Research Article - Biomedical Research (2017) Volume 28, Issue 14
Surgical model for ocular ischemic syndrome in mice
Yu Ling, Zhiyong Fu and Yanling Wang*
Department of Ophthalmology, Beijing Friendship Hospital, Capital Medical University, PR China
- *Corresponding Author:
- Yanling Wang
Department of Ophthalmology
Beijing Friendship Hospital
Capital Medical University, PR China
Accepted on June 16, 2017
Abstract
Background: To better understand the pathogenesis of Ocular Ischemic Syndrome (OIS), several animal models were established last decades. Our purpose is to characterize the functional and morphologic changes in a murine model of OIS caused by Bilateral Internal Carotid Artery Occlusion (BICAO).
Methods: Adult mice were submitted to BICAO or sham surgery. Changes in ocular blood flow and retinal circulation after surgery were evaluated by MRA to ensure the retinal blood flow occlusion. The retinal hypoperfusion was demonstrated 7 d after BICAO by both ocular fundus photography and fluorescein angiography. The OCT images were taken to measure the thicknesses of the various retinal layers, and then eyes were enucleated and embedded in paraffin for morphological studies.
Results: MRA images showed that the ligation of both internal carotid arteries significantly reduced ocular blood flow and narrowed the blood vessels. BICAO for 7 d was explored by both ocular fundus photography and fluorescein angiography finding that the retinal hypoperfusion persisted in the mice of BICAO group. The total retinal thickness and retinal ganglion cell density decreased compared with Sham group. However, no changes were evident in IPL layer in BICAO group, which was in agreement with the results obtained from OCT images.
Conclusion: We successfully visualized the occlusion of blood flow after the BICAO by MRA, and angiography could also demonstrate that BICAO for 7 d was sufficient to maintain the retinal ischemia and induce the morphological changes. We suggest that this protocol might be used as a mouse model for OIS in the future.
Keywords
Ocular ischemic syndrome, Bilateral internal carotid artery occlusion, Mice.
Introduction
Ocular Ischemic Syndrome (OIS) is characterized as visual loss, orbital pain and a series of signs of various anterior and posterior segments [1,2]. The pathological mechanism of OIS is still unknown. It is generally recognized that atherosclerosis is the main cause of OIS [1,3]. The chronic hypoperfusion due to stenosis or occlusion of common or internal carotid arteries induced by atherosclerosis likely leads to the development of OIS. More than 90% of patients with OIS suffer from a stenosis of the common or internal carotid arteries, and OIS is the initial clinical presentation of internal carotid stenosis in as many as 69% of patients [4,5].
To investigate the pathogenesis of OIS and to evaluate the efficacy of novel therapeutic strategies, several rodent models have been established. Slakter et al. initially demonstrated that Bilateral Common Carotid Arteries Occlusion (BCCAO) in the rat resulted in an incomplete retinal ischemia, chronic ganglion cell loss, and other cerebral degeneration [6]. Barnett et al. challenged that the reduction of blood flow by BCCAO for 7 d caused a metabolic stress to the retina and induced gliosis without evident histological damage [7]. As extending BCCAO to 30 d, Daniel et al. demonstrated the functional and morphological damage of the retina in rats [8]. In the rat model of BCCAO, cerebral ischemia is induced in addition to OIS, and severe brain injury induces the exacerbation of systemic conditions and increases the mortality in rats. Moreover, when the bilateral CCAs are blocked, a compensatory blood is supplied to the area of cerebral ischemia through the circle of Willis, but not to the ophthalmic artery.
Recently, a new murine retinal ischemic model demonstrated that both ligations of the Pterygopalatine Artery (PPA) and the External Carotid Artery (ECA) induced more severe retinal ischemic injury than single PPA ligation did [9]. ECA might contribute to the vascular network between the PPA and the ophthalmic artery. Therefore, in this study, we ligated the Internal Carotid Artery (ICA) to induce the ocular ischemia in mice, and the blood from ECA might compensate the blood supply to PPA with time, which mimicked the hypoperfusion induced by atherosclerosis in patients.
