- Biomedical Research (2007) Volume 18, Issue 2
Serum status of selected biochemical parameters in malaria: An animal model
Adekunle A.S.1,*, Adekunle O.C.2, Egbewale B.E.31Department of Biochemistry, Ladoke Akintola University of Technology, Ogbomoso, Nigeria
2Department of Medical Microbiology and Parasitology, Ladoke Akintola University of Technology, Osogbo, Nigeria
3Department of Community Health Ladoke Akintola University of Technology, Osogbo, Nigeria.
- *Corresponding Author:
- Adekunle Adeniran
Box 14066, U.I. Post Office Ibadan
OYO State Nigeria
Tel: 00234-080-6096-7829
E-mail: kunleniran@yahoo.com
Accepted date: February 21, 2007
Abstract
Studies on the effect of malaria on serum lipid profile, total proteins alkaline phosphatase, certain micronutrients and some organs of the body were carried out using mice as experimental animal. Serum total cholesterol, HDL-cholesterol, LDL-cholesterol, triglyceride, total protein, alkaline phosphatase, manganese, zinc and copper were assessed in both control and test subjects. Significant decrease (P<0.05) was observed for serum total proteins in malaria infected mice when compared with control. Although, increased concentrations were observed for total cholesterol, HDL-C, triglyceride, LDL-C, alkaline phosphatase and zinc, however, the levels are not significant (P>0.05). Reduced concentrations were observed for manganese and copper, though not significant (P>0.05). Our observations support the hypothesis that malaria infections affect serum biochemical profiles.
Keywords
malaria, serum lipid profile, alkaline phosphatase, micronutrients, HDL-cholesterol, LDL-cholesterol
Introduction
Malaria is the most important parasitic disease that afflicts human today. The World Health Organization (WHO) estimates that 270 million new malaria infections occur worldwide along with 110 million cases of illness and 2 million deaths where 25% of childhood deaths in Africa are attributed to malaria [1]. It is caused by protozoan parasites of the genus plasmodium. The disease is characterized by fever, chills, myalgia, headache, nausea, vomiting and diarrhea.
Humans can be infected by the four species of malarial parasites identified, however, over hundred species of plasmodia have been identified as infectious with cases identified in birds, reptiles, monkeys, higher apes and rodents [2].
Anemia in cerebral malaria is one of the most common and severe outcomes, though in areas of perennial transmission of malaria, severe anemia may be more common than cerebral malaria [3] .Micronutrients deficiencies and malnutrition have been observed in malaria and these micronutrients are very essential for various metabolic and immune processes in the body. For instance, zinc supplementations have been observed to increase resistance against malaria while manganese play important role in the activities of metalloenzymes. The essence of this study is to assess the serum lipid profile, the total protein and alkaline phosphatase in malaria as well as serum concentrations of micronutrients and try to determine the relationships between these biochemical parameters using experimental rodents.
Material and Methodology
The study population consisted of 40 mice. The mice were randomly distributed into 2 groups designated as Group 1, control mice and Group 2, malaria infected mice.
The mice were purchased from the commercial breeders with body weight ranging from 30-35 gm.
Preparation and Innoculation of Plasmodium Yeolli
Malaria parasites, P. yeolii were obtained from the Institute of Medical Research and Training, University of Ibadan, Nigeria. The parasites were inoculated into 2 mice and the level of parasitemia was assessed after 4 days, by slide preparation using the method described elsewhere [4]. On the 4th day, parasitaemia level was found to be very high.
After the achievement of high level of parasitaemia in the mice, blood samples ware collected from them and diluted in normal saline at the ratio of 75% parasitized blood and 25% normal saline. The diluted parasitized blood was then inoculated into the mice of group 2 via intraperitoneal route.
Mice in group I were not inoculated with malaria parasites i.e. they served as control.
