Research Article - Biomedical Research (2017) Volume 28, Issue 14
Relationship between miR-206 expression in the myocardium of hypothyroidism rat models and IGF-1 expression
Qian Xing1#, Silei Zhang1,2#, Haicheng Zhou1 and Jianling Du1*
1Department of Endocrinology, the First Affiliated Hospital of Dalian Medical University, Dalian, PR China
2Blood Purification Center, Dalian Sixth Peoples Hospital Affiliated of Dalian Medical University, Dalian, PR China
#These authors Contributed equally to this work
- *Corresponding Author:
- Jianling Du
Department of Endocrinology
The First Affiliated Hospital of Dalian Medical University, PR China
Accepted date: June 24, 2017
Abstract
Objective: To determine the mechanism by which muscle-specific microRNA (miR-206) and the miR-206 target gene insulin-like growth factor (IGF-1) cause myocardial damage in hypothyroidism rat models.
Methods: Wistar rats were grouped into normal (C) and Hypothyroidism (H) groups. Sixteen weeks after successful modeling, eight rats were picked randomly from the hypothyroidism group for levothyroxine (L-T4) treatment (HL). The patterns of myocardial tissue pathology were elucidated; the expressions of α-Myosin Heavy Chain (MHC), β-MHC, miR-206, and IGF-1 in the myocardial tissue were also measured.
Results: Rat myocardial α-MHC and IGF-1 protein expression in group H rats displayed a clear decrease (0.325 ± 0.027 and 0.101 ± 0.004, respectively, in H vs. 1.008 ± 0.137 and 0.149 ± 0.003 in C; P<0.01). In contrast, myocardial β-MHC expression showed a 2.539-fold increase in group H compared to group C (3.695 ± 1.516, P<0.05). Rat myocardial α-MHC expression was significantly increased in group HL compared to group H (1.078 ± 0.274 vs. 0.325 ± 0.027, P<0.01). However, β-MHC expression showed a clear decrease. The expression of miR-206 was also significantly decreased (1.488 ± 0.946, P<0.05) and that of IGF-1 protein increased compared to that seen in group H (0.160 ± 0.001 vs. 0.101 ± 0.004, P<0.01).
Conclusion: Myocardial miR-206 in hypothyroidism rat models inhibited IGF-1 transcription, which could be a partial cause of hypothyroidism-mediated cardiac disease. These changes in the hypothyroidism rat myocardium could be corrected using L-T4.
Keywords
Hypothyroidism, Myocardium, miR-206, IGF-1
Introduction
Hypothyroidism-induced cardiac functional changes are caused by myocardial systolic functional changes [1,2]. The cardiac Myosin Heavy Chain (MHC) α subtype expression is regulated by thyroid hormones; the combined influence of T3 and the nuclear receptor TH adjusts α-MHC and β-MHC expression at the transcriptional level. The pathological process of hypothyroidism-induced heart disease is mainly regulated by the decline in, and dysfunction of, myosin ATP enzyme activity. However, the effects of other elements on this process remain to be elucidated [3,4].
Studies have shown that miR-206 expression shows a 58.9-fold increase in the hypothyroidism rat model, compared to the normal rat model [5]. Although the abnormal expression of miR-206 was only observed in the muscle tissue, studies have indicated that it can also be expressed outside the muscle tissue, which results in the manifestation of relative pathological changes [5-9].
The potential regulated targeting of miR-206-insulin-like growth factor-1 (IGF-1) results in the expression of many pathological processes related to cardiac disease, such as alteration of the cardiac ion channel, stimulation of the growth of myocardial cells, increases in cardiac output, and promotion of cardiac blood discharge [10-12]. Simultaneously, IGF-1 also facilitates the relaxation of blood vessels, protects the ischemic myocardium, promotes cardiomyocyte hypertrophy, and enhances myocardial contractility [13-15]. IGF-1 at a low level could be an independent risk factor for cardiovascular diseases [10,16]. The role of IGF-1 in myocardial infarction and hypertension has been extensively researched in recent years. However, the effect of IGF-1 on hypothyroidism has not yet been reported.
In this study, the expressional changes occurring in the myocardial pathological conditions of hypothyroidism rat models were evaluated. In addition, the changes in miR-206 and the miR-206 target gene (IGF-1) expression in the myocardium of hypothyroidism rat models were analysed to determine their roles in myocardium damage. These results could serve as a basis for further discussions and investigations regarding the elements affecting hypothyroidism-based myocardial diseases.
