Research Article - Biomedical Research (2016) Health Science and Bio Convergence Technology: Edition-I
The protective effects of melatonin and vitamin c against renal ischemiareperfusion injury in rats
Yüksel Yıldız1*, Akın Soner Amasyalı2, Ferhat Şirinyıldız1, Gül Taşlı Yeşilçayır1, Cenk Orak1, Kemal Ergin3, Mustafa Yılmaz4, Gökhan Cesur1, Rauf Onur Ek11Department of Physiology, Medical Faculty, Adnan Menderes University, Aydin, Turkey
2Department of Urology, Medical Faculty, Adnan Menderes University, Aydin, Turkey
3Department of Histology and Embryology, Medical Faculty, Adnan Menderes University, Aydin, Turkey
4Department of Biochemistry, Medical Faculty, Adnan Menderes University, Aydin, Turkey
- *Corresponding Author:
- Yuksel Yildiz
Department of Physiology
Faculty of Medicine
Adnan Menderes University
Aydin, Turkey
Accepted date: May 10, 2016
Abstract
The present study was undertaken to determine the protective effects of Melatonin and Vitamin C in combination against renal I/R injury in rats. 40 wistar albino female rats were divided into five groups: Sham, I/R, I/R+Mel, I/R+VitC and I/R+Mel+VitC. While nothing was done in left kidney in Sham group, all I/R groups were subjected to I/R procedure (45 min occlusion and 60 min reperfusion). In I/R +Mel group, all rats were injected by Melatonin 50 mg/kg i.p. for 3 days plus morning of operation day. In I/R+VitC group, all rats were injected by Vitamin C 500 mg/kg/day i.p. 24 hours before I/R procedure. In I/R+Mel+VitC group, all rats were injected by Melatonin and Vitamin C by the same dose and intervals as in I/R+Mel and I/R+VitC groups. After 3 days, rats were exposed to I/R procedure following right nephrectomy. After sacrification, blood and left kidney parts were subjected to biochemical and histopathological examinations. The I/R significantly increased serum urea, creatinine and IMA levels and renal MPO activity and MDA levels, and decreased renal SOD and CAT activities. Pretreatment with Melatonin and Vitamin C markedly reversed these findings. In histology, while cortical necrotic changes of the epithelium lining tubules and glomeruli as well as asymmetrical and shrunken glomeruli were observed in IR group, there was a marked regression of the histopathological changes in combined pretreatment group. In conclusion, combined pretreatment with Melatonin and Vitamin C seems to have higher anti-inflammatory and antioxidant properties than individual pretreatments with either agent alone.
Keywords
Renal ischemia/reperfusion, Melatonin, Vitamin C, Oxidative stress.
Introduction
As in many other tissues, ischemia reperfusion causes also the injury in kidney in a various conditions such as kidney transplantation, partial nephrectomy, cardiopulmonary bypass surgery, accidental trauma, sepsis, some urologic interventions and hydronephrosis. Renal I/R injury may cause the renal failure, tubular necrosis, the lower glomerular filtration and an increase in renal vascular resistance.
Although it is essential for the survival of ischemic tissue, reperfusion itself also causes cellular injury. Ischemia/ reperfusion (I/R) of an organ or tissue results in many cellular events influencing the structure and function of virtually every organelle and subcellular system of the affected cells [1,2]. A number of processes including generation of reactive oxygen species (ROS), depletion of adenosin 5′-triphosphate (ATP), neutrophil infiltration, phospholipase activation and membrane lipid alteration, cytoskelatal dysfunction, and intracellular calcium accumulation have been implicated in the pathogenesis of renal I/R induced cell injury [2-6]. Thus, the mechanisms underlying renal I/R injury are multifactorial and interdependent, involving hypoxia, inflammatory responses and free-radical-induced damage [2,7].When the free radical formation following the reperfusion exceeds the capacity of endogenous antioxidant defence systems of the kidneys, freeradical mediated cellular injury occurs. Thus, free radical scavengers are proposed to be useful in the renal I/R injury [8].
