- Biomedical Research (2010) Volume 21, Issue 1
The prototype of Desmosomal junctions? proteins during embryonic development of ecodermal derived organ
M. Alfayez*Department of Anatomy, College of Medicine, King Saud University, Kingdom of Saudi Arabia, Riyadh.
- *Corresponding Author:
- M. Alfayez
Department of Anatomy
College of Medicine
King Saud University
Kingdom of Saudi Arabia, Riyadh
E-mail: Alfayez@ksu.edu.sa
Accepted date: July 26 2009
Abstract
During development, mammary gland epithelium goes through long and complicated devel-opmental process that involves proliferation, migration and morphological restructuring. Desmosomal junctions have been linked to these crucial developmental events. In this study the change in the desmosome and hemidesmosome during mammary gland development was examined through examining desomosmal proteins expression pattern using immuno-histochemistry and electron microscopy techniques. The results support the suggestion that desmosomes and hemidesmosomes are remodelled during mammary gland development. It also suggests that early mammary gland develop-ment stages, the cells require stronger adhesive mechanisms than at later stages. Such physical support is maintained by the desmosomes that hold the cells in the centre of the growing cells of developing mammary gland.
Keywords
Mammary gland, desmosome, hemidesmosome, mammogenesis.
Introduction
There are two main types of cell-to-cell anchoring junc-tions, the adherens junctions and the desmosome junc-tions. These share many structural and functional similari-ties, for example both have transmembrane glycoproteins belonging to the cadherin family of calcium-dependant adhesion molecules. However, they also differ in various ways, for example the adherens junctions are association with the cells’ (actin) microfilaments while desmosome junctions are associated with the intermediate filaments, usually keratins.
During development, mammary gland epithelium goes through a long and complicated developmental process that involves proliferation, migration and morphological restructuring. Desmosomal junctions have been linked to these crucial developmental events.
Runswick and colleagues have shown that desmosomes are involved in epithelial and myoepithelial positioning [1]. In their experiments they first showed that lumenal and myoepithelial cells isolated from human mammary gland are capable of reaggregating and reorganizing themselves within 24h in suspension culture, into a core of lumenal cells surrounded by myoepithelial cells, re-sembling their natural positioning in vivo. In these struc-tures the central lumenal epithelial cells express desmo-collin-2 and desmoglein-2 only, whilst the surronding myoepithelial cells express desmocollin-3 and desmo-glein-3. The authors showed that if anti-adhesion peptides were applied, corresponding to the cell adhesion recogni-tion (CAR) of desmocollin-2 and desmoglein-2 together and not individually, reaggregation of the cells was sig-nificantly prevented. In contrast combining anti-adhesion peptides for desmocollin-3 and desmoglein-3 permitted aggregate formation but disrupted cell positioning, and aggregates of disordered lumenal and myoepithelial cells were formed [1]. These results provide the strongest evi-dence of desmosomal junction involvement in the forma-tion of multilayered structural tissue.
The expression of desmosomal junctions in mammary gland development has also been examined during em-bryonic development [2]. Here, the expression pattern was studied through three important stages of embryonic development. Nanba, Nakanishi, Hieda [2] showed that desmocollins, desmogleins, plakoglobin and desmoplakin were all expressed by the mammary buds and the overly-ing developing epidermis of E13 mice. By E15 all of these proteins (desmocollins, desmogleins, plakoglobin and desmoplakin) were downregulated in the epidermis, yet they seemed to be retained in most parts of the devel-oping mammary duct except the deep downgrowing half. The authors also reported that staining for four hemides-mosomal molecules including plectin was positive in the basement membrane around the whole mammary bud at E13, but by E15 to E17 the staining was much reduced around the body of the developing mammary duct and completely absent from its downgrowing tip of the main duct and other secondary ducts.
Electron microscopy showed that at E13 the mammary bud cells possess well-developed desmosomes and hemidesmosomes [2]. However, at a similar stage it has also been reported that there are only a very few of these junctions [3,4]. By E15 these cell junctions were rarely seen, yet mammary cells at E17 showed restored desmo-somal structures [2]. This observation suggests a remodel-ling of desmosomal junctions during mammary gland development, which enforces the probability that desmo-somes play important roles in morphogenesis that are still not quite clear.
The aim of this study was to study the change in the des-mosome and hemidesmosome during mammary gland development.
