This study clearly showed that adipose-derived stem cells formed spheroids in the silicon elastomer-based concave microwells. A higher number of stem cells led to spheroids with larger diameters. Most of the cells in the cell spheroids emitted green fluorescence. The relative Cell Counting Kit-8 showed that higher values were seen with higher stem cell numbers. The increase of alkaline phosphatase activity assays with longer incubation time and mineralized extracellular deposits were evenly noted in each group.
The effects of adipose-derived stem cells on bone regeneration have been reported previously [15-17]. The expanded, human adult adipose-derived stem cells can generate ectopic bone through endochondral ossification, as previously reported for bone marrow stromal cells [15]. It was reported that adipose-derived stem cells showed inferior osteogenesis when compared with bone marrow-derived stem cells [16]. Conversely, adipose-derived stem cells showed higher differentiation potential when compared with bone marrow-derived stem cells in certain conditions [17]. Further research studies using three-dimensional culture models relying on adipose-derived stem cells and bone marrow stem cells may reveal the comparison results.
Three-dimensional system enabled the maintenance and directed differentiation of pluripotent stem cells under defined conditions [18]. It should be emphasized that many primary or progenitor cells showed significantly enhanced viability and functional performance when grown as spheroids [19]. Grafting human adipose-derived stem cells in spheroids produced effective angiogenesis via angiogenic cytokine secretion, extracellular matrix component preservation, and apoptotic signal regulation in ischemic tissue [20,21]. The co-culture method can be applied for increased survival and functionality [19,22]. The previous results suggest the co-culture of endothelial cells with mesenchymal stem cells induces both osteogenesis and angiogenesis [22]. The paracrine effects of multiple cells and adhesion molecules have attributed multiple signals, which lead to increased functionality [23]. Multicellular spheroids were suggested to be ideal building units for tissue reconstruction [19].
Three-dimensional cultures can be made using the hanging drop method [24]. Microfluidic bioreactors were used for the fabrication of three-dimensional stem cells [25]. In previous report, microwells were used for spheroid fabrication with stem cell and progenitor cell co-cultures [26]. The different types of surface characteristics can be applied for the microwells, including polyethylene glycol and polydimethylsiloxane [26,27]. In this report, the silicon elastomer-based concave microwells were used for making the stem cell spheroids. These spheroids can further be applied for tissue regeneration [28,29].
Various scaffolds have been used with adipose-derived stem cells [30-34]. This increased the survival and function of mesenchymal stem cell spheroids entrapped in alginate hydrogels for bone tissue engineering applications [30]. Gelatin has been used for osteogenic cell sheet fabrication using adipose-derived stem cells [31]. The enhanced osteogenesis of adipose-derived stem cells occurred by the synergistic effect of aligned fibers containing collagen I [32]. Catechol-functionalized hyaluronic acid hydrogels served as effective scaffold for adipose-derived stem cells due to improved biocompatibility and strong tissue adhesiveness [1]. Polycaprolactone-based scaffolds provided a candidate for an advantageous environment for the osteogenic differentiation of human adipose-derived stem cells [33]. Adipose-derived mesenchymal stem cells were delivered with poly-ε- caprolactone/β-tricalcium phosphate composite scaffolds in bone defects [34].
This study clearly showed that adipose-derived stem cells formed spheroids in the silicon elastomer-based concave microwells and that osteogenic differentiation was achieved with higher activity and longer incubation time. Spheroid-based cell delivery could be used as a simple and effective strategy for stem cell therapy.
Acknowledgment
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, Information and Communication Technology & Future Planning (NRF-2017R1A1A1A05001307).
References
- Park HJ, Jin Y, Shin J, Yang K, Lee C, Yang HS. Catechol-functionalized hyaluronic acid hydrogels enhance angiogenesis and osteogenesis of human adipose-derived stem cells in critical tissue defects. Biomacromolecules 2016; 17: 1939-1948.
- Tang Y, Xu Y, Xiao Z, Zhao Y, Li J, Han S. The combination of three-dimensional and rotary cell culture system promotes the proliferation and maintains the differentiation potential of rat BMSCs. Sci Report 2017; 7: 192.