Materials and Methods
In this study, Six to eight-week-old male C57/BL6 mice obtained from the Animal House of the Capital Medical University were housed in a 12 h reverse light cycle with free access to food and water. All procedures were conducted in accordance with the principles of The Association for Research in Vision and Ophthalmology. The animal research protocol was approved by the Animal Experimentation Ethics Committee of the Capital Medical University. Male mice used in the experiment were randomly divided into surgical and sham surgical groups. The surgical group was named as BICAO, and the sham surgical group as the control group.
BICAO procedure and magnetic resonance angiography (MRA)
BICAO was modified following the retinal ischemia procedure previously described [9]. The mice were anesthetized by pentobarbital (0.4 g/kg) through IP injection and placed on the heating pad in a supine position to maintain the body temperature at 37.0°C to 37.5°C by small animals gating and monitoring system (SA Instruments, Stony Brook, NY 11790, USA). A midline neck skin incision was made, and the left and right Common Carotid Arteries (CCAs) were exposed, then Internal Carotid Arteries (ICA) was ligated. Magnetic resonance angiography helped to identify the ocular ischemia after 24 h occlusion of ICAs (Bruker Corp. Billerica, MA 01821, USA). The MRA were performed by using 7 Telsa MRI scanner (Bruker Corp. Billerica, MA 01821, USA). The ischemia was maintained for 7 d after surgery, each eye was enucleated for the further analyses.
Ocular fundus photography and fluorescein angiography
Ocular fundus imaging is a simple, non-invasive technique for retinal vasculature, which was obtained using a retinal imaging microscope (Micron III, Phoenix Research Laboratories, Inc. Bioprobes Ltd. USA). 7 d after BICAO, the mice were anesthetized using pentobarbital (0.4 g/kg) through IP injection, and then mydriatic eye drops (1% atropine) was applied in order to obtain a better view of the fundus of the eyes. In the sodium fluorescein angiography [10]. Mice in anesthesia were injected with sodium fluorescein (40 mg/kg) through tail vein bolus injection. The retinal vessels began filling about 30 s after fluorescein administration. When the camera was ready, retracted the mouse eyelids with two fingers, orientated the field of view by visualizing the optic disc until you got the best fundus view, and then the temporal measurements of the different fluorangiographic phases were performed on all subjects for 30 min, including Arm-Retina Time (ART), Arteriovenous Transit Time (AVT) and Venous Filling Time (VFT). The captured images were quantified with picture analyzing system. The images of ocular fundus photography and fluorescein angiography were captured respectively.
Tissue preparation and histology
Seven days after BICAO, the mice were anesthetized and perfused by 10% formalin through the circulation. Bilateral eyes were enucleated from the mice, and then immersed for at least 24 h at 4°C in 10% formalin. After graded alcohol dehydration, xylene cleaned and infiltrated with wax, eyes were embedded in paraffin and then cut at a thickness of 5 μm by using a microtome. Slices were stained with hematoxylin and eosin (H and E). The standard area for measurement was at the central retina, approximately 200 μm from the optic disc. The thickness of the Inner Plexiform (IPL), Inner Nuclear (INL), Outer Plexiform (OPL), and Outer Nuclear (ONL) layers, and the total thickness were measured as the mean value of five different measurements at three points per section [11,12].
Optical coherence tomography (OCT)
OCT has been most broadly implemented in ophthalmology and offers a non-invasive method for visualizing retinal and choroidal vasculature in mice [13]. According to this procedure, OCT (B-scans) images were acquired using a bidimensional scan (B-scan) after 7 d of BICAO, which were consisted of 2000 Ascans (lines) acquired over 35 deg (1100 μm) × 1 deg (32 μm) of visual angle at 25 frames/s acquisition speed. During the acquisition, mice were anesthetized by pentobarbital (0.4 g/kg) through IP injection, and pupils were dilated and cyclopleged with tropicamide and phenylephrine. At each B-scan position 30 repetitive B-scans were recorded to reduce the speckle noise and make the high-resolution images for analysis of retinal layer segmentation. A semi-automated segmentation software developed at Duke University was used to segment individual layers from the averaged OCT B-Scan [11,13,14].
