- Biomedical Research (2015) Volume 26, Issue 1
Role of Chloride Ion Channels in Human Lens Epithelial Cell Proliferation, Adhesion, and Migration.
Gui-Rong Li1, Qiong-Shu Li2, Xin-Yue Yang1, Miao-Miao Liu3, Yue Cheng3, Rui Fei3*, Ya-Ping Li1*1Department of Ophthalmology, Second Hospital, Jilin University, Changchun 130041, China
2Department of Immunology, School of Basic Medicine Science, Jilin University, Changchun 130021, China
3Department of Cell Biology, School of Basic Medicine Science, Jilin University, Changchun 130021, China
- *Corresponding Author:
- Rui Fei
Department of Cell Biology
School of Basic Medicine Science of Jilin University
Changchun 130021, China - Yaping Li
Department of Ophthalmology
Second Hospital, Jilin University
Changchun 130041, China
Accepted date: September 09 2014
Abstract
The aim of this study was to investigate the proliferation, adhesion, and migration function of chloride ion channels in HLEC B-3, a human lens epithelial cell line, and provide experimental basis for studying the mechanism of human after-cataract as well as the effective methods for controlling and treating it. MTT and Scratch assay methods were applied to detect the proliferation, adhesion, and migration of HLEC B-3 after applying 5, 10, 20, 50, 100, and 200 μmol/L of chloride ion channel blockers (NPPB). In the cell proliferation experiment, no apparent difference was found in the first 12 and 24 h after treatment with 10, 20, 50, 100 and 200 μmol/L NPPB or 48 h after treatment with 20, 50, 100 and 200 μmol/L NPPB. The cell proliferations were obviously inhibited compared with the control group(without NPPB treatment), and the difference was significant (P < 0.05 or P < 0.01). Cell adhesion experimental results showed that when the cells were treated with 10, 20, 50, 100, and 200 μmol/L NPPB for 12 or 24 h, cell adhesions were obviously inhibited. The difference was very significant (P < 0.05 or P < 0.01) compared with the control group. The activity of chloride ion channels can inhibit the proliferation, adhesion, and migration of LECs; therefore, inhibiting its activity can control the occurrence of human after-cataracts.
Keywords
Chloride ion channels, Lens epithelial cells, Cataract
Introduction
The chloride ion channels (CIC), located in the cell membrane or intracellular membrane, are made up of proteins that can transport chloride and other anions [1,2]. They are widely distributed in different tissues and cells and participate in maintaining the dynamic balance of cell volume, proliferation, secretion, and cell death, as well as in the regulation of all kinds of cell functions [3,4]. Studies have found that most CICs also exist in epithelial cells such as the corneal, ciliary, lens, and retinochrome epithelia of ocular tissues [5,6]. CICs figures prominently in the liquid transport of lens epithelial cells (LECs) and in maintaining lens transparency [7].
Cataract is one of the leading causes of human blindness. Current preferred methods for cataract treatment include extracapsular cataract extraction, phacoemulsification, and artificial lens implantation. However, issues such as surgical trauma and after-cataract, which are caused by rebounding proliferation, migration, and differentiation of residual LECs, often emerge because of such treatment methods, which seriously affect the visual recovery of patients [8]. Statistics on infant patients reports a 100% incidence rate of after-cataract induced by surgery [9-11].
LECs are single-layer cells located close to the anterior surface of the lens capsule. These cells are most actively involved in metabolism and are the only cells in the lens with the ability to divide. They are responsible for the growth, differentiation, and damage repair of the lens and primarily functions in maintaining lens transparency and internal environment stability [12,13]. Studies have shown that the formation of after-cataract originates from abnormal proliferation, differentiation, and migration of LECs [14]. Therefore, studying the function of CICs in the LECs is significant in revealing the mechanism of the after-cataract as well as the effective measures to control or treat it. In this paper, the effect of CIC is presented in the proliferation, adhesion, and migration process of LECs, which provides an experimental basis of studying the mechanism of after-cataract.
