Research Article - Biomedical Research (2018) Artificial Intelligent Techniques for Bio Medical Signal Processing: Edition-II
Retracted: Simultaneous identification of the three active constituents in lungventilating-regulating oral liquid by RP-HPLC
Lina Lu1,2*, Yanhua Zuo3, Yaoxia Wang3, Yongqing Shi1 and Zuochong Liang1
1Chemical Engineering Institute, Northwest University for Nationalities, Lanzhou, PR China
2Key Laboratory for Utility of Environment-Friendly Composite Material and Biomass, Universities of Gansu Province, Lanzhou, PR China
3Department of Pharmacy, the Affiliated Hospital of Qingdao University, Qingdao, PR China
- *Corresponding Author:
- Lina Lu
Chemical Engineering Institute
Northwest University for Nationalities, PR China
Accepted date: May 26, 2017
Abstract
Problem statement: Lung-Ventilating-Regulating Oral Liquid is specified for cough, antipathy to cold by fever, nasal obstruction or discharge, headache, anhidrosis, and pain. It has effects of releasing exterior syndrome, dissolving cold, smoothing lung, and get rid of cough.
Objective: To develop a High Performance Liquid Chromatography (HPLC) method for ephedrine, hesperidin, and baicalin in Lung-Ventilating-Regulating Oral Liquid.
Methods: The three active constituents were identified in an Agilent TC-C18 (2) chromatographic column (250 mm × 4.6 mm, 5 μm), with 0.2% phosphoric acid solution-methyl cyanides as mobile phase, which was performed at a gradient elution column temperature of 25ºC, and a flow rate of 0.8 ml•min-1. Then the eluate was detected at detection wavelengths of 207 nm (for ephedrine) and 278 nm (for hesperidin and baicalin).
Results: Under the chromatographic conditions, ephedrine, hesperidin, and baicalin were well separated, which showed good linear relationships at 0.158-2.370, 0.164-4.100, and 0.160-4.000 μg, respectively. And coefficients of recovery of these three kinds of samples showed 100.2%, 98.7%, and 97.8%, respectively.
Conclusion: The developed method is convenient, accurate, and well repeatable, and consequently can be applied for the quality control of Lung-Ventilating-Regulating Oral Liquid.
Keywords
Baicalin, Ephedrine, Hesperidin, High performance liquid chromatography (HPLC), Chromatographic conditions, Phosphoric acid solution, Lung-ventilating-regulating oral liquid
Introduction
Lung-Ventilating-Regulating Oral Liquid is an oral fluid, which is extracted and purified mainly from eleven kinds of Chinese medicinal materials [1,2]. It has effects of relieving exterior syndrome, dissipating cold, facilitating lung, and relieving cough. It is indicated for cough, aversion to cold with fever, nasal obstruction or discharge, headache, anhidrosis, and ache [3]. The formula includes cultivated purple perilla leaf (144 g), hogfennel root (96 g), balloonflower root (96 g), bitter apricot seed (fried) (72 g), Chinese ephedra herb (96 g), pinelliae tuber (fried with honey) (72 g), hoelen (96 g), bitter orange (96 g), baikal skullcap root (96 g), seasoned orange peel (96 g), and licorice root (72 g). The quality standard includes identification of ephedrine by HPLC, identification of seasoned orange peel and ephedrine by TLC, and inspections of liquid description, relative density, and pH value [4]. In the formula, ephedrine, the main extract of Chinese ephedra herb, has effects of relaxing bronchial smooth muscle and contracting blood vessel, as well as marked central excitation effect [5]; hesperidin, the main extract of seasoned orange peel, has effects of improving deficiency of QI and blood and lymphatic system hypofunction, expelling dampness, and eliminating sputu [6]; and baicalin, the main extract of baikal skullcap root, has effects of inhibiting bacterial growth, promoting urination, eliminating inflammation, anti-anaphylaxis, and relieving muscular spasm [7]. These effects are accordant with the functions and indications of the preparation. Currently, there are only reports on identification of ephedrine [8-10], hesperidin and baicalin [11] in Lung-Ventilating-Regulating Pills or Oral Liquid yet. While in this study, the amount of ephedrine, hesperidin, and baicalin in Lung-Ventilating- Regulating Oral Liquid was simultaneously determined by HPLC gradient elution [12,13], which provided reference foundations for better quality control of the preparation.