Materials and Methods
Animals
For the experiments described in this study, a total of 546 CD1 mouse embryos (E12=24, E12.5=56, E13=26, E13.5=31, E14= 46, E14.5=88, E15.5= 37, E16= 12, E16.5= 43, E17.5= 80 and E18.5= 103).
Generating embryos of defined ages
As mouse embryonic development is a very rapid process, it was important to study embryos of as similar develop-mental age as possible. Two different mating techniques were compared. One was based on a short (about 2hr) and defined time for mating, and the second was based on the more widely used technique where the animals were left together overnight to mate.
Dissection
Pregnant mice were euthanased by CO2 suffocation fol-lowed by dislocation of the neck. Individual embryos were collected and transferred into a glass Petri dish car-peted with Sylgard® 184 Kit, silicone elastomer (Sigma, UK), which provided a surface for pining down the em-bryos in order to stabilized them. Under the dissection microscope while the embryo was completely covered with DMEM, it was pinned outstretched using insect pins into the Sylgard®. Gradually the pins were moved closer to the body, as the limbs and tail were trimmed. By ad-justing the angle of the incident lights and tilting the em-bryo sideways, the mammary glands were identified ac-cording to their location and shape.
About 2-4mm2 fragments of the skin (depends on the age of the embryo), containing one or maximum two adjacent mammary glands, e.g. No.4 & 5, were dissected out.
Immunofluorescence
Skin fragments containing mammary glands were snap frozen in liquid nitrogen, while they were immersed in Tissue-Tek® (Agar, UK) within an appropriate size foil cup. Serial frozen sections (10μm) were cut in a cryostat at -20º C and collected on pre-coated slides (BDH, UK).
Immunoperoxidase
Pieces of tissue containing mammary glands were fixed by immersion in 10% neutral buffered formalin (VWR, UK) overnight at room temperature with one change (af-ter 1hr) of fixative. Then the tissue was dehydrated in a graded ethanol series. This was followed by 2 changes of pure xylene at room temperature for 10min to 1hr, de-pending on the size of the embryo. Thereafter specimens were embedded in wax blocks of appropriate size.
The wax blocks were carefully trimmed around the em-bedded tissue, and serial sections 7μm thick were cut us-ing a Reichert-Jung 035 microtome. The slides containing mammary gland sections were de-waxed by two changes in pure xylene at room temperature for 10min, followed by re-dehydration in a graded ethanol series. Slides were then processed for antigen retrieval using either a pur-pose-designed electric pressure cooker, the 2100 Re-triever (Pickcell Laboratories) supplied with glass slide holder, or a microwave. In the first technique i.e. the pres-sure cooker, the slides where incubated in 0.98% Antigen Retrieval Solution (DAKO, UK) in distilled water (dH2O) inside the glass holder within the electric pressure cooker and heated up to 160º C for about 5 min and then left overnight enclosed within the cooker while electric pres-sure cooker slowly cool down. In the second technique, i.e. using a microwave, after slides had been de-waxed they were transferred into citrate buffer in a plastic Coplin jar, which was covered with cling film, and then heated in a 750-watt microwave at full power for 20min. The jar was then taken out and left at room temperature to cool down.
The DakoCytomation EnVision® Dual Link System Per-oxidase kit (DAKO, UK) was used for immunohisto-chemistry, following the manufacturer’s recommended procedures. In this method, after washing in PBS the slides were incubated in the appropriate concentration of an antibody for plectin (a hemidesmosome protein) and desmoplakins 1&2 (desmosome proteins) during normal development; see Table 1 for details of antibodies used.
Overnight at -4° C, followed by two washes in PBS, each for 5 min. After that, the peroxidase-labelled polymer (DAKO, UK) was applied for 30 min followed by 5 min in PBS. Sections were then covered with substrate chro-mogen for 10 min, washed in running tap water, then counterstained with haematoxylin for 10-15 seconds. Af-ter counterstaining in both techniques, the slides were processed though de-hydration and mounting.
Transmission electron microscopy
Small fragments of skin (2-4mm2) containing mammary gland(s) were fixed by immersion in 3% glutaraldehyde and 2% paraformaldehyde in 0.1 mM cacodylate buffer pH 7.3 overnight at room temperature, then postfixed in 1% aqueous osmium tetroxide for 1h, dehydrated in graded ethanols and incubated in propylene oxide for 30 min. The fragments were then freed from the pins, which were holding them to the Sylgard® and keeping them flat. Then, and after 2 changes in CY212 (Agar, UK) each for 12 hrs, individual fragments were embedded in the Aral-dite CY212.