The animals were housed in cages, maintained on commercial feed and tap water for entire duration of the study. By the 4th day, thick blood film slide was prepared and the level of parasitaemia was assessed using the method described by Greenwood and Armstrong [4]. The level was found to be high. However, the mice were allowed to stay for another 2 days making a total duration of 6 days.
On the 6th day, the experimental mice (both control and infected) were made immobilized by cervical dislocation. Laparotomy was performed, blood samples were collected into appropriately labeled bottles for analysis of the following biochemical parameters: total protein, HDL-C, LDL-C, total cholesterol, triglyceride, alkaline phosphatase, manganese, zinc and copper.
Analysis of Biochemical Parameters
Total cholesterol, HDL-C, LDL-C, triglyceride and total proteins were analyzed using COJ-500-D Griffin Colorimeter (Griffin George Ltd, England).
HDL-C was determined by spectrophotometric method of Jacobs [5], at 500mm, 370C using 1cm light path cuvette. 500ml of serum and 1000ml of precipitant were added into a centrifuge tube. In another centrifuge tube, 500ml of standard and 1000ml of supernatant were added. Each of the tubes was allowed to sit for 10 minutes at room temperature. They were centrifuged for 10 min at 4000rpm. The supernatant was separated and estimated for HDL-C by the CHOD-PAP method. In this method, the measurement was done against reagent blank at 500mm and 370C using 1cm light path cuvette.
Total cholesterol concentration in the serum was determined using cholesterol oxidase method. The measurement was done at 546nm, 37°C using 1cm light path cuvette.
Triglyceride was equally determined using the colorimetric [COJ-500 D-Griffin colorimeter) (Griffing George Ltd, England)] method as described earlier [6] The measurement was done against the reagent blank at wavelength 546nm, using 1cm light path cuvette at 25°C.
The concentration of the LDL-C was determined using Friedwald equation i.e. LDL-C = Total cholesterol – HDL – C (0.20 X Triglyceride).
Total protein concentration was determined by using Biuret method [7]. This was determined at 25°C, 546nm using 1cm light path cuvette.
Alkaline phosphatase activity was determined using an optimized standard method. Into 1cm light path cuvette, 1.0 ml of reagent was pipetted. The initial reading was taken, 0.02ml of serum was then added into the cuvette and timer was started simultaneously. Readings were taken against air at 1, 2 and 3 minutes at Hg 405nm x 2760.
Direct measurements of the trace elements i.e. manga-nese, zinc and copper were carried out using atomic absorption spectroscopy (AAS) [Bulk Scientific, model 200A].
Results and Discussion
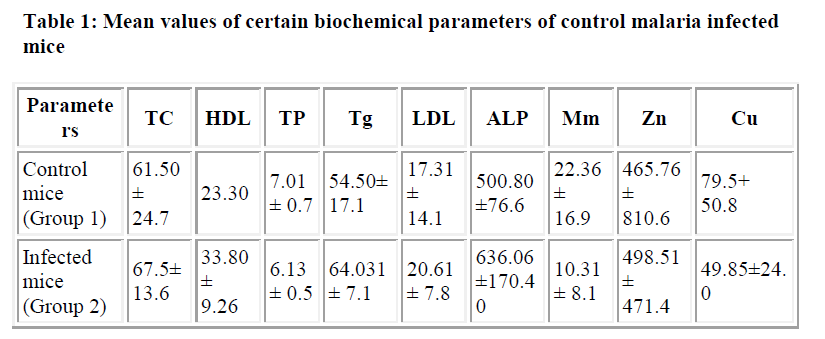
Means values of serum levels of the selected biochemical parameters in both the control and malaria-infected mice are mentioned in Table 1.