Materials and Methods
Instruments
Agilent 1200 high performance liquid chromatograph (equipped with online degasser, quaternionic pump, automatic sampler, column incubator, and PDA detector; American Agilent company), Advanced-I-24L ultrapure water machine (Chengdu Aike Water Treating Equipment Co., Ltd), FW135 Chinese medicinal herb crusher (Tianjing Taisite Instrument Co., Ltd), and AB204-S electronic analytical balance (Switzerland Mettler Toledo company).
Drugs
Ephedrine (lot No. 110749-200410), hesperidin (lot No. 110736-200933), and baicalin (lot No. 110715-201016) reference substances were all bought from National Institute for the Control of Pharmaceutical and Biological Products of China. Lung-Ventilating-Regulating Oral Liquid (specification: 10 ml/vial; Tongrentang Drug Plant of Beijing Tongrentang Corporation; lot numbers: 4260064, 4260087, and 4260094, respectively). Methyl cyanides and methanol were both chromatographic pure (Chemical Industry Branch Office, Shangdong Yuwang Industry Co., Ltd). Water was bidistilled water. And phosphoric acid was analytical pure (Yantai Shuangshuang Chemical Industry Co., Ltd).
Results
Chromatographic conditions
The three active constituents were identified in an Agilent TCC18 (2) chromatographic column (250 mm × 4.6 mm, 5 μm), with 0.2% phosphoric acid water solution (A)-methyl cyanides (B) as mobile phase, which was performed at a gradient elution column temperature of 25ºC (0-5 min, 100% A; 5-10 min, 97% A; 10-11 min, 95% A; 11-20 min, 98% A; 20-25 min, 92% A; 25-30 min, 80% A; 30-45 min, 80% A; and 45-50 min, 75% A), and a flow rate of 0.8 ml•min-1. Then the eluate was detected at detection wavelengths of 207 nm (for ephedrine) and 278 nm for hesperidin and baicalin respectively.
Preparation of solution
Reference solution: An appropriate amount of ephedrine, hesperidin, and baicalin reference substances were precisely taken, respectively; and then diluted in analytical pure methanol to make reference stock solutions with concentrations of 0.7900, 0.8200, and 0.8000 g•L-1, respectively. And the stock solutions were stored at 4ºC. An appropriate amount of the above reference stock solutions were taken, respectively; then diluted in methanol to make a mixed reference working solution with concentrations of 0.1580, 0.1640, and 0.1600 g•L-1, respectively. And the working solution was stored at 4ºC for further use.
Test solution: 0.2% phosphoric acid water solution and methanol were made into a mixed solvent with a certain volume according to a proportion of 4:1. 4 ml of Lung- Ventilating-Regulating Oral Liquid with 3 lot numbers (4260064, 4260087, and 4260094) were precisely taken, respectively; and then infused into three 25 ml volumetric flasks, respectively. 20 ml of the mixed solvent was taken and added into the volumetric flasks. The volumetric flasks were treated with ultrasound in an ultrasonic cleaner for 30 min. The flasks were then taken out and cooled in open air to room temperature. The liquids were metered to volume with the mixed solvent. 2 h later, the flasks were treated with ultrasound in the ultrasonic cleaner for another 30 min. And the flasks were then taken out and cooled in open air. The solutions were filtrated. And the subsequent filtrates were taken and placed at 4ºC for further use.
Negative sample solution: In the study, the 3 constituents in Lung-Ventilating-Regulating Oral Liquid were identified, which were ephedrine, hesperidin, and baicalin, respectively, and were from Chinese crude drug Chinese ephedra herb, seasoned orange peel, and baikal skullcap root, respectively. Negative reference substances short of Chinese ephedra herb, seasoned orange peel, and baikal skullcap root were made according to the formula and preparation method of the product, respectively. Then negative sample solutions were made according to the preparation method of the test solution. The negative sample solutions were mixed and filtrated and the subsequent filtrate was taken and placed at 4ºC for further use.
