- Biomedical Research (2013) Volume 24, Issue 1
The Investigation of the Antiangiogenic Potential of Amiodarone HCl in the Chick Embryo Chorioallantoic Membrane Model
Oguz Karahan1*, Celal Yavuz1, Sinan Demirtas1, Ahmet Caliskan1 and Erhan Atahan21Medical School of Dicle University, Department of Cardiovascular Surgery, Diyarbakir, Turkey
2Medical School of Cumhuriyet University, Department of Cardiovascular Surgery, Sivas/Turkey
- *Corresponding Author:
- Oguz Karahan
Medical School of Dicle University
Department of Cardiovascular Surgery
Diyarbakir, Turkey
Tel: 905063929320
E-mail: oguzk2002@gmail.com
Accepted date: December 10, 2012
Abstract
Angiogenesis, which plays a significant role in a variety of physiological processes, such as embryonic growth and wound healing, is strictly delimited and finely tuned by a balance of proangiogenic and antiangiogenic factors. Cardiac rhythm disorders are diseases that are often accompanied by vascular pathologies. As such, the purpose of this study was to investigate the antiangiogenic effects of Amiodarone HCl in the chorioallantoic membrane model. In this study, the antiangiogenic effect of Amiodarone HCl was compared with a positive control group that was given pure paraffin and the vascular endothelial growth factor inhibitor Bevacizumab, as well as a negative control group in which clearly antiangiogenic activity was shown in this model previously. Concentrations of 10-4, 10-5, and 10-6M of each drug were administered. For the purpose of determining the antiangiogenic effects of the drugs, blood vessels of the chorioallantoic membranes were evaluated using a stereoscopic microscope. The antiangiogenic effect scores of Amiodarone HCl at the dose of 10-4 molar (M) were higher than those of 10-5M and 10-6M, but that result was statistically insignificant. The antiangiogenic effect scores of Bevacizumab at the concentrations of 10-4M and 10-5M were significantly higher than that of 10-6M. This effect of Amiodarone may be important for determining routine antiarrhythmic doses.
Keywords
Chorioallantoic membrane; angiogenesis; Amiodarone HCl; Bevacizumab
Introduction
Angiogenesis, which is described as the formation of new blood vessels, is a complex process that includes endothelial cell activation, controlled proteolytic degradation of the extracellular matrix, proliferation and migration of endothelial cells, and formation of capillary vessel lumina [1]. Under normal circumstances, the formation of new blood vessels occurs during wound healing, organ regeneration and in the female reproductive system during ovulation, menstruation, and the formation of the placenta [2]. It also plays a role in the pathophysiology of multiple disease processes, including tumour neovascularization, ischaemic recovery, and wound healing. In addition, angiogenesis and cardiovascular diseases are strongly related to providing the distal perfusion [3]. The investigations were focused on ways to obtain therapeutic benefits in low-perfusion situations with neo-angiogenesis. As such, research on therapeutic angiogenesis and antiangiogenic has become more frequent in recent studies [4].
Amiodarone is an effective antiarrhythmic agent; however, standard Amiodarone therapies can cause significant side effects. For example, the most common side effect of intravenous treatment is hypotension, which can be serious and lead to cardiovascular collapse, even resulting in fatality [5,6]. While many side effects have been reported previously, its effects on the developing heart and regenerating tissues have not been represented sufficiently [6]. This study was designed to clarify the relationship between angiogenesis and Amiodarone. In the present study, we aimed to demonstrate the antiangiogenic potency of Amiodarone by using the chick embryo chorioallantoic membrane (CAM) model.
Materials and Methods
Preparation of the pellets
In this trial, the possible antiangiogenic effect of Amiodarone HCl (Cordarone®, 150 mg/3ml Sanofi-Dogu Ilac AS, Istanbul, Turkey) was studied. The Amiodarone used was in its commercially available form as a soluble infusion. Agarose (Merck, Damstadt, Germany) was added to distilled water to obtain a 2.5% (w/v) solution, which was autoclaved at 121°C and under one atmospheric pressure to provide dissolution and sterilization. Subsequently, it was cooled to 37°C in a sterile container, at which point the study drug was added. The positive control group was constructed with the antiangiogenic agent Bevacizumab, which is known as a specific vascular endothelial growth factor inhibitor [7].