Resin blocks containing skin fragment with mammary glands (buds) were initially sectioned using a Lecia Ul-tracut UCT (ultramicrotome) in semi-serial manner. The sections were cut at 0.5μm thicknesses using a glass knife. At intervals of about 15μm, a few sections were collected, stained with 1% toluidine blue and examined using a Leitz Wetzlar light microscope before proceeding further in cutting. When a mammary bud or any sign of it was seen, the block was carefully trimmed further into trapezoid shape. Thin sections were then cut using a dia-mond knife (Diatome 45º), a ribbon of ultra-thin (60 nm) sections were collected from the water surface onto 2mmx1mm slot carbon-coated 3.05mm grids (Agar Sci-entific, UK), and stained with 3% uranyl acetate for 10 min followed by 10 min by lead citrate. Ultra-thin sec-tions were examined in a JEOL 1200EX Philips transmis-sion electron microscope, operating at accelerating volt-age of 80 kV.
Examination procedures
Samples were examined by fluorescent or bright field microscopy using a Zeiss Axioskop, fitted with a colour AxioCam digital camera which was used for collecting images (using objective lenses X4, X16, X25 and X40). The obtained digital images were processed using Adobe® Photoshop® 7.
Results
EM of desmosomes/hemidesmosomes in mammogenesis
The organization of the desmosomes and hemidesmo-somes in the prenatal mammary glands was first studied by electron microscopy. Ultra-thin sections of mammary glands of three main stages of development, the early peg stage (E13), early sprouting stage (E16) and at the sheath stage (E18), were examined.
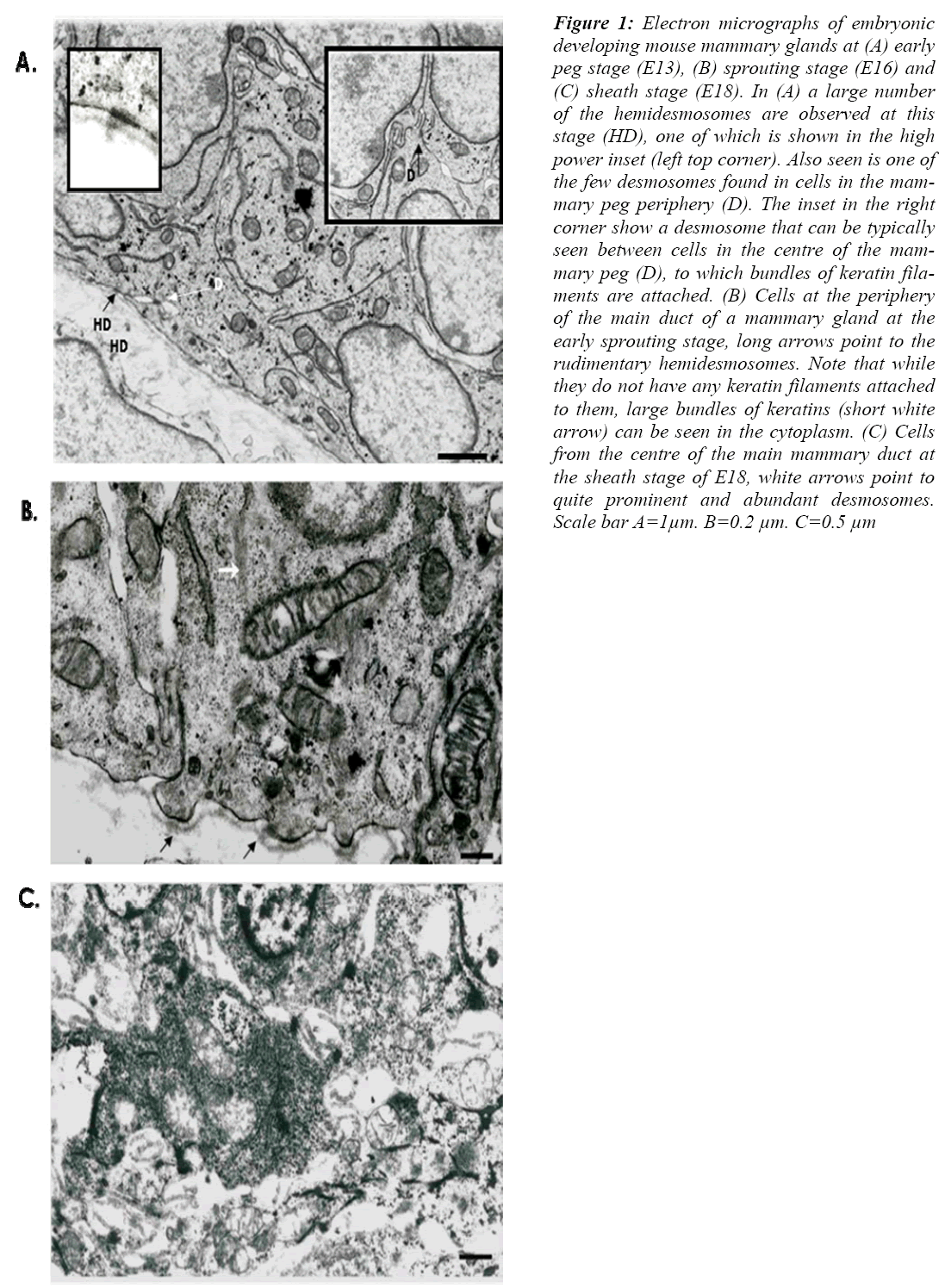