System suitability test and specificity test
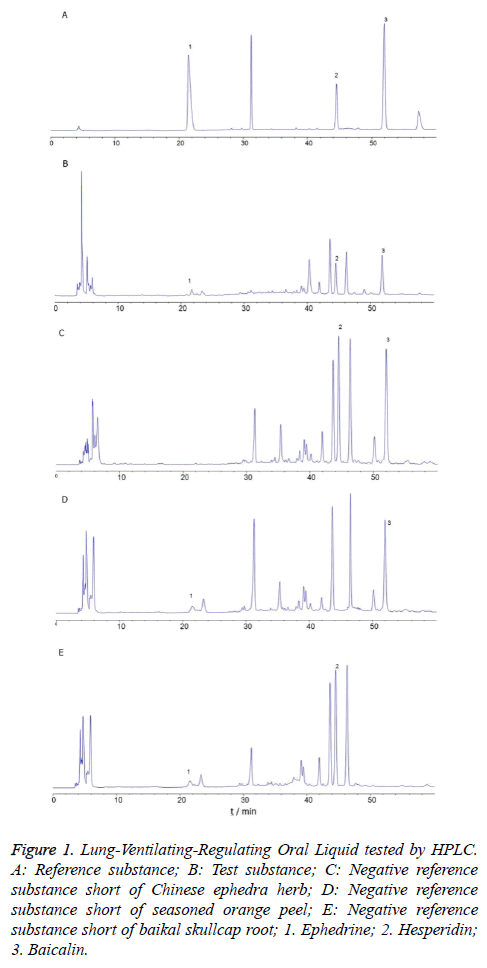
6 μL of the mixed reference solution, 12 μL of the test solution, and 12 μL of the negative sample solution were precisely taken, respectively. The three solutions were tested under the chromatographic conditions in Chromatographic conditions. Results: The separating degrees between the 3 targeted constituents and neighboring chromatographic peaks were all >1.5; symmetry factors were all 0.95-1.05; theoretical plate numbers were all >20000 calculated based on chromatographic peaks. The identification of the 3 constituents was not interfered by the other constituents in Lung- Ventilating-Regulating Oral Liquid, suggesting the method performed good specificity. The details were shown in Figure 1.
Figure 1: Lung-Ventilating-Regulating Oral Liquid tested by HPLC. A: Reference substance; B: Test substance; C: Negative reference substance short of Chinese ephedra herb; D: Negative reference substance short of seasoned orange peel; E: Negative reference substance short of baikal skullcap root; 1. Ephedrine; 2. Hesperidin; 3. Baicalin.
Linear relationship test
1, 3, 6, 9, 12, 15, 20, and 25 μL of the mixed reference solutions in 2.2.1 were precisely taken, respectively, which were then infused into a high performance liquid chromatograph, respectively. The solutions were tested under the chromatographic conditions in 2.1. With peak area as Yaxis and sample size as X-axis, the regression equations of ephedrine, hesperidin, and baicalin were Y=3001.6X - 80.029 (r=0.9998), Y=1369.3X - 21.673 (r=0.9999), and Y=3367.7X - 21.08 (r=1.0000), respectively. And the linear ranges were 0.158 - 2.370, 0.164 - 4.100, and 0.160 - 4.000 μg, respectively.
Precision test
6 μL of the mixed reference solution in 2.2.1 was taken precisely, and then infused into the high performance liquid chromatograph. The solution was tested complying with the chromatographic conditions in 2.1 for 6 continuous times. RSD of ephedrine, hesperidin, and baicalin calculated based on the peak area of each chromatographic peak were 1.48%, 1.25%, and 1.20%, respectively, suggesting the instruments had good precisions and could meet with the requirements of quantitative determination.
Repeatability test
6 samples of Lung-Ventilating-Regulating Oral Liquid with the same lot number (lot No.: 4260064) were precisely taken, and made into test solutions complying with the method in 2.2.2. The solutions were tested under the above-mentioned chromatographic conditions, with sample size of 12 μL. The peak area of each solution was recorded, and RSD calculated. Results: The amount of ephedrine, hesperidin, and baicalin was 0.1047, 1.3589, and 0.8066 g • L-1, respectively; and the RSD were 1.41%, 1.40%, and 0.84%, respectively. These suggested that the method had good repeatability.
Stability test
One sample of Lung-Ventilating-Regulating Oral Liquid with the same lot number (lot No.: 4260087) was precisely taken, and then made into a test solution complying with the method in 2.2.2. The solution was tested at 0, 2, 4, 6, 8, and 10 HPLC after preparation under the above-mentioned chromatographic conditions, respectively. The RSD of the areas of each chromatographic peak were 1.12%, 0.48%, and 0.23%, respectively, suggesting the test solution was stable within 10 HPLC after preparation.