Appropriate volumes of solutions were used to achieve three different concentrations of the drug (10-4, 10-5, and 10-6M). Approximately sixty pellets were used for each study set. Therefore, approximately 1 ml of combined agar and drug solution (10 μl×100 =1 ml) was prepared initially for the drug. The drug solutions with 100 IU, 10 IU and 1 IU/10 μl concentrations were prepared by diluting these initial mixtures ten-fold with the agarose solution again.
Using a micropipette, 10-μl drops of this mixed solution were placed on previously sterilized, vertical, cylindrical, stainless steel rods 5 mm in diameter to obtain circular pellets with the same diameter. The pellets were then left to solidify at room temperature in a sterile setting.
Chicken chorioallantoic membrane (CAM) assay
Ross 308 strain fertilized hen eggs were obtained from Yemsel Poultry Company (Kayseri, Turkey). The study protocol was approved by the Local Animal Ethics Committee.
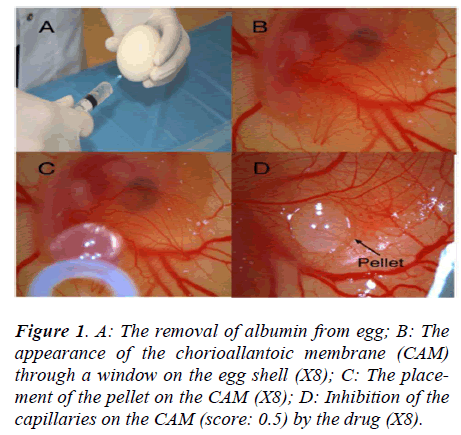
The fertilized hen eggs were incubated in a horizontal position, with environmental conditions of 37.5°C temperature and 80% relative humidity. On the fifth day of the incubation period, 5 ml of albumen were taken through the eggshell with a syringe (Figure 1A), and a shell piece 2-3 cm in diameter was removed from the opposite side of the eggs. Normal development of the CAM was verified (Figure 1B), and malformed or dead embryos were excluded. The windows on the eggshells were sealed with gelatin, and the eggs were incubated for 72 more hours, until the CAM reached 2 cm in diameter. Subsequently (on day 8), the seal was removed and the pellets were placed on the chorioallantoic membrane of each egg (Figure 1C). The seal was replaced and the eggs were incubated for 24 hours. The angiogenesis level was evaluated after that period.
There were five group created; pellet group without drug were utilized as negative control group (Group I), Bevacizumab was used for positive control group (group II), three different dosage of drug (10-4, 10-5, and 10-6M) groups were classified as group III-IV-V respectively. For each tested drug solution, 20 eggs were used. All the tests were duplicated. Eggs in which the pellets caused inflammation and embryo toxicity were excluded. Totally 200 eggs were used for five created groups (20 for each group, 20 for confirmation each test). Negative control group consisted with pellet without drug, did not demonstrate any antiangiogenic behaviour.
Angiogenesis scoring
The inhibitory effects of the drugs on angiogenesis in chorioallantoic membrane were evaluated under a stereoscopic microscope and assessed according to the scoring system used previously in several studies [8,9]. In this scoring system, the change in the density of the capillaries around the pellet and the extent of the effect are evaluated (Figure 1D). In the initial scoring of each subject, a score of 0 indicated the absence of any demonstrable antiangiogenic effect (normal embryo and no difference in surrounding capillaries); 0.5 indicated a very weak antiangiogenic effect (no capillary-free area, but an area with reduced density of capillaries not larger than the pellet area); 1 indicated a weak moderate antiangiogenic effect (a small capillary-free area or a small area with significantly decreased density of capillaries, less than double the size of the pellet); and 2 indicated a strong antiangiogenic effect (a capillary-free area around the pellet equal to or more than double the size of the pellet). The equation used for the determination of the average score was as follows:
Average score = [Number of eggs (Score 2) × 2 + Egg Number (Score 1) × 1] / [Total number of eggs (Score 0, 1, 2)].
