- Biomedical Research (2015) Volume 26, Issue 2
Simultaneous determination of two biflavones in Biyanling Tablets by HPLC.
Yang Yang, Xinye Tang, Hongbing Yao*Department of Otolaryngology, Children's Hospital of Chongqing Medical University, Chongqing 400014, China
- *Corresponding Author:
- Hongbing Yao
Department of Otolaryngology
Children's Hospital of Chongqing
Medical University Chongqing 400014 China
Accepted January 26 2015
Abstract
Objective of the present study is to develop analytical methods for simultaneous determination of two major biflavones, amentoflavone and heveaflavone, in Biyanling Tablets, thereby providing methodological reference for quality control of anticancer drug Biyanling Tablets. Samples are chromatographed on Diamonsil C18 (4.6 mm × 250 mm, 5 μm) column with a mobile phase of acetonitrile (B)–0.5% acetic acid solution (A); other chromatographic conditions are as follows: elution gradient 0~4 min, B: 35%→45%; 4~20 min, B: 45%→50%, detection wavelength 270 nm, flow rate 1.0 mL/min, and column temperature 30?. Amentoflavone and heveaflavone show good linear relationships (r≥0.9998 and 0.9996) within a range of 1.56~100 μg/mL, average recoveries are 98.2% and 96.5%, with RSDs of 2.1% and 2.4 % (n = 6), respectively. The method is simple, fast and reproducible, which provides a quantitative analytical method for quality control of Biyanling Tablets.
Keywords
Biyanling; biflavone; content determination; HPLC
Introduction
Biyanling Tablets are a compound preparation made from Herba Selaginellae Doederleinii, Radix Sophorae Subprostratae, Poria cocos, Radix Trichosanthis, etc. [1], which has heat clearing, detoxifying, hard lump resolving, node dispelling, Qi supplementing and Yin nourishing actions. It is used for symptoms like chest and diaphragm wind-heat, phlegm-fire depression, heat-toxin up invasion, Qi consumption and body fluid impairment. Common symptoms include dry mouth, sore throat, dry and burning throat, hoarseness, headache, stuffy nose, purulent nasal discharge or blood in nasal discharge. It is also used in the treatment of acute and chronic pharyngitis and stomatitis, and as adjuvant treatment to radiotherapy and chemotherapy of nasopharyngitis and nasopharyngeal carcinoma [2-3].
Nasopharyngeal carcinoma is a malignancy occurring in the apex area and side wall of nasopharyngeal cavity. It is one of the world's most prevalent malignancies, whose incidence tops among otolaryngologic malignancies. Common clinical symptoms include nasal congestion, blood in nasal discharge, ear fullness, hearing loss, diplopia, headache, etc. Occurrence and development of nasopharyngeal carcinoma are closely linked to PI3K/AKT signaling pathway, of which PI3K, AKT and pAKT are associated with the metastasis and prognosis of nasopharyngeal carcinoma [4-8].
Biyanling Tablets are a potential cure for nasopharyngeal carcinoma, and biflavones are its active anticancer constituents [9-12]. So this paper determines the contents of amentoflavone and heveaflavone in Biyanling Tablets using HPLC, with the aim to effectively control their quality, and improve the original quality standard.
Materials
Agilent 1200 HPLC system (with quaternary low pressure mixing pump, autosampler, column oven and diode array detector). SBC1250 electronic balance (Nanjing Kademenqi Instrument Co., Ltd.); SSB-500A medical CNC ultrasonic cleaner (Zunyi Ultrasonic Instrument Co., power 200 W, frequency 40 kHz).
Amentoflavone and heveaflavone reference substances were purchased from Chengdu Must Biotechnology Co., Ltd. Purities of the above reference substances were validated to be over 99% by HPLC normalization. HPLC grade acetonitrile (Merck), acetic acid (Dalian Shenqi Chemical Reagent Co., Ltd.); purified water (laboratory-made); other reagents were all of analytical grade. All solvents were filtered through 0.45 μmol/L microporous membrane, and degassed ultrasonically. Biyanling Tablets were purchased from Beijing Fenghe Pharmacy.