Results
The OSI model induced by the occlusion of bilateral ICAs
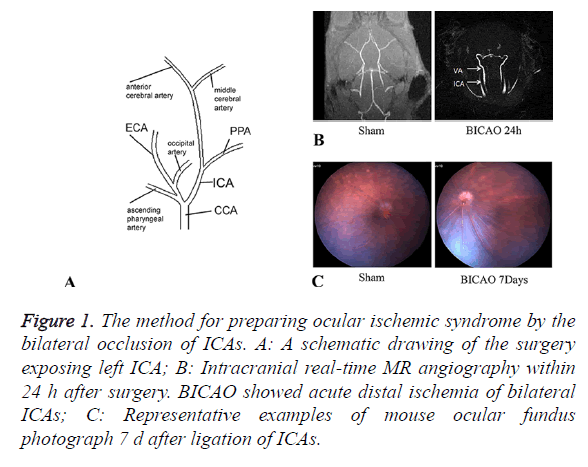
Twenty-six mice were performed BICAO surgery and ten to the sham procedure. The cervical arteries were clearly visualized in all mice. We could confirm the bilateral CCAs, External Carotid Arteries (ECAs), ICAs and Ophthalmic arteries (PPAs) entirely (Figure 1A). The proximal part of the ICAs was ligated selectively while leaving the ECAs intact. Magnetic resonance angiography was utilized to ensure complete distal interruption of blood flow after 24 h of BICAO (Figure 1B). After BICAO, the PPAs were filled retrogradely with the blood flow from the peripheral branch of the ECAs, which resulted in lower mortality compared with bilateral CCAs ligation [15]. Seven days after occlusion, images of ocular fundus were captured to demonstrate the ischemic condition compared with the sham mice, and affirm strongly the chronic ocular hypoperfusion (Figure 1C).
Figure 1. The method for preparing ocular ischemic syndrome by the bilateral occlusion of ICAs. A: A schematic drawing of the surgery exposing left ICA; B: Intracranial real-time MR angiography within 24 h after surgery. BICAO showed acute distal ischemia of bilateral ICAs; C: Representative examples of mouse ocular fundus photograph 7 d after ligation of ICAs.
Mouse fundus fluorescein angiography examination
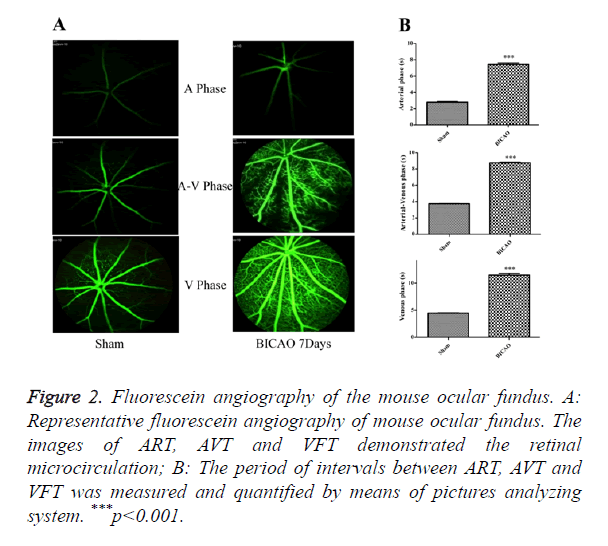
After video fluorescein angiography captured, the ART, AVT and VFT were measured by means of a picture analysis system to quantify the retinal microcirculation (Figure 2A). The ART consists of the period of time between the injection of sodium fluorescein into the tail vein and its first appearance in the retinal arteries. The AVP is defined as the period of time between the first influx of sodium fluorescein into retinal arteries and its first appearance in the corresponding retinal veins. The VFT is the period of time that all retinal veins appear fluorescein. Compared with the sham subjects, the AVP was significantly prolonged in BICAO models (p<0.001), while the AVP and VFT were also significantly longer in the BICAO models (p<0.001) (Figure 2B).
Figure 2. Fluorescein angiography of the mouse ocular fundus. A: Representative fluorescein angiography of mouse ocular fundus. The images of ART, AVT and VFT demonstrated the retinal microcirculation; B: The period of intervals between ART, AVT and VFT was measured and quantified by means of pictures analyzing system. ***p<0.001.