Materials and Methods
HLEC B-3 culture
HLEC B-3 cells were obtained from the Harbin Medical University (Harbin, China), cultured in a DMEM/F12 medium (Life Technologies Co., Taiwan, China), and supplemented with 10% inactivated FBS (Hangzhou Sijiqing Biological Engineering Materials Co. Ltd., Hangzhou, China) at 37 °C and in the presence of 5% CO2. Cell viability was determined using a 0.4% Trypan blue solution (Sigma-Aldrich Inc., Wisconsin, USA). Cell synchronization was achieved via the serum starvation method. After 2 to 3 days, the medium was refreshed once. Upon reaching full confluence, the cells were digested with 0.25% trypsin (Beijing Dingguo Biotechnology Co. Ltd., Beijing, China) and respectively cultured in different cell plates until the cell proliferation reached 70% or 80% completion for the experimental study.
Detection of HLEC B-3 proliferation
HLEC B-3 cells were cultured in individual wells on three different 96-well plates (8×103/well) at 37 °C in a 5% CO2 incubator. After 24 h, all wells were filled with 200 μL of the medium at final concentrations of 0, 5, 10, 20, 50, 100, and 200 μmol/L NPPB (Sigma-Aldrich Inc., Wisconsin, USA), respectively, with each concentration replicated in three wells. After the cells were cultured for 12, 24, and 48 h, each well was added with 20 μL of thiazolyl blue tetrazolium bromide (MTT, Sigma-Aldrich Inc., Wisconsin, USA) solution (5 g/L) and incubated at 37 °C with a CO2 incubator. Four hours later, the supernatants of each well were removed, 150 μL of dimethyl sulfoxide (DMSO, Beijing Dingguo Biotechnology Co. Ltd., Beijing, China) was added, and the plates were agitated by shaking for 10 min at a constant speed. Finally, the optical density (OD) of each well was determined at a wavelength of 570 nm (Enzyme Microplate Elx800), and the proliferation rates were calculated and compared.
Detection of HLEC B-3 adhesion
The cells were cultured and treated using a similar methodology as in section 2.2. After culturing the cells for 12, 24, and 48 h respectively, each well was gently washed twice with PBS to remove the supernatant cells. Finally, the optical density (OD) of each well was determined via MTT assay, and the adhesion rates were calculated and compared.
Detection of HLEC B-3 migration
HLEC B-3 cells were respectively cultured in individual wells on 24-well plate (1×105/well) at 37 °C in a CO2 incubator until the cells fully covered the wells. The central bottom of each well was then streaked using a special tip. After the wells were gently washed twice with PBS, the scratch width (Wi) of each well was captured by a 100X optical microscope. The wells were then filled with 500 μL of the medium at final concentrations of 0, 5, 10, 20, 50, 100, and 200 μmol/L NPPB. Cultivation was then resumed for 24 and 48 h. The scratch widths Mi for 24 h and Ni for 48 h were measured, and the relative migrations ratio of the cell was calculated using the equation:
Vi= (Wi-Mi (Ni))/Wi,
Where i refers to the different concentration groups, and i=0 for the blank group.
Statistical analysis
Data was analyzed using the SPSS11.5 software and presented
as mean and variance (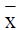 ± s) . The cross group
means and variances were compared using analysis of
variance (ANOVA). Probability values (P < 0.05 or P ≤
0.01) were selected to denote statistical significance.
± s) . The cross group
means and variances were compared using analysis of
variance (ANOVA). Probability values (P < 0.05 or P ≤
0.01) were selected to denote statistical significance.