Several experimental studies suggest that antioxidant vitamins, including ascorbic acid (AA), produce cytoprotective effects due to reduction of the ROS [9]. Thus, one major function of the AA is to protect tissues from harmful oxidation products. Furthermore, the AA prevents leukocyte activation, platelet aggregation, and leukocyte adhesion to microvascular endothelium [10]. Also, it has been demonstrated that the antioxidant and free radical scavenger ascorbic acid [11] has a protective effect against drug-induced nephrotoxicity in animals [12,13]. In addition, other previous studies showed that the antioxidant ascorbic acid attenuated renal damage caused by a variety of insults, such as postischemic stress, cisplatin, aminoglycosides, and potassium bromate in animals [14,15].
Melatonin and its metabolites have potent antioxidant/antiinflammatory properties and have been proven to be highly effective in a variety of disorders linked to inflammation and oxidative stress [16-18]. Melatonin not only neutralizes the RNS and the ROS species, but also acts through stimulation of several antioxidant enzymatic systems and stabilizing cell membranes [19,20]. Furthermore, melatonin treatment has been shown to have a protective effect against the I/R induced histopathological changes [21]. Melatonin is the major hormone secreted by the pineal gland in human body [19,22]. The exogenous melatonin was shown to preserve renal function by reducing lipid peroxidation, increasing glutathione levels, and preventing the increase in nitrite levels induced by renal I/R [23]. The aim of this study was to determine whether pretreatment with combination of Melatonin and Vitamin C have a significant effect on renal I/R injury.
Materials and Methods
All experiments on animals were performed in accordance with the ethical guidelines for the Care and Use of Laboratory Animals of the United States National Institutes of Health. The study protocol was approved by the ethical animal research committee at Adnan Menderes University. All animals were kept in individual cages in a controlled room (at 25°C, 75 % humidity, 12 hr light / dark cycle). The rats were fed ad libitum with standard rat food and tap water. Rats were deprived of food for overnight before experimentation but allowed free access to tap water throughout. The study was performed using female wistar albino rats (weighing 200-250 g).
Experimental design
40 wistar albino female rats were randomly divided into five groups: Sham, I/R, I/R+Mel, I/R+VitC and I/R+Mel+VitC (n=8). The rats were anesthetized by intraperitoneal injection of ketamine hydrochloride (50 mg/kg) and xylazine (10mg/kg). While nothing was done in left kidney in Sham group, all the I/R groups were subjected to the I/R procedure (45 min occlusion and 60 min reperfusion). In the I/R+Mel group, all rats were injected by Melatonin (Sigma Chemical Co., St Louis, MO, USA) 50 mg/kg i.p. for 3 days plus the morning of operation day. In the I/R+VitC group, all rats were injected by Vitamin C 500 mg/kg/day i.p. 24 hours before the I/R procedure (Redox-C Ampul 500 mg/5 ml Bayer). In the I/R +MEL+VitC group, all rats were injected by Melatonin and Vitamin C by the same dose and intervals as in the I/R+Mel and the I/R+VitC groups. After 3 days, rats were exposed to renal I/R following right nephrectomy. After sacrification, blood and left kidney parts were subjected to biochemical and histopathological examinations.
Biochemical study
Determination of serum Urea, creatinine and ischemia modified albumin (IMA) levels: After collecting, the blood samples were centrifuged at 1000 g for 7 minutes in cold. The serum was removed and stored at -80°C until analysis. Serum levels of creatinine and urea were determined with a chemiluminescent assay by routine an autoanalyzer (Architect C8000, Abbott, IL, USA). The IMA levels in the serum samples were determined via commercial SunRed Rat ELISA kit, (Sunred Biological Technology, Baoshan District, Shanghai, China). The test results were calculated by bioelisa reader Elx800 using standart curve on via 450 nm. Procedures were performed according to manufacturer’s instruction.
Measurement of tissue the MDA level and the MPO, the SOD, the GPx and the CAT activities: The sample tissues were homogenized on ice in the buffers provided/described in the corresponding assay kits (The MDA Lysis Buffer (the MDA); PBS having 0.1% NP40 (the MPO); 0.1M Tris/HCl, pH 7.4 containing 0.5% Triton X-100, 5mM β -ME, 0.1mg/ml PMSF (the SOD); and cold assay buffer (the GPx and the CAT)). Then the homogenates were centrifuged at 13,000 x g, 10 min for the MDA and the MPO assays; at 14,000 X g, 5 min at 4°C for the SOD activity assay and at 10,000 x g for 15 min at 4°C for the GPx and the CAT activity assays. In order to determine the MDA level and the MPO, the SOD, the GPx and the CAT activities, the supernatants were analyzed with their corresponding assay kits according to the manufacturer’s instructions. (All kits were from BioVision, Milpitas, CA, USA).