Histopathologic evaluation of retinal ischemia injury
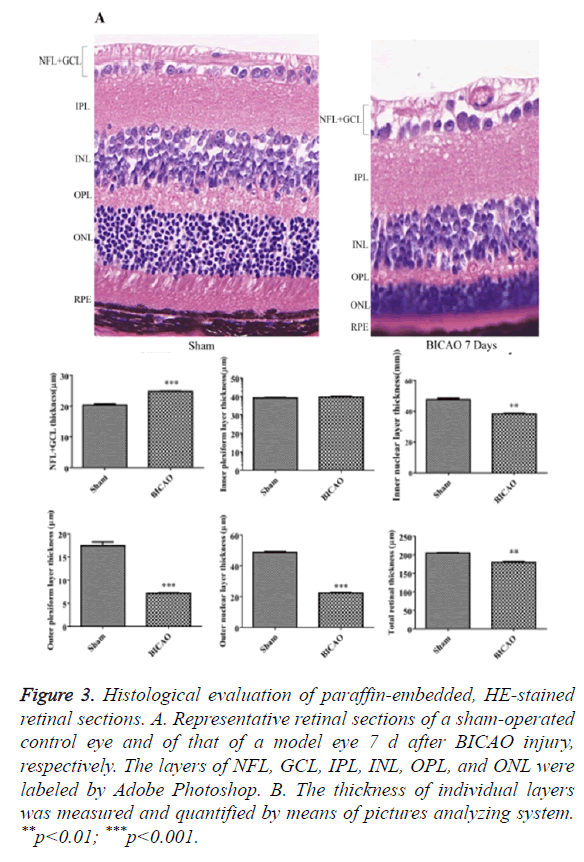
Figure 3A showed retinal sections of H and E staining. Compared with sham group, there was a significant decrease in the total thickness of the retina after 7 d of BICAO (p<0.01). The thickness of the NFL and GCL was also reduced considerably (p<0.001). Moreover, the thickness of INL, OPL, ONL and RPE were markedly reduced due to the ischemia injury (Figure 3B).
Figure 3. Histological evaluation of paraffin-embedded, HE-stained retinal sections. A. Representative retinal sections of a sham-operated control eye and of that of a model eye 7 d after BICAO injury, respectively. The layers of NFL, GCL, IPL, INL, OPL, and ONL were labeled by Adobe Photoshop. B. The thickness of individual layers was measured and quantified by means of pictures analyzing system. **p<0.01; ***p<0.001.
Retinal ischemia injury based on OCT images
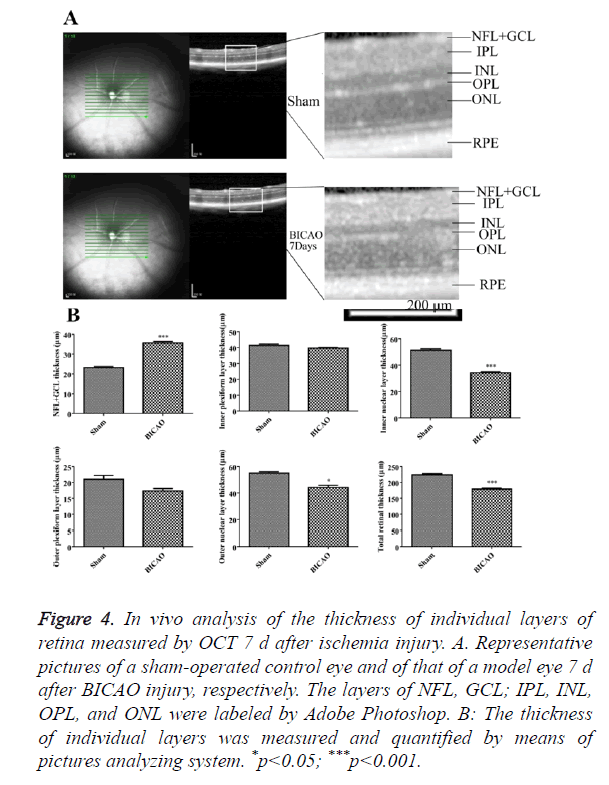
OCT images, approximately centered on the optic nerve, were shown in Figure 4A. All retinal layers were visible in the sham group. 7 d after BICAO, the total thickness of retina was dramatically decreased (p<0.001). The individual changes of NFL, GCL, INL and PRE layers corresponded well with the morphology studies in H and E staining. However, the boundary of OPL and ONL became intangible and appeared to merge into a single layer, and the thickness of ONL also decreased (p<0.05, Figure 4B).