Test of coefficients of recovery of the three kinds of samples
2 ml of Lung-Ventilating-Regulating Oral Liquid (lot No.: 4260064) with known amount (the amount of ephedrine, hesperidin, and baicalin was 0.1047 g•L-1, 1.3589 g•L-1, and 0.8066 g•L-1, respectively) was precisely taken and then placed in a 25 ml volumetric flask. 2ml of 0.158 g•L-1 ephedrine reference solution (by diluting 0.790 g•L-1 ephedrine reference stock solution in methanol), 3 ml of hesperidin reference stock solution, and 2 ml of baicalin reference stock solution were precisely added, respectively. Then the solution was metered to volume with the mixed solvent of 0.2% phosphoric acid water solution-methanol (4:1). And an applied test solution was made complying with the method in 2.2.2. 12 μL of the applied test solution was precisely taken, and then infused into the liquid chromatograph. The peak area was determined under the chromatographic conditions in 2.1, and then the coefficient of recovery was calculated. The results suggested the method had good recovery, and the details were shown in Table 1.
| Constituent | Amount in sample (mg) | Added amount (mg) | Determined Amount (mg) | Coefficient of recovery (%) | Average (%) | RSD (%) |
|---|---|---|---|---|---|---|
| Ephedrine | 0.2094 | 0.316 | 0.52 | 98.29 | 100.2 | 2.6 |
| 0.2094 | 0.316 | 0.5214 | 98.73 | |||
| 0.2094 | 0.316 | 0.5239 | 99.53 | |||
| 0.2094 | 0.316 | 0.532 | 102.09 | |||
| 0.2094 | 0.316 | 0.5189 | 97.94 | |||
| 0.2094 | 0.316 | 0.54 | 104.62 | |||
| Hesperidin | 2.7178 | 2.46 | 5.1709 | 99.72 | 98.8 | 1.9 |
| 2.7178 | 2.46 | 5.0602 | 95.22 | |||
| 2.7178 | 2.46 | 5.1468 | 98.74 | |||
| 2.7178 | 2.46 | 5.2019 | 100.98 | |||
| 2.7178 | 2.46 | 5.1547 | 99.06 | |||
| 2.7178 | 2.46 | 5.153 | 98.99 | |||
| Baicalin | 1.6132 | 1.6 | 3.1906 | 98.59 | 100.1 | 2.5 |
| 1.6132 | 1.6 | 3.1801 | 97.93 | |||
| 1.6132 | 1.6 | 3.2456 | 102.03 | |||
| 1.6132 | 1.6 | 3.2698 | 103.54 | |||
| 1.6132 | 1.6 | 3.2332 | 101.25 | |||
| 1.6132 | 1.6 | 3.1723 | 97.44 |
Table 1. Test of coefficients of recovery of the 3 constituents in lungventilating- regulating oral liquid.
Sample test: Appropriate amounts of 3 samples of Lung- Ventilating-Regulating Oral Liquid with 3 different lot numbers (3 samples each lot number) were precisely taken and made into test solutions complying with the method in 2.2.2. A total of 12 μL of the test solutions were applied, separately, and then tested under the chromatographic conditions in 2.1. The amount was calculated. The results are shown in Table 2.
| Lot No. | Ephedrine | Hesperidin | Baicalin | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Amount (g•L-1) | Mean Amount (g•L-1) | RSD (g•L-1) | Amount (g•L-1) | Mean Amount (g•L-1) | RSD (g•L-1) | Amount (g•L-1) | Mean Amount (g•L-1) | RSD (g•L-1) | |
| 4260064 | 0.1065 | 0.1046 | 1.66 | 1.3359 | 1.3605 | 1.72 | 0.7974 | 0.8041 | 0.72 |
| 0.1044 | 1.3823 | 0.808 | |||||||
| 0.1031 | 1.3634 | 0.8069 | |||||||
| 4260087 | 0.0976 | 0.0982 | 0.7 | 1.4316 | 1.4391 | 0.59 | 0.825 | 0.8265 | 0.21 |
| 0.0982 | 1.4373 | 0.8262 | |||||||
| 0.0989 | 1.4484 | 0.8283 | |||||||
| 4260094 | 0.1088 | 0.1098 | 0.89 | 1.4661 | 1.472 | 0.48 | 0.8759 | 0.8819 | 0.58 |
| 0.1108 | 1.47 | 0.8849 | |||||||
| 0.1098 | 1.4799 | 0.8848 | |||||||
Table 2. Identification of the 3 constituents in lung-ventilating-regulating oral liquid.
Discussion
Determination of detection wavelengths
The test substance and reference substance were detected in a PDA detector, and it was discovered that the maximum absorptions of ephedrine, hesperidin, and baicalin were at 207 nm, 280 nm, and 275 nm, respectively, and the later two also had strong absorptions at 278 nm, where only a few interfering peaks were present. Therefore, dual wavelength was selected to simultaneously determine the amount of ephedrine, hesperidin, and baicalin (207 nm for ephedrine and 278 nm for hesperidin and baicalin).