The results show that at the early peg stage mammary cells possess a number of well-formed desmosomes (Fig. 1 A), with bundles of filaments clearly associated. The desmosomes were seen mainly between cells at the centre of the mammary body, and occasionally between cells at the periphery (Fig. 1 A). Small hemidesmosomes with no clear keratin filament associations were also found (Fig 1 A).
Figure 1: Electron micrographs of embryonic developing mouse mammary glands at (A) early peg stage (E13), (B) sprouting stage (E16) and (C) sheath stage (E18). In (A) a large number of the hemidesmosomes are observed at this stage (HD), one of which is shown in the high power inset (left top corner). Also seen is one of the few desmosomes found in cells in the mam-mary peg periphery (D). The inset in the right corner show a desmosome that can be typically seen between cells in the centre of the mam-mary peg (D), to which bundles of keratin fila-ments are attached. (B) Cells at the periphery of the main duct of a mammary gland at the early sprouting stage, long arrows point to the rudimentary hemidesmosomes. Note that while they do not have any keratin filaments attached to them, large bundles of keratins (short white arrow) can be seen in the cytoplasm. (C) Cells from the centre of the main mammary duct at the sheath stage of E18, white arrows point to quite prominent and abundant desmosomes. Scale bar A=1μm. B=0.2 μm. C=0.5 μm
At the early sprouting stage, rudimentary desmosomes and hemidesmosomes were observed in relatively large number (Fig 1B). Although large bundles of keratin fila-ments were seen in the cell cytoplasm, fewer filaments seemed to be attached to the rudimentary hemidesmo-somes.
At the sheath stage, about E18, the cells of the main duct were less compact than at earlier stage, especially in the centre of the main duct (Fig. 2 C). However numerous desmosomes were observed in-between all the cells espe-cially those at the periphery.