Figure 4. In vivo analysis of the thickness of individual layers of retina measured by OCT 7 d after ischemia injury. A. Representative pictures of a sham-operated control eye and of that of a model eye 7 d after BICAO injury, respectively. The layers of NFL, GCL; IPL, INL, OPL, and ONL were labeled by Adobe Photoshop. B: The thickness of individual layers was measured and quantified by means of pictures analyzing system. *p<0.05; ***p<0.001.
Statistics
The results are presented as the means ± SEM. Statistical comparisons were made using paired t-test using Graphpad prism version 5.0. P<0.05 was considered to indicate statistical significance.
Discussion
It is well known that Human and Murine surprisingly share many common genetic features more than 95%. There are several rat ischemic animal models published last decades [16]. Compared with rats, mice can be genetically manipulated for the gain-of-function and loss-of-function studies. In this case, mouse model can yield more reliable insights into etiology and pathogenesis and lead us to better understanding OIS. In this study, we have established a mouse model of retinal ischemia through BICAO, and the functional and morphologic changes were comparable with human OIS.
We have demonstrated that the mouse model of OIS was different with the recent published by Fumiya Ishizuka, etc. [17]. They claimed blood flow from ECA could compensate to irrigate the ocular tissue after the occlusion of PPA. In this study, 24 h after BICAO, MRA was utilized to observe the status of ocular hypoperfusion. The pictures captured by MRA showed the blood flow to ocular was entirely occluded. We supposed the ECA was not ready to supply the blood flow to the ICA in the early phase. We observed the ocular fundus at 7 d after BICAO, recording the ART, AVT and VFT after injection of sodium fluorescein. The prolongation of ART, AVT and VFT demonstrated that the chronic hypoperfusion of central retinal artery was maintained, at least for the first 7 d.
As central retinal artery occlusion gave rise to the neuronal cell death, histological analysis was performed in order to demonstrate morphological changes. BICAO for 7 d evidently induced morphological damage to the retina with decreases in NFL, GCL, INL and ONL thickness and retinal ganglion cell density, which was consistent with other findings [9,18,19]. In all cases, the acute phase of central retinal artery occlusion is typically characterized by swelling and followed by thinning of the involved layers in the chronic phase. After fixation and dehydration, morphological studies are not the optimal method to define the particular phases. OCT imaging, as a clinical diagnostic tool, offers a non-invasive imaging method for ocular cross-sectional examination, and is therefore suited for observing the pathological changes in living mice. The thickness of involved retinal layers obtained from OCT images conformed to the results of H and E sections. These results indicated that both inner retinal layers (including the GCL, IPL, and INL) and the outer retinal layers (including the OPL and ONL) developed morphological damage after 7 d of BICAO. However, the morphological damage was different with OIS induced in spontaneously hypertensive rats [16]. We assumed that the difference shown in rats after BCCAO might be related to species difference.
OIS is the syndrome that results from chronic ocular hypoperfusion. According to our results, it was suggested that simply ligation of ICA might be sufficient to induce the acute retinal ischemia, while after the acute phase, the central retinal artery constantly maintained the circulation disorder. However, we retained the blood perfusion of ECA, and the blood flow from ECA would contribute to the vascular network between the PPA and the ophthalmic artery. The BICAO might be possible to induce the chronic hypoperfusion in the later phase. Unfortunately, we did not provide long-term experimental data here. Further studies about the long-term effects are necessary for this BICAO mouse model, including the vascular permeability, residential inflammation and the death pathways of neurocytes, etc.
In conclusion, a murine model of retinal ischemia was successfully developed, which exhibited the prolonged disturbance in the blood supply, retinal dysfunction, and histological damage. This procedure offered a simple and useful animal model for investigating mechanisms of OIS in genetically altered mice and for developing novel therapeutic drugs for retinal neurodegenerative disease [20].
Acknowledgements
Foundation: This work was supported by grants from the National Natural Science Foundation of China (81173412).