In our study, the serum status of total cholesterol, HDL-C, LDL-C and triglyceride in malaria infected mice was higher than those in uninfected mice. This finding is consistent with those in other studies that showed elevated levels of lipoproteins like HDL, LDL, total cholesterol and triglyceride in patients suffering from malaria infection [8,9]. Incidentally, cholesterol is synthesized in the liver which happens to be the major site of plasmodium infection and this raises some questions whether there is any relationship between the cholesterol synthesis by the liver and the plasmodium infection of the liver. Although, the parasite has ways that enables it to thrive and multiply using nutrients from the host, they still can not synthesize majority of their own lipids and cholesterol in vivo. In view of this, one would have to expect that the serum lipid levels to be low compared with the uninfected group. However, in our infected mice the serum lipid levels were rather found to be higher compared with the uninfected group.
However, the evidence of higher concentrations of serum lipids in the infected group despite the requirement of lipids for the growth of the parasite, could be explained from the recent findings which suggest that the plasmodium genome contains genes encoding enzymes of phospholipids metabolism, allowing de novo synthesis of phosphatidy choline via the kneddy pathway and necessitating only the uptake of the small choline molecule (10). In addition, the genome of the parasite contains genes similar to those for type II fatty acid synthesis pathway. The protein products of these genes are located within the apicoplast and allow for the production of fatty acids, some of which are unique to the parasite [11]. Thus the parasite may be able to meet many of its lipid requirements from its own biosynthetic pathways.
The nonsignificant difference in concentrations of the serum cholesterol between the infected and uninfected groups is consistent with a study carried out by [12], who observed no significant changes in plasma cholesterol during and after the infection of malaria.
Serum cholesterol plays an important role in atheromatous disease. In conditions with elevated concentrations of oxidized LDL particles, especially small LDL particles, cholesterol promotes atheroma plaque deposits in the walls of arteries, a condition known as atherosclerosis, which is a major contributor of the disease. In contrast, HDL particles have been the only identified mechanism by which cholesterol can be removed from atheroma. Therefore, an increased concentration of HDL-C as is observed in our study, correlates with lower rates of atheroma progression, even regression. In our study, we observed higher HDL-cholesterol than LDL-cholesterol. There is a universal trend that lower total cholesterol levels tend to correlate with lower rate of atherosclerotic event. However, the primary association of atherosclerosis with cholesterol has always been specific with cholesterol transport patterns, but not on total cholesterol level. For instance, if total cholesterol can be low, yet made up primarily of small LDL and small HDL particles and atheroma growth rates are high. In contrast, however, if LDL particle number is low and a large percentage of the HDL particles are large, then atheroma growth rates are usually low, even negative, for any given total cholesterol concentration.
In this study, elevated serum alkaline phosphatase in infected group was observed when compared with the serum level in uninfected group. The observed elevated serum level corroborates with the earlier findings [13,14], who observed higher serum level of alkaline phosphatase in malaria infection. Alkaline phosphatase is a membranebound enzyme which catalyses the hydrolysis of a number of phosphate esters and one of the major sites where it is bound to the membrane is the liver. However, in terms of pathogenesis, the host liver is among the organs affected in the early stage of falciparum malaria [15] leading to significant alterations in host hepatocyte physiology and morphology [16]. Therefore, the observed elevation in serum alkaline phosphatase is an indication that the hepatic stage of the parasite’s life cycle in its host and is accompanied by significant perturbation of the hepatocyte membrane leading to leakage of this enzyme out of the liver cells. This finding is in agreement with earlier findings [13], that centrilobular liver damage is one of the factors involved in hepatic dysfunction in acute malaria infection, leading to hyperbilirubinaemia which is a direct consequence of the impaired drainage capacity of the liver. Considering the percentage magnitude observed in the serum level of alkaline phosphatase in the infected group which is less than 50% of control activity, it is not as high as that associated with cholestasis where serum level is over twice the normal limit. Also, other disease such as liver cirrhosis is associated with a 92% increase above the normal limits of serum alkaline phosphatase (17; 18), which is higher than what we have observed in our study. In view of these differences in the percentage increase in serum alkaline phosphatase activity in malaria relative to these diseases, the etiology of the increased activity of alkaline phosphatase can be claimed to be due to the malaria infection.