According to this scoring system, a score of 0.5 meant that there was no antiangiogenic effect, a score of 0.5 to 1 indicated a weak antiangiogenic effect, and a score of N1 implied a strong antiangiogenic effect.
Statistical analysis
The angiogenesis scores were compared with a Kruskal- Wallis ANOVA test and a Mann-Whitney U test. A p value of less than 0.05 was considered statistically significant.
Results
Drug-free pellets of 10 μL agarose solution were used as negative controls; there were no significant antiangiogenic effects observed in the negative controls (average antiangiogenic score=0.1). Bevacizumab was used as the positive control and had an average antiangiogenic score of 0.95 (very good antiangiogenic effect) in 10-6M concentrations. Each tested drug was evaluated separately, and the results with different solutions were compared.
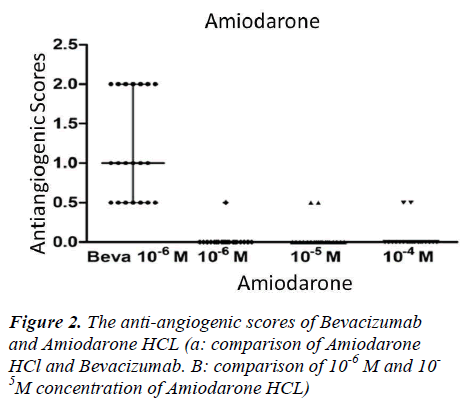
Our findings indicated that antiangiogenic behaviours were relatively increased with dose escalation. There were mildly antiangiogenic effects observed in 10-4M (M; Molar) concentration of Amiodarone, with antiangiogenic scores of 1.0 in one egg and 0.5 in two eggs. The 0.5 antiangiogenic scores in the two eggs were observed at 10-5M concentration of Amiodarone. The antiangiogenic score of 0.5 in the one egg was at a 10-6M concentration of Amiodarone (Fig 2). No statistically significant differences were observed between these dose-related antiangiogenic tendencies (p>0.05).
10-6M concentrations of Bevacizumab displayed significantly higher antiangiogenic effects than all the dosages of Amiodarone HCl (p<0.05) (Fig 2).
Discussion
Angiogenesis is a vital process that plays a role in embryonic development, menstrual cycle, and wound healing; it is controlled by proangiogenic and antiangiogenic factors [2,10]. Recently, the CAM model has long been preferred for the studies on tumour angiogenesis and metastasis, as well as the studies of the standard drug molecules with angiogenic or antiangiogenic activity [11]. The myocardium is a highly oxygen-dependent tissue, and the continuity of blood flow is critical for functional protection in acute ischaemic conditions. Thus, therapeutic angiogenesis for myocardial tissue has gained importance with recent advances. However, recent studies indicate that maintaining existing physiological angiogenesis response is important in stimulating neo-angiogenesis with therapeutic methods [12,13]. Both regulatory factors and basic mechanisms that enrolled at myocardial angiogenesis should be investigated for the defining possible therapeutic approaches [13].
Amiodarone HCl effectively controls a wide spectrum of atrial and ventricular arrhythmias. However, because of the wide spectrum of the mechanism of action of Amiodarone and the limiting possible side effects, such as thyroid dysfunction, pulmonary fibrosis, and dermatologic changes, its long-term use may be limited in some patients, and care should be taken when administering the drug [14]. These side effects have prompted researchers to observe other potential effects of Amiodarone HCl. Traupe et al. showed that Amiodarone rapidly accumulates in atherosclerotic vascular tissue, abolishes vascular autorhythmicity, and improves endothelium- dependent function in atherosclerotic arteries. The researchers suggested that these effects following Amiodarone administration might contribute to its antiarrhythmic effects [15]. Chen et al. found that Amiodarone treatment caused failure of cardiac valve formation in zebrafish embryos [6]. These complex effects have led to the notion that Amiodarone HCl can affect neo-angiogenesis in regenerating tissues such as the myocardium. As such, we investigated the possible antiangiogenic effects of Amiodarone HCl on a CAM model in this study; non-significant antiangiogenic effects were observed at a 10-4M dose of Amiodarone HCl, with antiangiogenic scores of 1.0 in one egg and 0.5 in two eggs (p>0.05). The antiangiogenic activity of Bevacizumab, known as a specific vascular endothelial growth factor inhibitor, was naturally higher than that of Amiodarone HCl (p<0.05).