Material and Methods
Experimental animals
Thirty 8 w old male Wistar rats weighing 200-220 g were obtained from the Dalian Medical University SPF Animal Center (experimental animal certificate number: SCXK (Liao) 2008-0002). These rats were maintained at the Dalian Medical University SPF Animal Center at a room temperature of 20 ± 2°C, relative humidity of 50%, and a light/dark cycle of 12/12 h. The rats were provided with free access to food and drinking water. The cages and bedding material were changed every day; the animal room and drinking bottles were also cleaned regularly. The experimental animals were maintained for 1 w, and then randomly divided into three groups, two primary groups and a secondary group. The primary groups were a normal control group (group C, n=8; fed with normal fodder and deionized water for 28 w) and a hypothyroidism group (group H, n=22; thyroid gland removed via surgery) whose Thyroid Stimulating Hormone (TSH) and Total Thyroxine 4 (TT4) content were measured after 4 w. Thyroid gland removal caused a significant increase in TSH expression and a corresponding decrease in TT4 content, which indicated success in modeling the hypothyroidism rat. These rats were fed with normal fodder and deionized water for an additional 24 w. From this group, a hypothyroidism Levothyroxine (LT4) treatment group (group HL (secondary), n=8) and a hypothyroidism group (group H, n=8) were randomly chosen 16 w after hypothyroidism was confirmed. The rats of group HL were injected with L-T4 (1.25 μg/100 g/d) every day for 8 w, while those of group H were maintained as above. All three groups were subjected to the same total observation period of 28 w; the myocardial tissue was taken from all rats during the 28th w.
The protocols in this study were approved by institutional review board and the Animal Care and Use Committee of the First Affiliated Hospital of Dalian Medical University (Dalian, China).
Serum thyroid function index measurement
Blood samples were collected from the venous plexus at the postorbital position of all rats at the fourth week (4 w after surgery for groups H and HL). Blood was also collected from the heart of each rat at the 28th w (28 w after surgery for groups H and HL). The serum was separated and the TSH and TT4 contents were measured at China Medical University using the immunofluorescence method. The sensitivity of TT4 was 1 μg/dL.
Histopathological analysis
The myocardial tissue was incubated (24°C) in 4% paraformaldehyde for 24-48 h, and transported to the pathology department of The First Affiliated Hospital of Dalian Medical University for pathology sampling.
Real-time PCR analysis of α-MHC, β-MHC, and IGF-1 mRNA expression in the myocardium
RNA was extracted using the TRIzol (KeyGEN BioTECH; NianJan, China) reagent kit, as per the manufacturer’s protocols. RNA concentration was calculated using the formula OD260 × dilution ratio × 0.04 μg/μL. The cDNA was prepared via reverse transcription, using the PrimeScript® RT Master Mix Perfect Real Time reagent kit (Takara Bio Inc.; Shiga, Japan). A response system was set, and the process performed in accordance with the reverse transcription response conditions: 37°C for 15 min and 85°C for 5 s. The SYBR Green PCR amplification kit was used to prepare the real-time PCR samples; the ABI 7500 PCR platform was used for sample amplification. The primer sequences used for PCR included β-actin (Forward 5’- GCCCTAGACTTCGAGCAAGA-3’; Reverse 5’- AGGAAGGAAGGCTGGAAGAG-3’), α-MHC (product 87 bp; Forward 5’-GGACAAGCTGCAGTTGAAGGTG-3’; Reverse 5’-CGGAACTTGGACAGGTTGGTG-3’), β-MHC (product 144 bp; Forward 5’- ACATTGCCGAGTCCCAGGTC-3’; Reverse 5’- GGTGCTGTTTCAAAGGCTCCA-3’), and IGF-1 (product 148 bp; Forward 5’-GCACTCTGCTTGCTCACCTTTA-3’; Reverse 5’-TCCGAATGCTGGAGCCATA-3’). The configuration of the real-time PCR response system was set according to that of the SYBR® Premix Ex Taq TM (Tli RNase H Plus; Takara Bio Inc.) reagent kit. The samples were analysed using the ABI PRISM® 7500 Real Time PCR System (Applied Biosystems; Waltham, MA, USA). The PCR conditions were set as follows: 40 cycles of pre-degeneration at 95°C for 30 s, degeneration at 95°C for 5 s, and annealing at 60°C for 34 s.