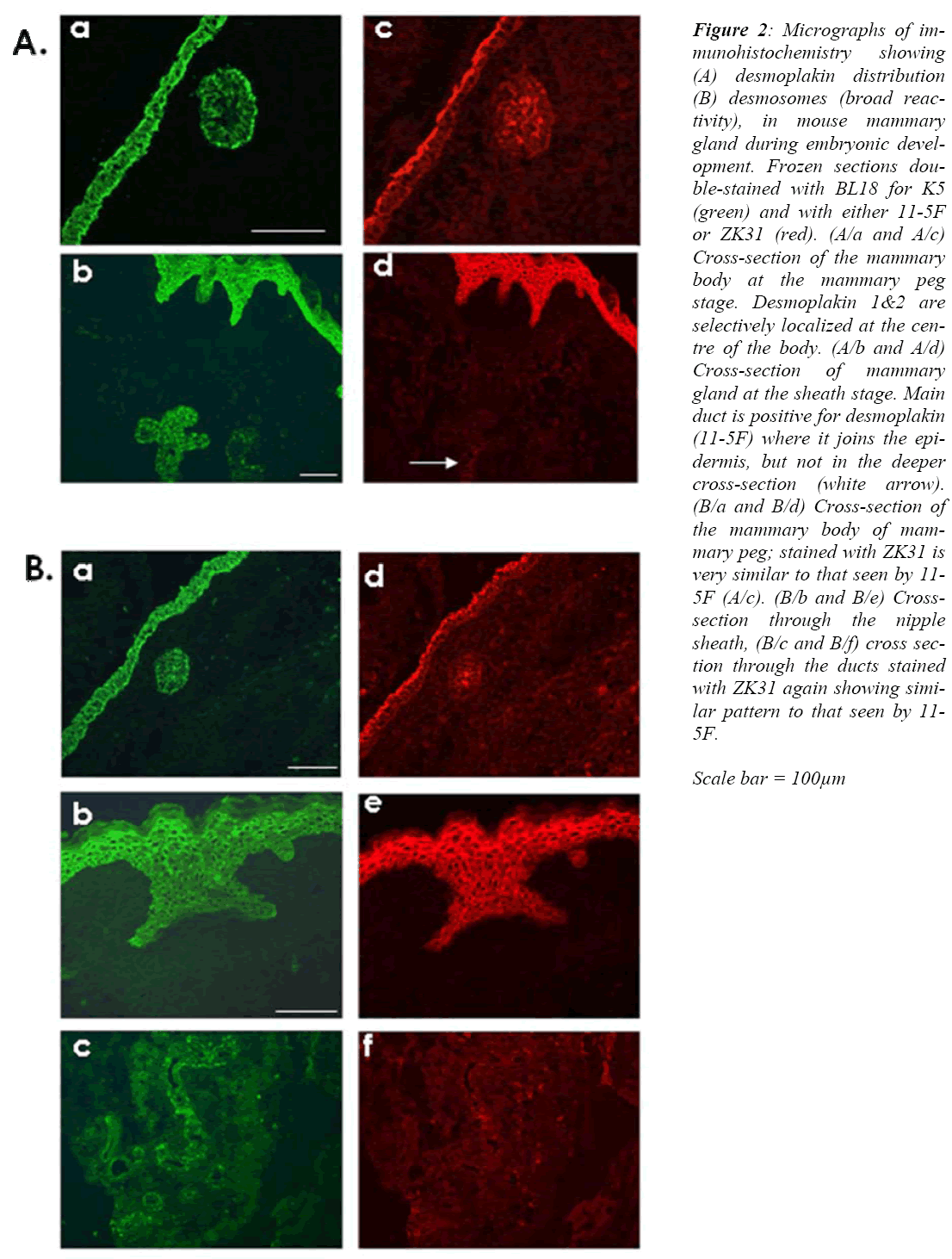
Figure 2: Micrographs of im-munohistochemistry showing (A) desmoplakin distribution (B) desmosomes (broad reac-tivity), in mouse mammary gland during embryonic devel-opment. Frozen sections dou-ble-stained with BL18 for K5 (green) and with either 11-5F or ZK31 (red). (A/a and A/c) Cross-section of the mammary body at the mammary peg stage. Desmoplakin 1&2 are selectively localized at the cen-tre of the body. (A/b and A/d) Cross-section of mammary gland at the sheath stage. Main duct is positive for desmoplakin (11-5F) where it joins the epi-dermis, but not in the deeper cross-section (white arrow). (B/a and B/d) Cross-section of the mammary body of mam-mary peg; stained with ZK31 is very similar to that seen by 11-5F (A/c). (B/b and B/e) Cross-section through the nipple sheath, (B/c and B/f) cross sec-tion through the ducts stained with ZK31 again showing simi-lar pattern to that seen by 11-5F.
Scale bar = 100μm
Expression of desmoplakin 1&2
The desmoplakin 1&2 expression pattern, as seen by monoclonal antibody 11-5F, shows that during the early peg stage of E13 only cells at the middle of the mammary body are positive (Fig. 2 A/c), while cells at both the mammary neck and the periphery of the mammary body were all negative. In the overlying ectoderm only cells of the periderm were positive.
Later on, at about E16, as the mammary gland develops deeper into the mesenchyme, none of the mammary cells are positive for 11-5F. At this stage the periderm layer retained its positive reactivity, but other layers of the de-veloping epidermis were still negative.