Histopathology
All kidneys were fixed with 4% formaldehyde, dehydrated in ethanol series, cleared in xylene and finally embedded into paraffin blocks. Serial sections (5 μm) were taken randomly from these blocks by a microtome (Leica RM 2135; Leica, Germany) and were stained with hematoxylin-eosin. After mounting with entellan medium slides were examined under the Olympus BX51 light microscope (Olympus Co., Tokyo, Japan) attached with a DP20 camera (Olympus Co., Tokyo, Japan). The histological parameters evaluated in the kidney tissues were; cortical necrosis, glomerulosclerosis, hyperemia and tubuler damage. Histopathological changes in the kidney were graded on a score; 0: none, 1: mild, 2: moderate and 3: severe damage.
Statistical analysis
All results were expressed as median and range for all groups. The data were analyzed using SPSS 17.0 program. The analytic assessment of comparisons between twain groups was carried out by Mann Whitney U test. Differences of p<0.05 were regarded as significant.
Results
In blood biochemical analyses, the higher urea and creatinine levels (69.50 (65.25-80.25) and 0.74 (0.71-0.78)) in the IR group were significantly decreased in the IR+VitC (60.50 (48.25-64.00)) and in the IR+Mel (0.71 (0.69-0.73)) groups respectively (p<0.05). Also, the IMA levels in the IR+Mel (13.90 (12.83-19.20)) and the IR+Mel+VitC (15.15 (14.15-17.25)) groups were remarkably lower compared to the IR group (18.40 (17.33-26.63) p<0.05).
In tissue biochemical analyses, the MDA levels of the IR+Mel (9.67 (3.09-12.21)), the IR+VitC (11.74 (8.44-13.42)) and the IR+Mel+VitC groups (10.33 (3.40-11.15)) were lower than the IR (15.17 (13.43-18.45)) and Sham control groups (12.96 (6.63-15.76), p<0.05). All individual and combinatorial pretreatments decreased the MDA levels compared to the IR and Sham groups (p<0.05). But combined pretreatment of Melatonin and Vitamin C showed more efficient results in preventing of lipid peroxidation (Table 1).
| Sham | IR | IR+Mel | IR+VitC | IR+Mel+VitC | |
|---|---|---|---|---|---|
| Histology Score | 0.0 (0.0-0.0)# | 8.0 (4.0-8.00) | 5.0 (4.0-6.0) | 3.0 (2.0-7.0) | 3.0 (2.0-5.0)* |
| MDA (nmol/mg) | 12.96 (6.63-15.76) | 15.17 (13.43-18.45) | 9.67 (3.09-12.21)* | 11.74 (8.44-13.42)* | 10.33 (3.40-11.15)* |
| MPO (mU/ml) | 11.63 (10.37-14.85)# | 15.72 (15.49-17.89) | 13.97 (11.10-15.78)* | 14.16 (12.98-16.63) | 12.85 (11.56-14.37)* |
| SOD(U/ml) | 28.10 (11.55-46.65) | 16 (11.35-18.38) | 35.95 (22.20-39.33)* | 32.60 (16.90-37.60) | 46.35 (37.68-58.48)* |
| CAT (mU/ml) | 3.05 (2.13-4.30) | 2.70 (1.95-3.40) | 2.75 (2.10-3.60) | 3.05 (2.85-4.93) | 4.7 (3.7-5.9)* |
| GPx (mU/ml) | 79.2 (53.3-117.2) | 124.2 (111.9-150.6) | 99.70 (69.38-140.5) | 109.0 (65.68-236.5) | 153.7 (103.3-285.7) |
| Creatinine (mg/dl) | 0.67 (0.57-0.73)# | 0.74 (0.71-0.78) | 0.71 (0.69-0.73)* | 0.72 (0.65-0.75) | 0,73 (0.65-0,76) |
| Urea (mg/dl) | 67.00 (54.00-74.00) | 69.50 (65.25-80.25) | 69.50 (66.25-75.50) | 60.50 (48.25-64.00)* | 69.50 (60.00-74.00) |
| IMA (U/ml) | 21.60 (16.70-24.80) | 18.40 (17.33-26.63) | 13.90 (12.83-19.20)* | 18.90 (15.00-20.30) | 15.15 (14.15-17.25)* |
Table 1: Histological and biochemical results of all groups. IR, ischemia reperfusion group; IR+Mel, 50 mg/kg Melatonin pretreated group; IR +VitC, 500 mg/kg Vitamin C pretreated group; IR+Mel+VitC, 50 mg/kg Melatonin and 500 mg/kg Vitamin C pretreated group; MDA, Malondialdehyde; MPO, Myeloperoxidase; SOD, Superoxide dismutase; CAT, Catalase; GPx, Glutathione peroxidase; IMA, Ischemia modified albumin.