Results
HLECB-3 proliferation
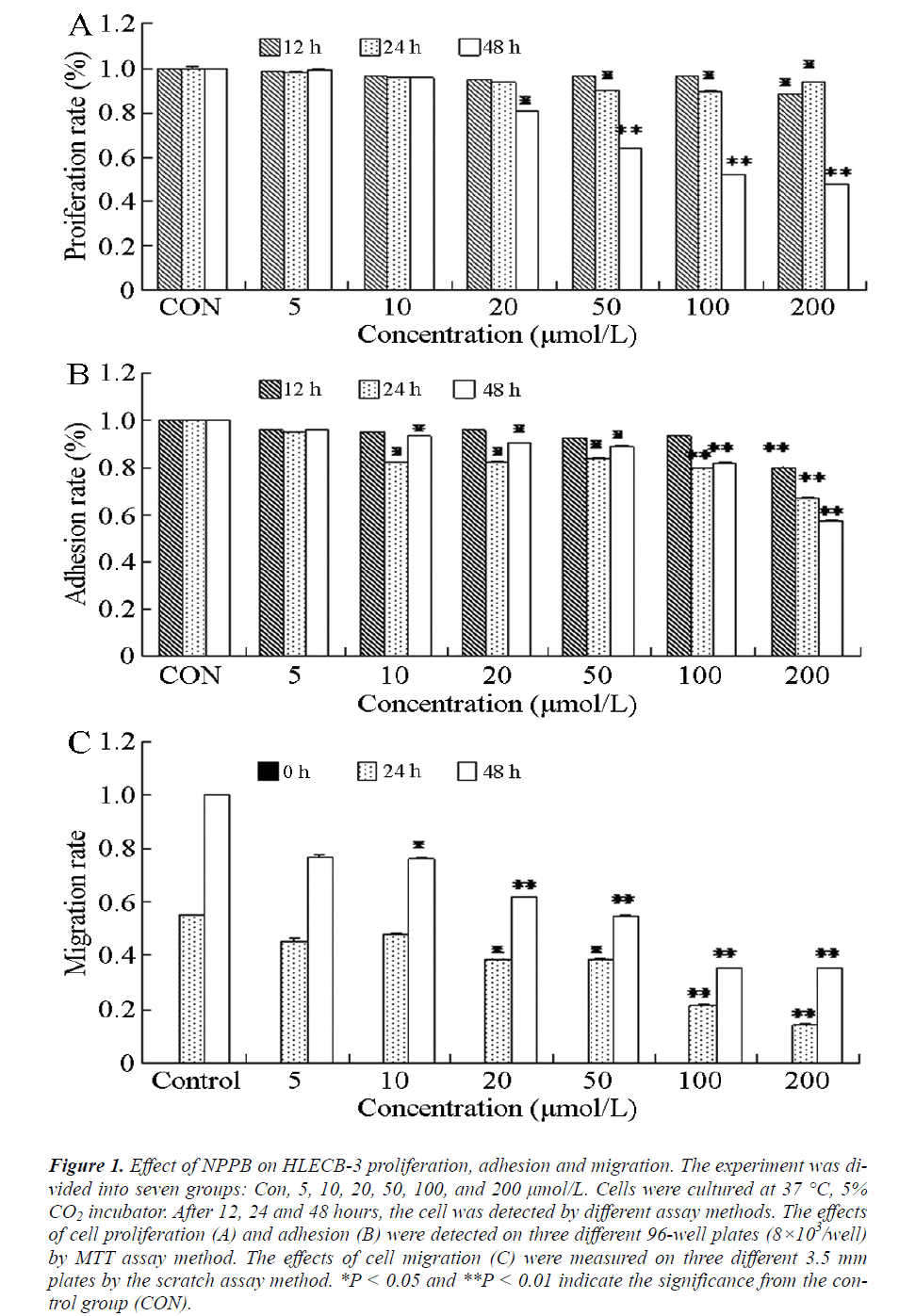
The activity of CIC can reportedly affect cell proliferation and consequently cause after-cataract [15,16]. To study the relation between CIC and human after-cataract, MTT assays were performed to detect the proliferation activity of HLEC B-3. The results showed (Figure 1A) that in cells treated with different concentrations of NPPB for 12 h, the proliferation was inhibited at 200 μmol/L NPPB (P < 0.05) compared with the control group. After treatment with 50, 100, and 200 μmol/L NPPB for 24 h or with 20 μmol/L NPPB for 48 h, cell proliferations were also inhibited (P < 0.05). The cells treated with 50, 100, and 200 μmol/L NPPB for 48 h demonstrated marked inhibition of proliferation (P < 0.01). These results indicated that NPPB could inhibit human LEC proliferation, which was most apparent at concentrations greater than 50 μmol/L NPPB at 48 h treatment.
Figure 1: Effect of NPPB on HLECB-3 proliferation, adhesion and migration. The experiment was divided into seven groups: Con, 5, 10, 20, 50, 100, and 200 μmol/L. Cells were cultured at 37°C, 5% CO2 incubator. After 12, 24 and 48 hours, the cell was detected by different assay methods. The effects of cell proliferation (A) and adhesion (B) were detected on three different 96-well plates (8×103/well) by MTT assay method. The effects of cell migration (C) were measured on three different 3.5 mm plates by the scratch assay method. *P < 0.05 and **P < 0.01 indicate the significance from the control group (CON).