At the sheath stage of prenatal mammary gland develop-ment, the suprabasal layers of the embryonic epidermis are now positive (Fig. 2 A/d), along with cells at the cen-tre of a small segment only of the main mammary duct that is close to the epidermis.
Identical results were obtained using a monoclonal anti-body ZK31 antibody, which is a an antibody with broad reactivity for desmosome proteins. At E13 only cells in the middle of the mammary body were positive (Fig 2 B/d). From as early as E15, these central mammary glands loose their positive reactivity for ZK31 antibody. The negative reaction with ZK31 continue throughout the subsequent prenatal mammogenesis stages up to and in-cluding the sheath stage (Fig 2 B/e and B/f).
Expression of Plectin during mammary development
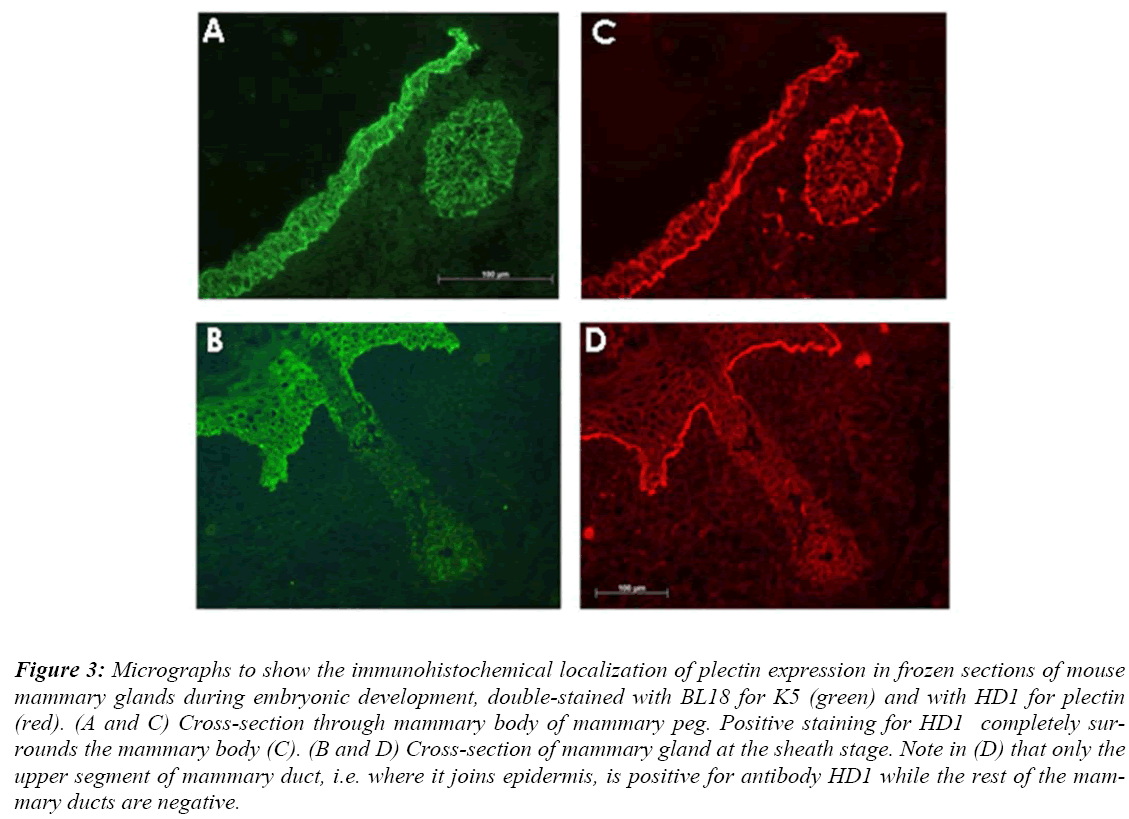
Antibody HD1 was used for immuonohistochemistry in-vestigations in order to examine the pattern of hemides-mosomes during prenatal mammary gland development. The results of these investigations revealed that during early stages of mammary gland development including the bud and peg stages, positive staining by HD1 antibody completely surrounds the mammary buds and pegs (Fig. 3 C).
Figure 3: Micrographs to show the immunohistochemical localization of plectin expression in frozen sections of mouse mammary glands during embryonic development, double-stained with BL18 for K5 (green) and with HD1 for plectin (red). (A and C) Cross-section through mammary body of mammary peg. Positive staining for HD1 completely sur-rounds the mammary body (C). (B and D) Cross-section of mammary gland at the sheath stage. Note in (D) that only the upper segment of mammary duct, i.e. where it joins epidermis, is positive for antibody HD1 while the rest of the mam-mary ducts are negative.