The GPx activity in combinatorial pretreatment group (153.7 (103.3-285.7)) was higher than all other groups (p>0.05). None of the pretreatment groups (the IR+Mel (99.70 (69.38-140.5)), the IR+VitC (109.0 (65.68-236.5)), the IR+Mel+VitC (153.7 (103.3-285.7)) caused significant change in the GPx activity compared to the IR group (124.2 (111.9-150.6)). The CAT activity was significantly increased in the IR+Mel+VitC group (4.7 (3.7-5.9)) compared to the IR group (2.70 (1.95-3.40), p<0.05). Although the CAT activity level was also increased in the IR+Mel (2.75 (2.10-3.60)) and the IR+VitC (3.05 (2.85-4.93)) groups, but it did not reached the significant level. While the MPO activity of the IR group (15.72 (15.49-17.89)) were higher than all groups (Sham 11.63 (10.37-14.85), VitC 14.16 (12.98-16.63)), it was remarkably decreased in the IR +Mel (13.97 (11.10-15.78)) and the IR+Mel+VitC (12.85 (11.56-14.37)) groups by the Melatonin and Melatonin +Vitamin C pretreatment compared to the IR group (p<0.05).
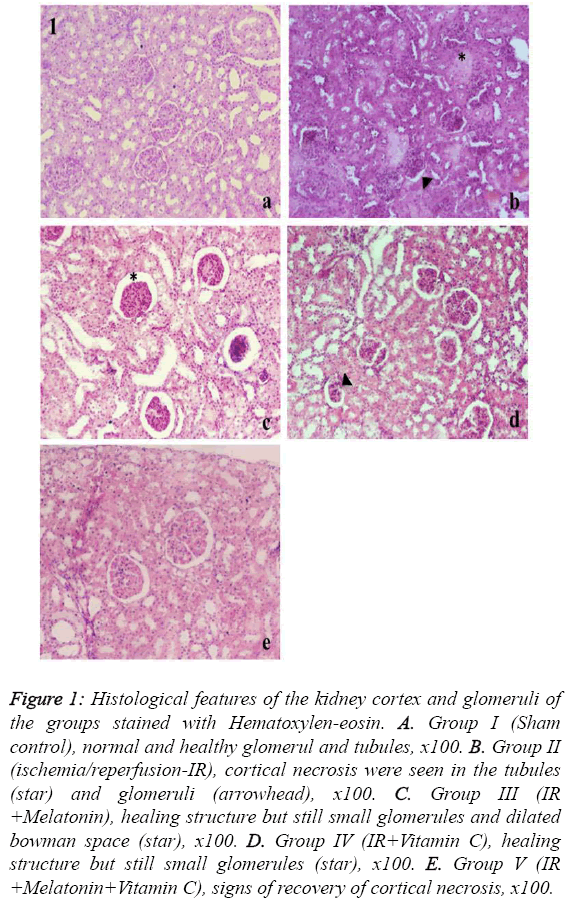
A marked increase in the SOD activity levels were observed in the IR+Mel+VitC (46.35 (37.68-58.48)) and the IR+Mel (35.95 (22.20-39.33)) groups compared to the IR group (16.00 (11.35-18.38)) (p<0.05). Overall, the best results in biochemistry were observed in the I/R+MEL+VitC and the I/R +MEL groups. But, combined application of Melatonin and Vitamin C revealed more efficient results in almost all parameters. The results of the histological examination showed that while there was no difference among the microscopic injury scores of the IR+Mel (5.0 (4.0-6.0)), the IR+VitC (3.0 (2.0-7.0)) and the IR+Mel+VitC groups (3.0 (2.0-5.0), P >0.05), the microscopic injury scores of theIR+Mel+VitC groups were significantly different from the microscopic injury scores of the IR group (8.0 (4.0-8.00), P<0.05) (Table 1). Combinatorial pretreatment with the Melatonin and Vitamin C (3.0 (2.0-5.0)) resulted in a substantially lower microscopic injury score compared to that in the IR group (P<0.05) (Table 1). While cortical necrosis were seen in the tubules and glomeruli of the IR group, there was healing structure as well as small glomeruli and dilated bowman space in the IR+VitC and the IR+Mel groups respectively. The best recovery signs of cortical necrosis were observed in the IR+Mel+VitC group (Figure 1).