- Yao W, Lay YE, Kot A, Liu R, Zhang H, Chen H, Lam K, Lane NE. Improved Mobilization of Exogenous Mesenchymal Stem Cells to Bone for Fracture Healing and Sex Difference. Stem Cells 2016; 34: 2587-2600.
- da Silveira Gerzson A, Machado DC, Marinovic DR, Pagnoncelli RM. Assessment of adhesion and proliferation of bone marrow mesenchymal stem cells in polymer matrices with rhGH. Int J Oral Maxillofacial Implants 2017; 32: e183-e189.
- Zhang J, Chen J. Bone tissue regeneration - application of mesenchymal stem cells and cellular and molecular mechanisms. Curr Stem Cell Res Ther 2016.
- Wagner DR, Wecht S, Rojas M. Mesenchymal stem cells in the treatment of chronic lung disease. J Tissue Eng Regenerat Med 2016; 21: 1366-1375.
- Gonzalez-Fernandez ML, Perez-Castrillo S, Sanchez-Lazaro JA, Prieto-Fernandez JG, Lopez-Gonzalez ME, Lobato-Perez S. Assessment of regeneration in meniscal lesions by use of mesenchymal stem cells derived from equine bone marrow and adipose tissue. Am J Vet Res 2016; 77: 779-788.
- Konno M, Hamabe A, Hasegawa S, Ogawa H, Fukusumi T, Nishikawa S. Adipose-derived mesenchymal stem cells and regenerative medicine. Develop Growth Differentiation 2013; 55: 309-318.
- Grada A, Falanga V. Novel stem cell therapies for applications to wound healing and tissue repair. Surg Technol Int 2016.
- Frese L, Dijkman PE, Hoerstrup SP. Adipose tissue-derived stem cells in regenerative medicine. Transfus Med Hemother 2016; 43: 268-274.
- Pers YM, Rackwitz L, Ferreira R, Pullig O, Delfour C, Barry F. Adipose mesenchymal stromal cell-based therapy for severe osteoarthritis of the knee: A phase I dose-escalation trial. Stem Cells Translat Med 2016; 5: 847-856.
- Bobi J, Solanes N, Fernández-Jiménez R, Galán-Arriola C, Dantas AP, Fernández-Friera L, Gálvez-Montón C, Rigol-Monzó E, Agüero J, Ramírez J, Roqué M, Bayés-Genís A, Sánchez-González J, García-Álvarez A, Sabaté M, Roura S, Ibáñez B, Rigol M. Intracoronary administration of allogeneic adipose tissue-derived mesenchymal stem cells improves myocardial perfusion but not left ventricle function, in a translational model of acute myocardial infarction. J Am Heart Assoc 2017; 6.
- Santini MT, Rainaldi G. Three-dimensional spheroid model in tumor biology. Pathobiol J Immunopathol Mol Cell Biol 1999; 67: 148-157.
- Kunz-Schughart LA, Kreutz M, Knuechel R. Multicellular spheroids: a three-dimensional in vitro culture system to study tumour biology. Int J Exp Pathol 1998; 79: 1-23.
- Osinga R, Di Maggio N, Todorov A, Allafi N, Barbero A, Laurent F. Generation of a bone organ by human adipose-derived stromal cells through endochondral ossification. Stem Cells Translat Med 2016; 5: 1090-1097.
- Liao HT, Chen CT. Osteogenic potential: Comparison between bone marrow and adipose-derived mesenchymal stem cells. World J Stem Cells 2014; 6: 288-295.
- Pagani S, Borsari V, Veronesi F, Ferrari A, Cepollaro S, Torricelli P, Filardo G, Fini M. Increased chondrogenic potential of mesenchymal cells from adipose tissue versus bone marrow-derived cells in osteoarthritic in vitro models. J Cell Physiol 2017; 232: 1478-1488.