Determination of extraction solvent
With the amount of the three active constituents, namely ephedrine, hesperidin, and baicalin, as targets, 6 kinds of extraction solvents, i.e. purified water, 0.2% phosphoric acid water solution, methanol, 0.2% phosphoric acid water solutionmethanol (1:1), 0.2% phosphoric acid water solution-methanol (3:2), and 0.2% phosphoric acid water solution-methanol (4:1), were investigated. And it indicated that the mixed solution of 0.2% phosphoric acid water solution-methanol (4:1) had the best extracting effects. The drug solution was extracted with ultrasound in the ultrasonic cleaner for 30 min, 60 min, and 90 min, respectively, and it was discovered that there was insignificant difference between extraction for 30 min and that for longer times. It suggested that extraction for 30 min for the second time after the first extraction and placement at a room temperature for 2 HPLC had better effects than single extraction for 30 min. Thus, the former method was selected.
Determination of mobile phase
Purified water-methyl cyanides was used for gradient elution in the past, but ephedrine was not eluted by this way all the time, and the chromatographic peak failed to meet the separating requirements. And then 0.2% phosphoric acid water solutionmethyl cyanides was used for gradient elution, and it suggested that the mobile phase had a weaker eluting power when 0.2% phosphoric acid water solution accounted for a big proportion, while the mobile phase had greater eluting power and produced smaller peak retention time when the proportion of methyl cyanides was appropriately increased. The gradient of the mobile phase was adjusted further to obtain good peak separating degrees of the 3 active substances and no interference from the other constituents.
Acknowledgement
This work was supported by the Fundamental Research Funds for the Central Universities of Northwest University for Nationalities (No: 31920150016) and funded under the Youth Foundation of Ganshu Province, grant number: 1506RJYA275. The authors are grateful to all study participants.
References
- Fan C, Li N, Cao X. Determination of chlorophenols in honey samples using in-situ ionic liquid-dispersive liquid–liquid microextraction as a pretreatment method followed by high-performance liquid chromatography. Food Chemistry 2015; 174: 446-451.
- Zhang XZ, Zhang PJ. Elevating the quality of Lung-Ventilating-Regulating Oral Liquid by ultrafiltration. Chinese J Med Pharmaceutical Industry 2002; 33: 283-285.
- No authors listed. Pharmacopoeia of the People's Republic of China – 2010, Ed 1. China Medical Science Press, Beijing, 2010.
- Liu Z, Shi Q, Yang Y, Li YJ, Zhu YY, Bai G. Comparison of antiasthma effects and mechanisms between ephedrine and pseudoephedrine. Chinese Traditional Herbal Drugs 2009; 40: 771-774.
- Hu ZJ, Chen JQ. Identification of hesperidin in different seasoned orange peel drugs by HPLC. Chinese J Exp Traditional Med Formula 2012; 18: 95-98.
- Yu Y, Wang YJ, Pi JX, Wang DH, Xuan XY, Zheng Y. Bacteriostasis of coadministration of baicalin-borneol on Staphylococcus epidermidis. Chinese J Exp Traditional Med Formula 2013; 19: 191-194.
- Wang Z. Identification of ephedrine hydrochloride in Lung-Ventilating-Regulating Pills. Shandong J Traditional Chinese Med 2008; 27: 490-491.
- Xie YY, Zhang J. Identification of ephedrine hydrochloride and pseudoephedrine hydrochloride in Lung-Ventilating-Regulating Granules. J Pediatric Pharmacy 2013; 19: 44-46.
- Xiao ST, Fu XT, Chang ZR. Identification method for ephedrine in Lung-Ventilating-Regulating Granules. Chinese J Informat Traditional Chinese Med 2011; 18: 53-55.
- Tang DZ. Identification of naringin, hesperidin, and baicalin in Lung-Ventilating-Regulating Capsules. Chinese J Exp Traditional Med Formula 2011; 17: 93-95.
- Zhang QH. Practical Manual of High-performance Liquid Chromatography. Chemical Industry Press, Beijing, 2008.
- Liu J, Dai SY, Ye LM, Fu CM. Study on the Chemical Profiling of Fangfeng Tongsheng Pills by HPLC. Indian J Pharmaceutical Sci 2016; 78: 344-351.
- Jin SE, Seo CS, Shin HK, Ha H. Traditional herbal formulas to as treatments for musculoskeletal disorders: Their inhibitory effects on the activities of human microsomal cytochrome p450s and udp-glucuronosyltransferases. Pharmacognosy Magazine 2016; 12: 241.