Figure 1: Histological features of the kidney cortex and glomeruli of the groups stained with Hematoxylen-eosin. A. Group I (Sham control), normal and healthy glomerul and tubules, x100. B. Group II (ischemia/reperfusion-IR), cortical necrosis were seen in the tubules (star) and glomeruli (arrowhead), x100. C. Group III (IR +Melatonin), healing structure but still small glomerules and dilated bowman space (star), x100. D. Group IV (IR+Vitamin C), healing structure but still small glomerules (star), x100. E. Group V (IR +Melatonin+Vitamin C), signs of recovery of cortical necrosis, x100.
Discussion
Ischemia-reperfusion (I/R) causes the generation of superoxide and other the ROS products such as hydrogen peroxide and hydroxyl radical. The early rise in the ROS causes to lipid peroxidation of the membranes, tissue damage and the loss of renal function in the I/R-induced models of acute kidney injury [24]. The I/R causes also generation of the proinflammatory cytokines and chemokines from renal endothelial and parenchymal cells [25]. These harmful effects of the I/R can be prevented by effective antioxidant and anti-inflammatory agents. In this regard, combination of melatonin and Vitamin C were used in the present study, and their combined protective effects were compared to their individual ones on the I/R injury in adult rats. In the present study, impaired renal functions were evidenced by elevated serum urea, creatinine and the IMA levels and elevated kidney tissue the MPO activity and the MDA levels as well as depleted kidney tissue the SOD and the CAT activities in the I/R group. On the other hand, asymmetrical and shrunken glomeruli, cortical necrotic changes of the epithelium lining tubules (star) and glomeruli (arrowhead) in renal histology clearly demonstrated the alteration in renal structure after the I/R injury (Figure 1).
Pretreatment with Melatonin and Vitamin C resulted in marked improvement in renal functions, manifested by significant decrease of kidney the MPO activity and the MDA level, and serum urea, creatinine and the IMA levels and significant increase in kidney the CAT and the SOD activities. Also, histopathological improvements were noticed in all pretreatment groups. But, regression of the histopathological changes caused by ischemia was remarkable in the combined pretreated group. Glomeruli and tubules appeared apparently healthy and also most of the tubules, with less necrosis and infiltration. In the present study, we used another serum marker, the IMA, in addition to urea and creatinine levels for detecting of the protective effects of Melatonin and Vitamin C alone and in combination against renal I/R injury. It has been already suggested that the IMA may serve as early biomarker for renal the IR injury. Amasyali et al. demonstrated that, the rising the IMA levels might be useful for the prediction of necrosis in renal the IR injury [26]. Our results on urea, creatinine and the IMA levels were in agreement with previous studies [26-28].
Furthermore, the renal tissue the MDA levels in the I/R group was increased compared to sham group, indicating the presence of enhanced lipid peroxidation due to the I/R injury. There was a significant reduction of kidney tissue the MDA levels in the ischemic rats pretreated with Melatonin and Vitamin C alone and in combination. But combined pretreatment of Melatonin and Vitamin C demonstrated more efficient results in preventing of lipid peroxidation (Table 1). The MDA results of the present study were in agreement with previous studies [27-29]. In addition, significant increase in renal the SOD and the CAT activities were evident in the combined pretreated groups. These findings on the SOD and the CAT activities were in agreement with studies of Singh and Chander and Mohammed and Lasheen [2,30]. These increasing effects on the SOD and the CAT activities could be attributed to the reduction of the ROS and subsequently reduction of any depressor effect of the ROS on gene expression of these enzymes [2]. Moreover, none of the treatment groups caused a significant change in the GPx activity compared to the IR group. Although the highest the GPx activity level was in the combination group, but it did not reached the significant level. This could be due to insufficient reduction of depressor effect of the ROS on gene expression of this enzyme [2].