- Zujur D, Kanke K, Lichtler AC, Hojo H, Chung UI, Ohba S. Three-dimensional system enabling the maintenance and directed differentiation of pluripotent stem cells under defined conditions. Sci Adv 2017; 3: e1602875.
- Lin RZ, Chang HY. Recent advances in three-dimensional multicellular spheroid culture for biomedical research. Biotechnol J 2008; 3: 1172-1184.
- Bhang SH, Cho SW, La WG, Lee TJ, Yang HS, Sun AY. Angiogenesis in ischemic tissue produced by spheroid grafting of human adipose-derived stromal cells. Biomaterials 2011; 32: 2734-2747.
- Lee JH, Han YS, Lee SH. Long-duration three-dimensional spheroid culture promotes angiogenic activities of adipose-derived mesenchymal stem cells. Biomol Ther 2016.
- Gurel Pekozer G, Torun Kose G, Hasirci V. Influence of co-culture on osteogenesis and angiogenesis of bone marrow mesenchymal stem cells and aortic endothelial cells. Microvascular Res 2016; 108: 1-9.
- Bates RC, Edwards NS, Yates JD. Spheroids and cell survival. Crit Rev Oncol Hematol 2000; 36: 61-74.
- Emmert MY, Wolint P, Wickboldt N, Gemayel G, Weber B, Brokopp CE. Human stem cell-based three-dimensional microtissues for advanced cardiac cell therapies. Biomaterials 2013; 34: 6339-6354.
- Sart S, Agathos SN, Li Y, Ma T. Regulation of mesenchymal stem cell 3D microenvironment: From macro to microfluidic bioreactors. Biotechnol J 2016; 11: 43-57.
- Futrega K, Atkinson K, Lott WB, Doran MR. Spheroid coculture of hematopoietic stem/progenitor cells and monolayer expanded mesenchymal stem/stromal cells in polydimethylsiloxane microwells modestly improves in vitro hematopoietic stem/progenitor cell expansion. Tissue Eng Part C, Methods 2017; 23: 200-218.
- Karp JM, Yeh J, Eng G, Fukuda J, Blumling J, Suh KY. Controlling size, shape and homogeneity of embryoid bodies using poly(ethylene glycol) microwells. Lab Chip 2007; 7: 786-794.
- Lee SI, Ko Y, Park JB. Evaluation of the shape, viability, stemness and osteogenic differentiation of cell spheroids formed from human gingiva-derived stem cells and osteoprecursor cells. Exp Thera Med 2017; 13: 3467-3473.
- Park JB, Lee JY, Park YJ, Rhee SH, Lee SC, Kim TI. Enhanced bone regeneration in beagle dogs with bovine bone mineral coated with a synthetic oligopeptide. J Periodontol 2007; 78: 2150-2155.
- Ho SS, Murphy KC, Binder BY, Vissers CB, Leach JK. Increased survival and function of mesenchymal stem cell spheroids entrapped in instructive alginate hydrogels. Stem Cells Translat Med 2016; 5: 773-781.
- Kim AY, Kim Y, Lee SH, Yoon Y, Kim WH, Kweon OK. Effect of gelatin on osteogenic cell sheet formation using canine adipose tissue derived mesenchymal stem cells. Cell Transplant 2016.
- Chen H, Qian Y, Xia Y, Chen G, Dai Y, Li N. The enhanced osteogenesis of ADSCs by the synergistic effect of aligned fibers containing collagen I. ACS Appl Materials Interfaces 2016.
- Ruminski S, Ostrowska B, Jaroszewicz J, Skirecki T, Wlodarski K, Swieszkowski W. 3D printed PCL-based scaffolds provide an advantageous environment for osteogenic differentiation of human adipose-derived stem cells. J Tissue Eng Regenerat Med 2016.
- Kim Y, Lee SH, Kang BJ, Kim WH, Yun HS, Kweon OK. Comparison of Osteogenesis between Adipose-Derived Mesenchymal Stem Cells and Their Sheets on Poly-ε-Caprolactone/β-Tricalcium Phosphate Composite Scaffolds in Canine Bone Defects. Stem Cells Int 2016; 2016: 8414715.