Real-time PCR analysis of miR-206 expression
The overall quantity of RNA extracted and the measured concentration and purity of the RNA were determined using the procedures outlined in the previous section. cDNA formation by reverse transcription was accomplished using reaction conditions of 37°C for 60 min and 85°C for 5 s, with the One Step PrimeScript® miRNA cDNA Synthesis reagent kit (Takara Bio Inc.) response system. The samples for realtime PCR were prepared using the SYBR green PCR amplification kit. The ABI 7500 PCR platform was used for amplification; the primers for amplification, miR-206 and U6, were obtained from Takara Bio Inc. (no sequences provided). The PCR system was configured according to the standards set by the SYBR Premix Ex Taq™ II (Perfect Real Time, Takara Bio Inc.) reagent kit response system. Consequently, the following reaction conditions were applied to obtain the required response: 40 cycles of pre-degeneration at 95°C for 30 s, degeneration at 95°C for 5 s, and annealing at 60°C for 34 s.
Western blot analysis of IGF-1 protein expression
Approximately 50 mg of rat myocardial tissue was mixed with 100 μL protein extraction reagent (KeyGEN BioTECH; NianJan, China) and protease inhibitor. The sample was ground on ice and incubated for 30 min. This was centrifuged at 9,500 Xg and 4°C for 20 min. The supernatant was extracted, mixed with a coloring agent (KeyGEN BioTECH; NianJan, China), and incubated at room temperature (24°C) for 10 min. The absorbance of this solution was measured at 595 nm. The quantity of sample protein (mg/ml) was calculated using the equation: quantity=OD sample/OD standard × standard concentration. Based on the values obtained, the sample protein concentration was adjusted using a lysis buffer. Fifty microliters of the protein (loading quantity) was added to a buffer solution (5X) and boiled for 5 min. The supernatant was separated by 13% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (Thermo Scientific ABgene; Waltham, MA, USA) using Bio-Rad electrophoresis plates (Bio-Rad; Hercules, CA, USA) at plate conditions of 150 V and 30 mA for 2 h. A nitrocellulose membrane was equilibrated in blotting buffer for 10 min and then glued onto the negative pole and the membrane of the positive pole. The membrane was subjected to 120 mA of wetting for 1.5 h, and subsequently washed once with Tryptone-Buffered Saline and Tween-20 (TBST). The sample was sealed with TBST containing 5% bovine serum albumin (BSA) and agitated vigorously in a shaker at room temperature for 1 h. The membrane was removed and washed thrice with TBST for 5 min. IGF-1 (Abcam; Cambridge, UK)/β-actin (Santa Cruz Biotechnology; Santa Cruz, CA, USA) primary antibodies (1:400) were added to the membrane, agitated at room temperature for 1 h, and incubated overnight at 4°C. The membrane was incubated with secondary alkaline phosphatase IGF-1/β-actin antibodies (ZSGB-BIO; Beijing; China) (1:1000) for 1 h at room temperature. The membrane was subsequently washed thrice with TBST for 5 min. According to the size of the membrane, equal sample volumes from solutions A and B of the Enhanced Chemiluminescence (ECL) reagent kit (Beyotime-BIO; Shanghai, China) were mixed evenly. The membrane was transferred to a dark room, placed on a plastic wrap membrane, mixed with the blended ECL solution, and allowed to rest for 3 min to allow for ample contact between the membrane and the solution. The membrane was subsequently removed and the redundant solution absorbed; the membrane was then wrapped in another plastic wrap membrane. All bubbles and folds were smoothed out with a glass rod; the membrane was then placed in the dark with an X-ray film fixed on top of it. The time of exposure was estimated according to the brightness of the protein bands. The film was placed into a processing machine to develop the images, then the film was scanned using the FluorChem V 2.0 Gel Imaging Analysis Software (ProteinSimple; San Jose, CA, USA); the gray value of each protein electrophoresis band was analysed and recorded for quantitative analysis.
Statistical analysis
The data were processed and analysed using the SPSS 20.0 statistical software (IBM; Armonk, NY, USA). All values are presented as the mean ± standard deviation. The mean in each group was then compared by single-factor analysis of variance; comparison between two groups was made with the LSD method, and all comparisons within groups were made with paired t-tests. Differences of P<0.05 were considered statistically significant.