Serum total proteins were observed to be reduced in malaria infected group when compared with the serum concentration in control groups. Since most individual proteins except albumin contribute little to the total protein concentration, quite a large percentage change in the concentration of one may not cause a detectable change in the total protein concentration. In view of this, low serum total protein concentration observed in this study may be be due to reduced concentration of albumin i.e. hypoalbuminemia. Serum proteins are synthesized in the liver, which, incidentally is one the major sites infected by the malaria parasite and any illness such as malaria which may cause infection of the liver may lead to fall in plasma albumin concentration due to decreased synthesis of albumin. Also illnesses such as malaria may result in increased catabolism of albumin and therefore leads to nitrogen loss.
The increased in the serum lipid concentrations observed in the malaria infected group in this study may be due to reduced serum concentration of albumin. Albumin (reduced plasma concentration of which lead to reduced total serum proteins) is required to bind to neutral fats (triglyceride, and cholesterol) for these lipids to be transported in the plasma (because the lipids are hydrophobic in nature and therefore requires some forms of hydrophilic adaptation in the form of lipoproteins). However, infection can lead to a fall in serum albumin due to decreased synthesis of albumin and/or increased catabolism of albumin, consequently causes reduction in the binding capacity, and leading to increased plasma free concentration of these analytes i.e. the lipids.
A similar explanation is offered for the increase in concentration of zinc in the group with malaria. Concentration of carrier molecule i.e. albumin is diminished during infection leading to increased plasma free concentration of zinc. Zinc is an integral component of nearly 300 enzymes in different species of all phylla [19]. Important zinc containing metalloenzymes include alkaline phosphatase. Zinc dependent enzyme such as alkaline phosphatase, is a useful indicator of zinc status. In this study, a correlation between serum zinc status and serum alkaline phosphatase activity is evident. Depression of alkaline phosphatase activity in either serum or neutrophils has been observed in animals and in a number of human zinc deficiency conditions. However, a study of zinc deficient with zinc supplementation showed a definite increase in alkaline phosphatase activity that paralleled with the degree of zinc repletion [20].
Several studies have shown reduced serum levels of micronutrients in malaria infection compared to noninfected con-trols [18,20]. The decrease in serum concentrations of copper and manganese observed in this study corroborate with the previous findings [22,23]. This may be due to significant decrease in the serum concentration of their carrier molecule, that is, albumin. Copper plays an important role in iron metabolism. Deficiency of copper impairs iron absorption which could be the consequence of malaria infection.
In this study, reduced concentration of serum copper is evident with an increased concentration of serum lipids. The relationship between copper deficiency and coronary heart disease has been reviewed [24]. Subclinical copper depletion contributes to an increased risk of coronary heart disease through instability of heart rhythm and hyperlipidemia. Experimental animals fed on copper deficient diets and human subjects fed diet with marginal copper deficiency have exhibited many signs closely related to coronary heart disease such as hypercholesterolemia, eletrocardiograms, glucose intolerance and hypertension [19].
In summary, our study have determined the effects of plasmodium yeolli on serum profiles of lipids, total proteins, alkaline phosphatase, and certain trace elements using animal model. On the basis of our findings, we do suggest that along with antimalarial drugs, other medications that may improve the serum status of the affected biochemical parameters should be incorporated in the treatment strategy during and after malaria infection. This is imperative in view of the immense importance of the serum components affected.
References
- Jean-Philippe S. Malaria: http//www.malaria test.com/malaria.html); 2005.
- Bruce-Chwatt L,.Peters W. chloroquine resistant plasmodium falciparum in Africa. Lancet 1979; II: 1374 1375.
- Beales PF. Anemia in malaria control: a practical approach. Ann. Trop Med. Parasite 1997; 91: 713-718.