HLECB-3 adhesion
To prove further the effect of CIC in after-cataract formation and to elucidate its mechanism, MTT assay was performed to detect the adhesion effect of NPPB. The experimental results are shown in Figure 1B. After 12 h of treatment with 200 μmol/L NPPB, cell adhesion was significantly inhibited (P < 0.01) compared with the control group. Cell adhesion was also inhibited (P < 0.05) by treatment with 10, 20, and 50 μmol/L NPPB for 24 h. The difference between 100 μmol/L and 200 μmol/L NPPB was even more significant after 24 h treatment (P < 0.01). Treating the cells with different concentrations of NPPB for 48 h yielded the same results as the 24 h experiments. These results indicated the time- and concentrationdependence of the human LECs adhesion inhibition by NPPB, with the highest inhibition at concentrations more than 100 μmol/L NPPB in the 24 h treatment.
HLECB-3 migration
Cell migration is an important indicator in estimating cell viability and functions prominently, in multiple physiological and pathological processes. Abnormal migration of LECs can lead to human after-cataract [17]. In the present study, the migration effect of HLECB-3 was detected via scratch assay after adding NPPB. In Figure 1C, cell migration was significantly inhibited (P < 0.05) after subjecting the cells to 24 h treatment with 20 and 50 μmol/L NPPB or 48 h with 10 μmol/L NPPB. The most significant cell inhibition effect (P < 0.01) was observed in cells treated with 100 μmol/L and 200 μmol/L NPPB for 24 h or with 20, 50, 100, and 200 μmol/L NPPB for 48 h. These results showed that different concentrations of NPPB could variably inhibit human LECs migrations at different durations of treatment. The inhibition was greatest at concentrations of more than 20 μmol/L NPPB for 48 h.
Discussion
After-cataract, which is also known as secondary cataract, is a common disease after extracapsular cataract extraction and perforated trauma and is the major cause of malignant postoperative vision. Currently, the pathogenesis of after-cataract is unclear because of the migration, proliferation, and collagen deposition of remaining LECs post-operation as well as the thickened basement membrane and tissue fibrosis [18]. Recent studies have revealed the complexity and diversity of CIC and have shown that the transformation of function of CIC directly influences the ionic balance of the cell exterior and interior and results in disease progression [19,20].
NPPB is a sensitive CIC blocker that can hinder Cl- by inducing the lipid membrane to change the channel protein conformation [21]. NPPB could inhibit the proliferation of ovarian cancer cell in a concentration-dependent manner, increase the amount G0-phase cells amount, and decrease the number of S-phase cells. Cell proliferation is inhibited when CIC is blocked [22]. In our experiment, NPPB was used to investigate the function of CIC on human LECs proliferation. The results showed that after the LECs were treated with different concentrations of NPPB for 24 or 48 h, cell proliferation was significantly inhibited. Decreasing the duration of treatment with NPPB could alleviate this inhibition. These results suggest that CIC could regulate the proliferation of LECs.
Cell adhesion and migration are characteristic of living cells throughout their life cycle that help to maintain normal cellular activities and are involved in many physiological and pathological processes [23]. CIC was found to participate in cell morphology and is closely related with cell adhesion and migration. Morphological changes in rat glial cells could induce Cl- current. RGD peptide could inhibit the adhesion and migration of human LECs, and Cl- channel blockers such as NPPB or Nolvadex could block the shrinkage and migration of glioma cells [24]. These studies proved that CIC was related with cell adhesion and migration. Our results suggested that when the HLEC B-3 cells were treated 24 or 48 h with concentrations greater than 10 μmol/L NPPB, cell adhesion was significantly inhibited. Similarly, treatments for 24 or 48 h at concentrations greater than 20 μmol/L, NPPB also displayed apparent inhibition. These results showed that the Cl- channels function primarily in the adhesion and migration of human LECs, and that CIC had a significant regulatory function in these processes. The inhibition of CIC may prevent the adhesion and migration of human LECs.
In summary, our results prove that ClC functions prominently in the occurrence and development of aftercataract. The inhibition of CIC may prevent posterior capsular opacity as well as improve and restore vision. This study not only offers experimental basis for further investigations on the mechanism of the human after-cataract but also demonstrates the importance of effective control, treatment, and prognosis of after-cataract development.
References
- Mindell JA, Maduke M. ClC chloride channels. Genome Biol 2001; 2: REVIEWS 3003.
- Bennetts B, Parker MW. Molecular determinants of common gating of a ClC chloride channel. Nat Commun 2013; 4: 2507.
- Verkman AS, Galietta LJ. Chloride channels as drug targets. Nat Rev Drug Discov 2009; 8: 153-171.