Methods and Results
Preparation of solutions
Reference solution
10 mg of amentoflavone and heveaflavone reference substances were accurately weighed, respectively, placed in 5 mL volumetric flasks, ultrasonically dissolved by adding 80% ethanol, diluted to the mark, and shaken well to give the reference stock solutions.
Test solution
After film coating of Biyanling Tablets was removed, the remaining was pulverized, and passed through a 60 mesh sieve. 1 g of the resulting powder was accurately weighed, placed in a stoppered conical flask, added with 50 mL of petroleum ether (60~90°C), and ultrasonically extracted for 60 min (power 200 W, frequency 40 kHz). After discarding the supernatant, ultrasonic extraction was repeated once again. Drug residue was placed on filter paper, petroleum ether was evaporated to dryness at room temperature, and the remaining was transferred into a stoppered conical flask, added precisely with 50 mL of 70% ethanol, stoppered, weighed, ultrasonically extracted for 60 min (power 200 W, frequency 40 kHz), replenished with solvent, and filtered. 10 mL of the filtrate was accurately weighed, evaporated to dryness in a 58 ℃ water bath, redissolved in 80% ethanol, and diluted to the mark in a 10 mL volumetric flask for later use. Before injection analysis, the solution was filtered through a 0.45 μmol/L microporous membrane, and the subsequent filtrate was collected as the test solution
Chromatographic conditions
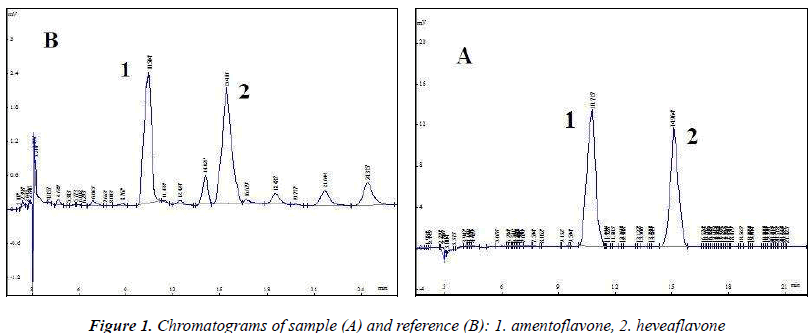
The above test solution was used to optimize chromatographic conditions. Diamonsil C18 (4.6 mm × 250 mm, 5 μm) column was used; acetonitrile (B)–0.5% acetic acid solution (A) were used as the mobile phase, optimization results revealed gradient elution conditions (0~4 min, B: 35% → 45%; 4~20 min , B: 45% → 50%); detection wavelength (270 nm), flow rate (1.0 mL/min) and column temperature (30 ℃), injection volume was 10 μL. Chromatographic analysis results are shown in Figure 1, amentoflavone and heveaflavone in Biyanling Tablets met the baseline separation conditions, in addition, chromatographic analysis time for single injection was less than 30 min.
Standard curve and limit of detection
Appropriate amount of two types of reference stock solutions under the item "Reference solution" was accurately weighed, placed on the same 2 mL volumetric flask, diluted to the mark with anhydrous ethanol, and shaken well to prepare the standard working solution containing the two constituents both with a concentration of 200 μg/mL. The above standard working solution was prepared by multiple dilution to standard serial working solutions (100, 50, 25, 12.5, 6.25, 3.12 and 1.56 μg/mL), and injected for analysis as per the chromatographic conditions under the item "Chromatographic conditions". Standard curve was plotted with concentration of reference substance (μg/mL) as the abscissa (X), and chromatographic peak area as the ordinate (Y), and the limit of detection (S/N≥3) and the limit of quantification (S/N≥10) were calculated, the results are shown in Table 1.
Precision test
An aliquot of reference solution (with concentrations of amentoflavone and heveaflavone both 12.5 μg/mL) was taken, and injected for six continuous times as per the chromatographic conditions under the item "Chromatographic conditions". Peak areas were recorded, and RSDs of amentoflavone and heveaflavone were calculated to be 1.5% and 2.0%, respectively.