Later when mammary gland reaches the sheath stage of development, positive staining was found to be restricted to the epidermal-dermal junction including that of the nipple sheath. The staining appears weaker around the small segment of the main mammary duct that lies close to the embryonic epidermis, and is completely absent from the rest of the main duct and the secondary ducts (Fig. 3 D).
Discussion
The studies described here have revealed that at the early peg stage, desmoplakins expression is mainly within the cells in the centre of the mammary body. At this stage the desmosomes looked normal and fully developed under electron microscopy. At later stages (early sprouting stage), the desmosomes become much less abundant and also look rather small and less defined. Desmosomes at the sheath stage looked fully mature and were more abun-dant. Hemidesmosome appearance was in line with that of the desmosomes, as they were present during the early peg stage and looked less defined in the early sprouting stage.
However, in contrast with earlier reports in which it was stated that hemidesmosomes were fewer in the mammary body than in the mammary neck at the early peg stage [4], the present study found no obvious differences between the number or the distributions of hemidesmosomes be-tween the mammary body and the neck. This is probably due to the developmental status of the mammary peg: in the present study, all mammary pegs used were at early peg stage development while in the study by Hogg [3], the mammary pegs were of E15 mice, i.e. at much more advanced stage of development. Nonetheless, the results of ultrastructural investigations of the desmosomes and hemidesmosomes during prenatal mammary gland devel-opment are in general in line with earlier studies [5,2].
Immuonohistochemistry staining of sections of different mammary glands at different stages of embryonic devel-opment for desmoplakin 1&2 and HD1 has revealed that these proteins are first present at the peg stage, then they disappear from the mammary gland at least during early sprouting stage (E16). This confirms the observations of Nanba and colleagues [2]. Interestingly, this pattern seems to coincide with the changes in the desmosome and hemidesmosome distribution observed by electron mi-croscopy as discussed above.
These results present further support for the suggestion that desmosomes and hemidesmosomes are remodelled during mammary gland development. It also suggests that early mammary gland development stages (the bud and the peg), the cells require stronger adhesive mechanisms than at later stages. Such physical support is maintained by the desmosomes that hold the cells in the centre of the growing bud or peg. This is further supported by the fact that deficiency in either plectin or desmoplakin, which appear to be a main major components of the desmosome and hemidesmosme lead to skin fragility [5, 6].
Finally, these results also indicate that there might be a compensatory relationship between certain keratins and desmosomal junction proteins. This is most evident in the cells located at the boundary of downgrowing mammary structure (the advancing edge). These cells probably need to be resilient in order to counter the direct resistance from the underlying mesenchyme, at the same time these cells need to be relatively flexible in order to navigate through the mesenchyme. These cells were found to ex-press K15 and K17 (for reinforcement), but do not ex-press desmoplakin 1&2 (allowing for more flexibility).
References
- Runswick SK, O'Hare MJ, Jones L, Streuli CH, Gar-rod DR. Desmosomal adhesion regulates epithelial morphogenesis and cell positioning. Nat Cell Biol 2001; 3 (9): 823-830.
- Nanba D, Nakanishi Y, Hieda Y. Changes in adhesive properties of epithelial cells during early morphogene-sis of the mammary gland. Dev Growth Differ 2001; 43 (5): 535-544.
- Hogg NAS. Lumen formation in the developing mouse mammary gland. J Embryol Exp. Morph 1983; 73: 39-57
- Nanba D, Hieda Y, Nakanishi Y. Remodeling of des-mosomal and hemidesmosomal adhesion systems dur-ing early morphogenesis of mouse pelage hair folli-cles. J Invest Dermatol 2000; 114 (1): 171-177.
- Smith FJ, Eady RA, Leigh IM, McMillan JR, Rugg EL, Kelsell DP, et al. Plectin deficiency results in muscular dystrophy with epidermolysis bullosa. Nat Genet 1996; 13 (4): 450-457.
- Vasioukhin V, Bowers E, Bauer C, Degenstein L, Fuchs E. Desmoplakin is essential in epidermal sheet formation. Nat Cell Biol 2001; 3: 1076-1085.