Results
Rat weight and thyroid function tests
TT4 levels decreased significantly, TSH levels increased significantly, and weight decreased significantly in rats belonging to the hypothyroidism group (group H) compared to the normal control group (group C). The administration of LT4 supplements helped to restore/correct thyroid function; however, the resultant weight gain was not significant (Table 1).
| Groups | 4 w after successful modeling | 28 w after successful modeling | Weight at 28 w (g) | ||
|---|---|---|---|---|---|
| TSH (mIU/L) | TT4 (μg/dL) | TSH (mIU/L) | TT4 (μg/dL) | ||
| Group C (n=8) | 0.053 ± 0.014 | 4.495 ± 0.454 | 0.083 ± 0.04 | 3.945 ± 0.713 | 530.500 ± 39.300 |
| Group H (n=8) | 6.588 ± 1.621* | 1.745 ± 1.007* | 7.763 ± 4.32* | 1.417 ± 0.295* | 333.800 ± 61.000* |
| Group HL (n=8) | 4.955 ± 1.011* | 1.710 ± 0.750* | 0.420 ± 0.15Δ | 3.075 ± 1.263D | 378.000 ± 51.800* |
Table 1. Weight and thyroid function changes in the three groups.
Histological changes (light microscopy)
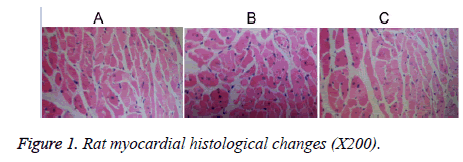
The cardiac muscle fibers of group C rats were observed to have lined up, with the nuclear chromatin displaying uniform distribution (oval nuclear chromatin at the central spot and myocardial cells located close to each other; Figure 1a). The cardiac cells of rats from group H displayed an irregular arrangement and distance between myocardial cells; several of the myocardial cells were widened with atrophy (Figure 1b). Myocardial cells from group HL rats also showed atrophy; however, the atrophy was less severe than that seen in group H rats (Figure 1c).
Myocardial α-MHC, β-MHC, IGF-1 mRNA and miR-206 expression
At the 28th week, α-MHC mRNA expression in group H rats decreased significantly, β-MHC expression increased significantly (P<0.01), and that of miR-206 had a 2.539-fold increase compared to the normal control group (P<0.05; Table 2). On the other hand, α-MHC expression showed a significant increase, and β-MHC and miR-206 expression displayed a significant decrease (P<0.01 and 0.05, respectively) in group HL, compared to that seen in group H. No significant differences were observed among the three rat groups in terms of the IGF-1 mRNA expression in the myocardium (P>0.05).
| Groups | α-MHC | β-MHC | MIR-206 | IGF-1 mRNA |
|---|---|---|---|---|
| C (n=8) | 1.008 ± 0.137 | 1.027 ± 0.265 | 1.044 ± 0.352 | 1.015 ± 0.185 |
| H (n=8) | 0.325 ± 0.027* | 2.661 ± 0.896# | 3.695 ± 1.516# | 1.298 ± 0.412 |
| HL (n=8) | 1.078 ± 0.274** | 0.721 ± 0.082** | 1.488 ± 0.946Δ | 1.029 ± 0.397 |
Table 2. α-MHC/β-MHC mRNA expression in the rat myocardium.
Myocardial IGF-1 protein expression
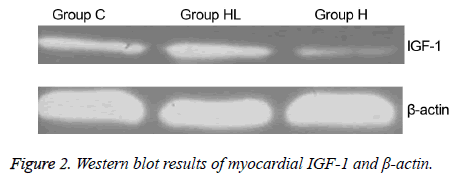
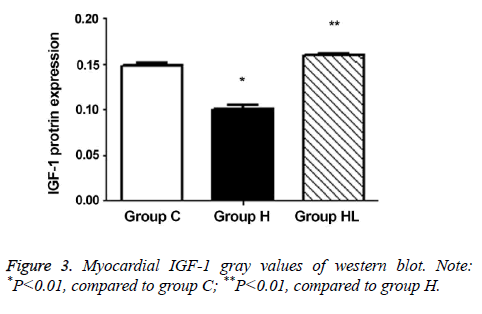
The expression of IGF-1 protein in the myocardium (Figures 2 and 3) was compared to that observed in group C (0.149 ± 0.003); IGF-1 expression decreased significantly in group H (0.101 ± 0.004; P<0.01). In addition, the IGF-1 expression in group HL showed a significant increase compared to that seen in group H (0.160 ± 0.001; P<0.01).