In renal ischemia reperfusion injury, the neutrophils play a major role in oxidant injury of the kidney via NADPH oxidase and myeloperoxidase (MPO) activities of neutrophils. Therefore, inhibition of neutrophil recruitment to ischemic tissues during reperfusion and diminishing of the tissue the MPO activity, which is an index of tissue neutrophil infiltration, are essential in the prevention and treatment of ischemia reperfusion injury. In a number of oxidative stress models, such as liver, renal and skeletal muscle ischemia reperfusion, it has been demonstrated that Melatonin and Vitamin C treatment reduce the increase in the MPO activities [31-33]. In the present study, elevated levels of the MPO activity was inhibited by Melatonin and Vitamin C either alone or in combination (Table 1). But combined application of Melatonin and Vitamin C revealed more efficient results in reduction of the MPO activity. Ischemia reperfusion promotes also generation of the proinflammatory cytokines and chemokines from renal endothelial and parenchymal cells [25]. The cytokines and the ROS produced by the I/R injury upregulate the expression of adhesion molecules [34]. The combination of chemokines, cytokines and adhesion molecules lead to recruitment, activation and sequestration of leukocytes, which generate further the ROS and cytokines and potentiated the injury [35]. In this regard, while several studies demonstrated that proinflammatory cytokines such as the TNF- α, IL-β and IL-6 levels in kidney increased by experimental renal I/R [36-38], but Kurcer et al showed that renal ischemia reperfusion did not cause an increase in the TNF-α, IL-β and IL-6 levels and based on these results they suggested that protection effect of Melatonin from renal I/R injury is not mediated by proinflammatory cytokines [27]. In relation to this, because we did not investigate any proinflammatory parameters, we don’t know which one is true for our experimental conditions. However, it is obviously true that a portion of the protective effect of melatonin and Vitamin C in the present study comes from reduction in the MPO activity and the ROS generation of neutrophils, indicating that the protective effect of both agents is neutrophil dependent.
Melatonin and Vitamin C have been used as antioxidant and anti-inflammatory agent in many previous studies individually or in combination with another agent [13,30,39-42]. To the best of our knowledge, this is the first study showing of their combination in renal I/R injury in rats. The biochemical and histopathological results in the present study suggest that Melatonin and Vitamin C pretreatments prevented renal damage induced by I/R, but combined application of Melatonin and Vitamin C revealed more efficient results in preventing renal cell damages (Table 1 and Figure 1). This substantial protection in the combination group could possibly originate from the synergy between antioxidant and the antiinflammatory effects of the both molecules. However, the nature of this synergy remains to be demonstrated. In addition, since the treatment mechanisms of Melatonin and Vitamin C were studied by many previous studies in detail in the literature, we aimed to investigate just the protective effects of combined application of Melatonin and Vitamin C against renal I/R injury by exploring the antioxidant and antiinflammatory effects in the present study. However, we believe that the mechanisms suggested in the literature so far are also strongly likely in the present study.
In conclusion, combined pretreatment with Melatonin and Vitamin C seems to have higher anti-inflammatory and antioxidant properties than individual pretreatments with either agent alone. Thus, prophylaxis with Melatonin and Vitamin C has a potential to reduce the severity of renal injury in renal ischemia-reperfusion in the rat.
References
- Cole E, Naimark D, Aprile M, Wade J, Cattran D, Pei Y. An analysis of predictors of long-term cadaveric renal allograft survival. Clin Transplant 1995; 9: 282-288.
- Singh D, Chander V, Chopra K. Protective effect of the catechin on ischemia-reperfusion-induced renal injury in rats. Pharmacol Rep 2005; 57: 70-76.
- Bulkley GB. Free radical mediated reperfusion injury: a selective review. Br J Cancer 1987; 55: 66-73.
- Weight SC, Bell PR, Nicholson ML. Renal ischaemia-reperfusion injury. Br J Surg 1996; 83: 162-170.
- Chatterjee PK, Brown PA, Cuzzocrea S, Zacharowski K, Stewart KN, Mota-Filipe H. Calpain inhibitor-1 reduces renal ischemia/reperfusion injury in the rat. Kidney Int 2001; 59: 2073-2083.
- Paller MS. The cell biology of reperfusion injury in the kidney. J Invest Med 1994; 42: 632-629.