Conflicts of Interest
The author(s) declare(s) that there is no conflict of interest regarding the publication of this paper.
References
- Brown GCLEM. The ocular ischemic syndrome. Clinical, fluorescein angiographic and carotid angiographic features. Int Ophthalmol 1988; 11: 239-251.
- Sivalingam A, Brown GC, Magargal LE. The ocular ischemic syndrome. III. Visual prognosis and the effect of treatment. Int Ophthalmol 1991; 15: 15-20.
- Terelak-Borys B, Skonieczna K, Grabska-Liberek I. Ocular ischemic syndrome - a systematic review. Med Sci Monit 2012; 18: RA138-144.
- Mendrinos E, Machinis TG, Pournaras CJ. Ocular ischemic syndrome. Surv Ophthalmol 2010; 55: 2-34.
- Mizener JB, Podhajsky P, Hayreh SS. Ocular ischemic syndrome. Ophthalmology 1997; 104: 859-864.
- Slakter JS, Spertus AD, Weissman SS, Henkind P. An experimental model of carotid artery occlusive disease. Am J Ophthalmol 1984; 97: 168-172.
- Barnett NLNNO. Prolonged bilateral carotid artery occlusion induces electrophysiological and immunohistochemical changes to the rat retina without causing histological damage. Exp Eye Res 1995; 61: 83-90.
- Lavinsky D, Arterni NS, Achaval M. Chronic bilateral common carotid artery occlusion: a model for ocular ischemic syndrome in the rat. Graefes Arch Clin Exp Ophthalmol 2006; 244: 199-204.
- Ogishima H, Nakamura S, Nakanishi T. Ligation of the pterygopalatine and external carotid arteries induces ischemic damage in the murine retina. Invest Ophthalmol Vis Sci 2011; 52: 9710-9720.
- Chang B. Mouse models for studies of retinal degeneration and diseases. Methods Mol Biol 2013; 935: 27-39.
- Lee JY, Chiu SJ, Srinivasan PP. Fully automatic software for retinal thickness in eyes with diabetic macular edema from images acquired by cirrus and spectralis systems. Invest Ophthalmol Vis Sci 2013; 54: 7595-7602.
- Yin L, Geng Y, Osakada F, Sharma R, Cetin AH. Imaging light responses of retinal ganglion cells in the living mouse eye. J Neurophysiol 2013; 109: 2415-2421.
- Zam A, Zhang PF, Levine E. Evaluation of OCT for quantitative in-vivo measurements of changes in neural tissue scattering in longitudinal studies of retinal degeneration in mice. Opt Coherence Tomogr Coherence Domain Opt Meth Biomed 2014; 8934.
- Chiu SJ, Li XT, Nicholas P. Automatic segmentation of seven retinal layers in SDOCT images congruent with expert manual segmentation. Opt Express 2010; 18: 19413-19428.
- Tamaki M, Kidoguchi K, Mizobe T. Carotid artery occlusion and collateral circulation in C57Black/6J mice detected by synchrotron radiation microangiography. Kobe J Med Sci 2006; 52: 111-118.
- Wang Y, Fan Y, Zhang L. Bilateral common carotid artery occlusion in spontaneously hypertensive rats: a feasible animal model for ocular ischemic syndrome. Anat Rec (Hoboken) 2016; 299: 806-814.
- Ishizuka F, Shimazawa M, Umigai N, Ogishima H, Nakamura S. Crocetin, a carotenoid derivative, inhibits retinal ischemic damage in mice. Eur J Pharmacol 2013; 703: 1-10.
- Daugeliene L, Niwa M, Hara A. Transient ischemic injury in the rat retina caused by thrombotic occlusion-thrombolytic reperfusion. Invest Ophthalmol Vis Sci 2000; 41: 2743-2747.
- Ogishima H, Nakamura S, Nakanishi T. Ligation of the pterygopalatine and external carotid arteries induces ischemic damage in the murine retina. Investig Ophthalmol Vis Sci 2011; 52: 9710-9720.
- Masuda T, Shimazawa M, Ishizuka F. Tissue kallikrein (kallidinogenase) protects against retinal ischemic damage in mice. Eur J Pharmacol 2014; 738: 74-82.