Reproducibility test
Six aliquots of Biyanling Tablets with the same batch number were taken and prepared into six parallel test solutions following the method under the item "Test solution", and injected for analysis as per the chromatographic conditions under the item "Chromatographic conditions". RSDs of average amentoflavone and heveaflavone contents were measured to be 1.2% and 1.5%, respectively.
Stability test
An aliquot of test solution was taken, placed at room temperature and injected once every 0, 2, 4, 8, 10, 24, 36 and 48 h for analysis as per the chromatographic conditions under the item "Chromatographic conditions". Peak areas were recorded, and standard curves were employed to calculate the RSDs of average contents of amentoflavone and heveaflavone to be 0.2% and 0.5%, respectively. The results showed good stability of sample solutions within 48 h.
Recovery test
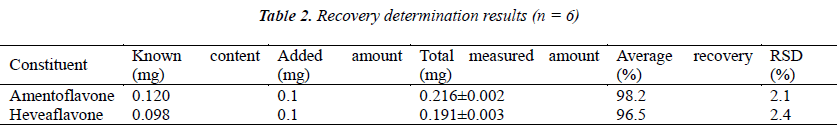
Six 1 g aliquots of samples with known content were accurately weighed, placed in stoppered conical flasks, added separately with 200 μL of amentoflavone and heveaflavone reference solutions (with concentration both 500 μg/mL), prepared into test solutions following the method under the item "Test solution", and injected for analysis as per the chromatographic conditions under the item "Chromatographic conditions", followed by calculation of recoveries, see Table 2.
Sample content determination
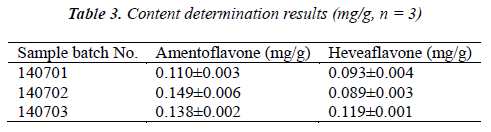
Test samples were prepared into test solutions as per the item "Test solution", and injected for analysis as per the chromatographic conditions under the item "Chromatographic conditions", followed by calculation of amentoflavone and heveaflavone contents in the samples by external standard method. The results are shown in Table 3.
Discussion
Selection of extraction method
Reflux extraction, Soxhlet extraction and ultrasonic extraction are investigated, respectively, in this experiment. Ultrasonic extraction is adopted owing to its highly efficiency, simplicity and fastness. During the experiment, different extraction solvents (50%, 70% and 100% ethanol) used for test solution preparation, different solvent volumes (30, 50, 75 and 100 mL) and different extraction time (15, 30, 60 and 90 min) are investigated, respectively. The results reveal that amentoflavone and heveaflavone are extracted relatively completely when the samples are extracted ultrasonically (50 mL × 60 min × 1 time) with 50 mL of 70% ethanol.
Selection of detection wavelength
Detector used in this study is a diode array detector, UV spectra recorded shows absorption peaks of amentoflavone at around 210, 288 and 335 nm, and absorption peaks of heveaflavone at around 210, 268 and 345 nm. Taking into account the durability of analytical method (such as using an ordinary UV detector), a single wavelength of 270 nm is selected as the detection wavelength [13-15].
Selection of mobile phase
Mobile phase systems with different proportions of methanol–water and acetonitrile–water are tested; gradient elution with acetonitrile–water system can achieve good separation effect. Besides, addition of 0.2% acetic acid in water can effectively improve the shapes of amentoflavone and heveaflavone chromatographic peaks, so acetonitrile–0.5% acetic acid system is selected.
Selection of Biyanling Tablets content determination indices
The author team's preliminary study isolated and purified two biflavones, amentoflavone and heveaflavone from Biyanling Tablets; and literatures have shown that these biflavones have good anti-cancer activities [4-7]. Therefore, the above two biflavones are likely to be the major active constituents contributing to the anticancer effect of Biyanling Tablets. In this paper, the two major active constituents in Biyanling Tablets are selected as the content determination indices, which can to some extent objectively reflect the quality of Biyanling Tablets preparation, and thus lay the foundation for research and development of Biyanling Tablets as well as anti-cancer medication.
References
- Pharmacopoeia Commission of the Ministry of Health of the PRC. Drug Standards of the Ministry of Health of the PRC. Traditional Chinese Medicinal Prepara- tions) 1993; XI: 205-206.