- Williams P, Lopez H, Britt D, Chan C, Ezrin A, Hottendorf R. Characterization of renal ischemia-reperfusion injury in rats. J Pharmacol Toxicol Methods 1997; 37: 1-7.
- Erdogan H, Fadillioglu E, Yagmurca M, Ucar M, Irmak MK. Protein oxidation and lipid peroxidation after renal ischemia-reperfusion injury: protective effects of erdosteine and N-acetylcysteine. Urol Res 2006; 34: 41-46.
- Taha MO, Souza HS, Carvalho CA, Fagundes DJ, Simoes MJ, Novo NF. Cytoprotective effects of ascorbic acid on the ischemia-reperfusion injury of rat liver. Transplantation Proceedings. 2004; 36: 296-300.
- Lehr HA, Frei B, Olofsson AM, Carew TE, Arfors KE. Protection from oxidised LDL-induced leukocyte adhesion to microvascular and macrovascular endothelium in vivo by Vitamin C but not by vitamin E. Circulation. 1995; 91: 1525-1532.
- Niki E. Action of ascorbicacid as a scavenger of active and stable oxygen radicals. Am J Clin Nutr 1991; 54: 1119S-1124S.
- Antunes LM, Darin JD, Bianchi MD. Protective effects of Vitamin C against cisplatin-induced nephrotoxicity and lipid peroxidation in adult rats: a dose-dependent study. Pharmacol Res 2000; 41: 405-411.
- Ocak S, Gorur S, Hakverdi S, Celik S, Erdogan S. Protective effects of caffeic acid phenethyl ester, Vitamin C, vitamin E and N-acetylcysteine on vancomycin-induced nephrotoxicity in rats. Basic Clin Pharmacol Toxicol 2007; 100: 328-333.
- Kadkhodaee M, Khastar H, Faghihi M, Ghaznavi R, Zahmatkesh M. Effects of co-supplementation of vitamins E and C on gentamicin-induced nephrotoxicity in rat. Exp Physiol 2005; 90: 571-576.
- Appenroth D, Fröb S, Kertsen L, Splinter K, Winnefeld K. Protective effects of vitamin E and C on cisplatin nephrotoxicity in developing rats. Arch Toxicol 1997; 71: 677-683.
- Carrillo-Vico A, Guerrero JM, Lardone PJ, Reiter RJ. A review of the multiple actions of melatonin on the immune system. Endocrine 2005; 27: 189-200.
- Mayo JC, Sainz RM, Tan DX, Hardeland R, Leon J, Rodriguez C. Anti-inflammatory actions of melatonin and its metabolites, N1-acetyl-N2-formyl-5-methoxykynuramine (AFMK) and N1-acetyl-5-methoxykynuramine (AMK), in macrophages. J Neuroimmunol 2005; 165: 139-149.
- Reiter RJ, Tan DX, Maldonado MD. Melatonin as an antioxidant: physiology versus pharmacology. J Pineal Res 2005; 39: 215-256.
- Rodriguez C, Mayo JC, Sainz RM, Antolı´n I, Herrera F, Martı´nV. Regulation of antioxidant enzymes: a significant role for melatonin. J Pineal Res 2004; 36: 1-9.
- Maldonado MD, Murillo-Cabezas F, Terron MP, Flores LJ, TanDX, Manchester LC. The potential of melatonin in reducing morbidity-mortality after cranio cerebral trauma. J Pineal Res 2007; 42: 1-11.
- Yurtcu M, Abasiyanik A, Avunduk MC, Muhtaroglu S. Effects of melatonin on spermatogenesis and testicular ischemia reperfusion injury after unilatera ltesticular torsion-detorsion. J Pediatr Surg 2008; 43: 1873-78.
- Ozturk G, Coşkun S, Erbaş D, Hasanoglu E. The effect of melatonin on liver superoxide dismutase activity, serum nitrate and thyroid hormone levels. Jpn J Physiol 2000; 50: 149-153.
- Rodriguez-Reynoso S, Leal C, Portilla-de Buen E, Castillo JC, Ramos-Solano F. Melatonin ameliorates renal ischemia/reperfusion injury. J Surg Res 2004; 116: 242-247.
- Fekete A, Vannay A, Ver A, Vasarhelyi B, Muller V, Ouyang N. Sex differences in the alterations of Na(+), K(+)-ATPase following ischaemia-reperfusion injury in the rat kidney. J Physiol 2004; 555: 471-480.