- Jentsch TJ, Stein V, Weinreich F, Zdebik AA. Molecular structure and physiological function of chloride channels. Physiol Rev 2002; 82: 503-508.
- Hartzell HC, Qu Z. Chloride currents in acutely isolated Xenopus retinal pigment epithelial cells. J Physiol 2003; 549: 453-469.
- A1-Nakkash L, Reinach PS. Activation of a CFTRmediated chloride current in a rabbit corneal epithelial cell line. Invest Ophthalmol Vis Sci 2001; 42: 2364-2370.
- Wang HS. The research progress about chloride ion channel: features of physiology & pharmacology including correlated diseases. Science & Technology Information 2012; 11: 489-491.
- Stein JD. Serious adverse events after cataract surgery. Curr Opin Ophthalmol 2012; 23: 219-225.
- Chang JR, Koo E, Agrón E, Hallak J, Clemons T, Azar D, Sperduto RD, Ferris FL 3rd, Chew EY; Age-Related Eye Disease Study Group. Risk factors associated with incident cataracts and cataract surgery in the age related eye disease study. Ophthalmology 2011; 118: 2113-2119.
- Churchill A, Graw J. Clinical and experimental advances in congenital and paediatric cataracts. Philos Trans R Soc Lond B Biol Sci 2011; 366: 1234-1249.
- Ramasubramanian A, Mantagos I, Vanderveen DK. Corneal endothelial cell characteristics after pediatric cataract surgery. J Pediatr Ophthalmol Strabismus 2013; 50: 251-254.
- Augusteyn RC. On the growth and internal structure of the human lens. Exp Eye Res 2010; 90: 643-654.
- McLean SM, Mathew MR, Kelly JB, Murray SB, Bennett HG, Webb LA, Esakowitz L, McLean JS. Detection of integrins in human cataract lens epithelial cells and two mammalian lens epithelial cell lines. Br J Ophthalmol 2005; 89: 1506-1509.
- Wang E, Reid B, Lois N, Forrester JV, McCaig CD, Zhao M. Electrical inhibition of lens epithelial cell proliferation: an additional factor in secondary cataract? FASEB J 2005; 19: 842-844.
- West-Mays JA, Pino G, Lovicu FJ. Development and use of the lens epithelial explant system to study lens differentiation and cataractogenesis. Prog Retin Eye Res 2010; 29: 135-143.
- Weidmann A, Kwittner S, Beck R, Teller J, Jonas L, Nebe JB. Prevention of Lens Epithelial Cell Growth In Vitro Using Mibefradil-Containing PLGA Micro Particles. Open Ophthalmol J 2008; 2: 112-118.
- Wei M, Fan YC. The mechanism of posterier capsular opacification in cytology and molecular biology. Practical J Clin Med 2005; 2: 77-80.
- Kalantan H. Posterior polar cataract: A review. Saudi J Ophthalmol 2012; 26: 41-49.
- Mazzone A, Eisenman ST, Strege PR, Yao Z, Ordog T, Gibbons SJ, Farrugia G. Inhibition of Cell Proliferation by a Selective Inhibitor of the Ca2+-activated Cl-Channel, Ano1. Biochem Biophys Res Commun 2012; 427: 248-253.
- Jentsch TJ. CLC chloride channels and transporters: from genes to protein structure, pathology and physiology. Crit Rev Biochem Mol Biol 2008; 43: 3-36.
- Dolovcak S, Waldrop SL, Fitz JG, Kilic G. 5-Nitro-2-(3-phenylpropylamino)benzoic acid (NPPB) stimulates cellular ATP release through exocytosis of ATPenriched vesicles. J Biol Chem 2009; 284: 33894-33903.
- Li M, Wang B, Lin W. Cl-channel blockers inhibit cell proliferation and arrest the cell cycle of human ovarian cancer cells. Eur J Gynaecol Oncol 2008; 29: 267-271.
- Dwane S, Durack E, Kiely PA. Optimising parameters for the differentiation of SH-SY5Y cells to study cell adhesion and cell migration. BMC Res Notes 2013; 6:366.
- Oharazawa H, Ibaraki N, Ohara K, Reddy VN. Inhibitory effects of Arg-Gly-Asp (RGD) peptide on cell attachment and migration in a human lens epithelial cell line. Ophthalmic Res 2005; 37: 191-196.