- Greenwood BM, Armstrong JRM. Comparison of two simple methods for determining malaria parasite density. Transactions Royal Society Tropical Medicine and Hygiene, 1991; 85 pp 186-188.
- Jacobs D. in Laboratory and Test Handbook: Jacobs, D.S; Kasten, B.L., De Mott, W.R., Wolfson, W.L., Eds; Lexi-Comp Inc: Hudson (Cleverland), 1990; p. 219.
- Faucher JF, Milana EN, Missinou MA, Ngomo R, Kombila M, Kremsner PG. The impact of malaria on common lipid parameters. Parasite Res 2002; 88: 1040-1043.
- Nilson EI, Nilson EP. Changes in plasma lipoproteins in acute malaria J. intern Med 1990; 227:151-155.
- Vial HJ, Eldin P, Tieliens AG, Vanhellmond JJ. Phospholipids in parasite protozoa. Mol. Biochem. Parasitol. 2003; 126:143-154
- Onongbu IC, Onyeneke EC. Plasma lipid changes in human malaria. Trepenmed Parasitol 1983; 34: 193-196.
- Maegraith B. Aspects of the pathogenesis of malaria. Southwest Asian J Trop Med. Pub. Health 1981; 12: 251-267. Serum status of biochemical parameters in malaria
- Ibrahim HG, Ubom G. Serum alkaline phosphatase activity as a potential biomarker for the integrity of the hepatic drainage system in acute falciparum malaria infection. The Internet journal of infectious Diseases 2005; 4: 1-5.
- Miller LH, Baruch DI, Marcsh K, Doumbo OK. The pathogenic basis of malaria. Nature 2002; 415: 673-679.
- White NJ, Ho M. The pathophysiology of malaria. Adv. Parasitol 1992; 31: 84-167.
- Nogochi T, Yamashits Y. The rabbit differs from other mammals in the tissues distribution of alkaline phosphatase isoenzyines. Biochem. Biophys Res. Commum 1987; 143: 15-19.
- Kechrid Z, Kenouz R. Determination of alkaline phosphatase activity in patients with different zinc metabolic disorders. Turk J Med Sci 2003; 33: 387-391.
- Vallee RL, Auid DS. Zinc coordination, function, and structure of zinc enzymes and other proteins. Biochemistry 1990; 29: 564-567.
- Tietz NW. Fundamentals of Clinical Chemistry 1987; W.B. Saunders Company Philadelphia, U.S.A.
- Das BS. Thurnham DI, Das DB. Plasma α- Tocopherol, retinal, and carotenoids in children with falciparum malaria. Am J Clin Nutri 1996; 640: 94-100.
- Davis B. Trace elements. Tietz Fundamentals of Clinical Chemistry. 4th edn 1993; 485-489. W.B. Saunders, A Division of Harcourt Brace & Company. Philadelphia, Pennysylvia, USA.
- Njoku OU, Ononogbu IC, Nwachukwu DE. Plasma cholesterol, α- carotene and ascorbic acid changes in human malaria J Comm Dis 1995; 27:: 186.
- Adelekan DA, Adeodu OO, Thurnham SI. Comparative effects of malaria and malnutrition on plasma concentration of antioxidant micronutrients in children. Ann Trop Pard 1997; 17; 223-227.
- Hautvast JLA, Tolboom JJM, West CE, Kafwembe EM, Sauerwein RW, Van Staveren WA. Malaria is associated with reduced serum retinol levels in rural Zambian children. Int J Vit Nutr Res 1998; 68: 384-388.
- Davis TME, Garcia-Webb P, Lin-Chun F, Spencer JL, Xing-Bo G. Antioxidant vitamins in acute malaria. Trans R Soc Trop Med Hyg 1993; 87: 596-597.
- Klevay LM. Ischemic heart disease: Towards a unified theory. In. Role of Copper in lipid metabolism KY Lei, TP Carr, edn Boca Raton FL, CRC Press 1990; pp 233-267.