- Bonventre JV, Zuk A. Ischemic acute renal failure: an inflammatory disease? Kidney Int 2004; 66: 480-485.
- Amasyali AS, Akkurt A, Kazan E, Yilmaz M, Erol B, Yildiz Y. The protective effect of tadalafil on IMA (IschemiaModifiedAlbumin) levels in experimental renal ischemia-reperfusion injury. Int J Clin Exp Med. 2015; 8: 15766-15772.
- Kurcer Z, Oguz E, Ozbilge H, Baba F, Aksoy N, Celik H. Melatonin protects from ischemia/reperfusion-induced renal injury in rats: this effect is not mediated by proinflammatory cytokines. J Pineal Res. 2007; 43: 172-178.
- Karaman A, Turkmen E, Gursul C, Tas E, Fadillioglu E. Prevention of renal ischemia/reperfusion-induced injury in rats by leflunomide. Int J Urol 2006; 13: 1434-41.
- Korkmaz A, Kolankaya D. The Protective effects of ascorbic acid against renal ischemia-reperfusion injury in male rats. Renal Failure 2009; 31: 36-43.
- Mohamed Abd E, Lasheen NN. Comparative study on the protective role of vitamin C and L-arginine in experimental renal ischemia reperfusion in adult rats. Int J Physiol Pathophysiol Pharmacol 2014; 6: 153-165.
- Sener G, Sehirli AO, Keyer-Uysal M, Arbak S, Ersoy Y, Yegen BC. The protective effect of melatonin on renal ischemia-reperfusion injury in the rat. J Pineal Res 2002; 32: 120-126.
- Rodriguez-Reynoso S, Leal C, Portilla E, Olivares N, Muniz J. Effects of exogenous melatonin on hepatic energetic status during ischemia-reperfusion: possible role of TNF-a and nitricoxide. J Surg Res 2001; 100: 141-149.
- Kearns SR, Daly AF, Sheehan K, Murray P, Kelly C, Bouchier-Hayes D. Oral Vitamin C reduces the injury to skeletal muscle caused by compartment syndrome. J Bone Joint Surg Br. 2004; 86: 906-11.
- Ysebaert DK, De Greef KE, De Beuf A, Van Rompay AR, Vercauteren S, Persy VP. T cells as mediators in renal ischemia/reperfusion injury. Kidney Int 2004; 66: 491-96.
- Kher A, Meldrum KK, Wang M, Tsai BM, Pitcher JM, Meldrum DR. Cellular and molecular mechanisms of sex differences in renal ischemia–reperfusion injury. Cardiovasc Res 2005; 67: 594-603.
- Kelly KJ, Williams WW Jr, Colvin RB, Meehan SM, Springer TA, Gutierrez-Ramos JC. Intercellular adhesion molecule-1-deficient mice are protected against ischemic renal injury. J Clin Invest 1996; 97: 1056-1063.
- Kielar ML, John R, Bennett M, Richardson JA, Shelton JM, Chen L. Maladaptive role of IL-6 in ischemic acute renal failure. J Am Soc Nephrol 2005; 16: 3315-3325.
- Jo SK, Sung SA, Cho WY, Go KJ, Kim HK. Macrophages contribute to the initiation of ischaemic acute renal failure in rats. Nephrol Dial Transplant 2006; 21: 1231-1239.
- Ozcan AV, Sacar M, Aybek H, Bir F, Demir S, Onem G. The Effects of Iloprost and Vitamin C on kidney as a remote organ after ischemia/reperfusion of lower extremities. J Surg Res 2007; 140: 20-26.
- Ahmadiasl N, Banaei S, Alihemati A, Baradaran B, Azimian E. Effect of a combined treatment with erythropoietin and melatonin on renal ischemia reperfusion injury in male rats. Clin Exp Nephrol. 2014; 18: 855-64.
- Takhshid MA, Tavasuli AR, Heidary Y, Keshavarz M, Kargar H. Protective effect of Vitamins E and C on endosulfan-induced reproductive toxicity in male rats. Iran J Med Sci 2012; 37: 173-80.
- Sinanoglu O, Sezgin G, Ozturk G, Tuncdemir M, Guney S, Aksungar FB. Melatonin with 1,25-dihydroxyvitamin D3 protects against apoptotic ischemia-reperfusion injury in the rat kidney. Ren Fail 2012; 34: 1021-6.