- Writing Group of Compilation of National Herbal Medicine. Compilation of National Herbal Medicine. People's Medical Publishing House 1975; I: 240, 339,340, 903.
- Yang JX. Anticancer TCM Herbal Preparations. Peo- ple's Medical Publishing House 1981; 107, 335, 336.
- Brigette BYM, Vivian WYL, Connie WCH, Cecilia PYL, Chi HW, Edwin PH, Margaret HLN, Suk HC, Sai WT, Chi-Man T, Crystal SFC, Kakiu H, Anthony TCC. Preclinical evaluation of the mTOR–PI3K inhibitor BEZ235 in nasopharyngeal cancer modelsOriginal Re-search Article. Cancer Letters 2014; 343: 24-32.
- Hong YZ, Kai FW, Carman KMI, Chris KCW, Nai KM, Kwok WL, Alice STW. Hepatocyte growth factor enhances proteolysis and invasiveness of human naso- pharyngeal cancer cells through activation of PI3K and JNK. FEBS Letters 2008; 582: 3415-3422.
- Anja P, Simone G, Anna KH, Guido P, Markus W, Ul- rich S, Martina R, Rudolf R. Survivin and pAkt as po- tential prognostic markers in squamous cell carcinoma of the head and neck Original Research Article Oral Surgery, Oral Medicine, Oral Pathology and Oral Radi- ology 2014: 117: 733-742.
- Joseph TB, Amen I, Christina T. Targeting the phos- phatidylinositol 3-kinase (PI3K)/AKT/mammalian tar- get of rapamycin (mTOR) pathway: An emerging treatment strategy for squamous cell lung carci- nomaReview Article. Cancer Treatment Reviews 2014; 40: 980-989.
- Ong CS, Zhou J, Ong CN, Shen HM. Luteolin induces G1 arrest in human nasopharyngeal carcinoma cells via the Akt–GSK-3β–Cyclin D1 pathwayOriginal Research Article Cancer Letters 2010; 298: 167-175.
- Lee NY, Min HY, Lee J, Nam JW, Lee YJ, Han AR, Wiryawan A, Suprapto W, Lee SK, Seo EK. Identifica- tion of a new cytotoxic biflavanone from Selaginella doederleinii. Chem Pharm Bull (Tokyo) 2008; 56: 1360-1361.
- Silva GL, Chai H, Gupta MP, Farnsworth NR, Cordell GA, Pezzuto JM, Beecher CW, Kinghorn AD. Cyto- toxic biflavonoids from Selaginella willdenowii. Phy- tochem 1995; 1: 129-134.
- Guruvayoorappan C, Kuttan G. Amentoflavone, a bi- flavonoid from Biophytum sensitivum augments lym-phocyte proliferation, natural killer cell and antibody dependent cellular cytotoxicity through enhanced pro- duction of IL-2 and IFN-gamma and restrains serum sialic acid and gamma glutamyl transpeptidase produc- tion in tumor-bearing animals. Journal of experimental therapeutics & oncology 2007; 6: 285-295.
- Lee JS, Lee MS, Oh WK, Sul JY. Fatty acid synthase inhibition by amentoflavone induces apoptosis and an- tiproliferation in human breast cancer cells. Biological & pharmaceutical bulletin 2009; 32: 1427-1432.
- Lu YH, Liu ZY, Wang ZT, Wei DZ. Quality evaluation of Platycladus orientalis (L.) Franco through simulta- neous determination of four bioactive flavonoids by high-performance liquid chromatographyOriginal Re- search Article Journal of Pharmaceutical and Biomedi- cal Analysis 2006; 41: 1186-1190.
- Bi WT, Tian ML, Row KH. Evaluation of alcohol- based deep eutectic solvent in extraction and determi- nation of flavonoids with response surface methodol- ogy optimizationOriginal Research Article. Journal of Chromatography A 2013; 1285: 22-30.
- Felicia BW, Lane CS, Stephen AW, James G. Develop- ment and evaluation of methods for determination of naphthodianthrones and flavonoids in St. John's wor- tOriginal Research Article. Journal of Chromatography A 2006; 1115: 93-102.