Our results revealed that Amiodarone HCl shows doserelated antiangiogenic effects. However, this effect is mild and is encountered at high doses. According to these data, the antiangiogenic effect of Amiodarone HCl seems to be safe for administration in different types of arrhythmias; however, the other possible side effects should not be ignored. Regarding antiangiogenic behaviour, Clinicians should be careful for only the continuous loading dose.
References
- Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003; 9(6):653-660.
- Hoeben A, Landuyt B, Highley MS, et al., De Bruijn E.A. Vascular endothelial growth factor and angiogenesis. Pharmacol. Rev. 2004; 56:549–580.
- Zhang J, SE Hughes. Role of the ephrin and Eph receptor tyrosine kinase families in angiogenesis and development of the cardiovascular system. J Pathol 2006; 208: 453–461.
- Losordo DW, Dimmeler S. Therapeutic angiogenesis and vasculogenesis for ischemic disease. Part I: angiogenic cytokines. Circulation. 2004 Jun 1;109(21):2487-91.
- Somberg JC, Cvetanovic I, Ranade V, et al., Comparative effects of rapid bolus administration of aqueous Amiodarone versus 10-minute cordarone I.v. infusion on mean arterial blood pressure in conscious dogs. Cardiovasc Drugs Ther. 2004 Sep; 18(5):345-51.
- Chen YH, Lee HC, Hsu RJ, et al., The toxic effect of Amiodarone on valve formation in the developing heart of zebrafish embryos. Reprod Toxicol. 2011 Dec 29. [Epub ahead of print]
- Narayana A, Kelly P, Golfinos J, et al., Antiangiogenic therapy using Bevacizumab in recurrent highgrade glioma: impact on local control and patient survival. J Neurosurg 2009; 110:173–180.
- Demirci B, Dadandi MY, Paper DH, et al., Chemical composition of the essential oil of Phlomis linearis Boiss. & Bal., and biological effects on the CAMassay: a safety evaluation. Z Naturforsch C 2003 Nov-Dec; 58(11–12):826–9.
- Bobek V, Kovarik J. Antitumor and antimetastatic effect of warfarin and heparins. Biomed Pharmacother 2004 May; 58(4):213–9.
- Kerbel R, Folkman J. Clinical translation of angiogenesis inhibitors. Nat Rev Cancer 2002; 2(10):727-739.
- Dogan OT, Polat ZA, Karahan O, et al., Antiangiogenic activities of bemiparin sodium, enoxaparin sodium, nadroparin calcium and tinzaparin sodium. Thromb Res. 2011 Oct;128(4):29-32.
- Wadhwa S. Therapeutic Myocardial Angiogenesis: A ray of hope for patients unsuitable for CABG/PTCA. Journal of Indian Academy of Clinical Medicine. 2000; 1:252- 255.
- Toyota E, Matsunaga T, Chilian WM. Myocardial angiogenesis. Mol Cell Biochem. 2004 Sep; 264(1-2):35-44.
- Singh BN. Amiodarone: a multifaceted antiarrhythmic drug. Curr Cardiol Rep. 2006 Sep; 8(5):349-55.
- Traupe T, Keller M, Fojtu E, et al., Antioxidant activity and sex differences of acute vascular effects of Amiodarone in advanced atherosclerosis. J Cardiovasc Pharmacol. 2007; 50(5):578-84.