Discussion
Thyroid hormones affect the activity and functionality of the myocardial myosin ATP enzyme by manipulating the protein synthesis mechanism. In other words, the major pathological process behind damaged myocardial function (resulting from hypothyroidism) results from a decrease in myocardial myosin ATP enzyme activity, which in turn causes dysfunction [17]. With hypothyroidism, there is a decrease in thyroid hormone expression and ATP enzyme activity. The calcium intake and transfer ability of the myocardial tissues also becomes reduced; this leads to a reduction in the myocardial excitation and relaxation ability, as well as the rate, direct positive variable force, and positive variable rate of myocardial contractility [18-20]. Myosin, which acts as the initiator of the abovementioned processes, consists of two heavy chains and four light chains; the heavy chains function as the major contractile protein of myocardium and display ATP enzyme activity. There are two main types of MHC structures, the α- and β- chains. However, the myosin ATP enzyme consists of three different enzymes: V1 (αα), V2 (αβ), and V3 (ββ). The major difference between the myosin ATP enzyme and its isozyme is the structure of the heavy chain. The activity of the myosin ATP enzyme depends on the quantity of the V1 and V3 enzymes within the myocardial cell. Therefore, relative changes in the quantities of the V1 and V3 enzymes could reflect myosin ATP enzyme activity and (consequently) myocardial contractility [21,22]. Thyroid hormones promote α- MHC expression and restrain β-MHC expression at the level of gene transcription; in addition, the promoting activity is more rapid than the restricting activity [23]. The expressions of the myocardial MHC subtypes are dependent on the thyroid hormone receptor (TR), because α-MHC and β-MHC are initially coupled to TRα1 and TRβ1 [24,25]. Hyposecretion of thyroid hormones can reduce the previously mentioned effects by converting the α-MHC to β-MHC (by turning). This results in the conversion of the V1 unit of the myosin ATP enzyme and isozyme to V3. This leads to a reduction in the myosin ATP enzyme activity and cardiac contractility, which in turn leads to cardiac failure [26,27]. The results of this study indicate that the reduction in thyroid hormone secretion and changes in the myocardial pattern are associated with changes in α-MHC and β-MHC (closely related to the myosin ATP enzyme) expression.
Recent research using immunohistochemistry, reverse transcription (RT)-PCR, and in situ hybridization has demonstrated that human thyroid cells produce the IGF-1 protein. IGF-1 is known to reinforce iodide symporter activity in the cellular membrane, which assists in the intake, transfer, and organization of iodide, and further promotes the release and combination of TH in thyroid cells [28]. Miell et al. [29] studied the conditions of withdrawal and retreatment during thyroid hormone therapy, which was applied following total thyroidectomy due to a thyroid tumor. Analysis of the data obtained in their study demonstrated that the level of blood circulation positively affected thyroid hormone conditions. Harkawa et al. [30] measured the IGF-1 mRNA level in rats affected by hypothyroidism to determine the effect of thyroid hormone on IGF-1. The results revealed that T4 treatment led to a reduction in the IGF-1 mRNA level, as well as normalization of hypothyroidism in the rat liver after 2 w, which indicated that the thyroid hormones affected the expression of liver IGF-1 mRNA. This study obtained similar results; there was no significant difference in the IGF-1 mRNA expression level among the three groups. However, IGF-1 protein expression decreased significantly in group H, while myocardial IGF-1 mRNA and protein expression did not differ significantly between group C and group HL. Therefore, it can be assumed that the thyroid hormones affect IGF-1 expression by adjusting the level of post-transcription within the rat myocardium.
In addition to the above-mentioned effects, TH is known to affect other micro processes. TH mainly plays a role at the nuclear level: the TR nuclear receptor interacts with TH in the cell nucleus, which in turn interacts with the specific target gene of the TH Thyroxine Reaction Element (TRE) as a single form, homodimer, or heterodimer (TRE, located upstream of the start point of transcription, initiates specific TH transcription of the response gene). This facilitates the THmediated promotion of the transcription of target genes. In addition, the mRNA level of transcription is altered; that is, target gene expression is altered at the transcriptional level. The experiments conducted by Dong et al. [9] indicated significant differences between the expression levels of the two miR-206 target genes, demonstrating that TH controls the expression of some genes by adjusting the miRNA. Although miR-206 is only expressed in muscular tissues under physiological conditions, it has been known to be expressed in non-muscle tissues under some specific physiological conditions. In this study, there was an increase in myocardial miR-206 expression in group H rats. However, this was significantly reduced after L-T4 treatment, to almost the same level as that seen in group C. Therefore, miR-206 expression in the myocardium of hypothyroidism rat models was controlled by TH, and showed an increase when the myocardial functions were damaged. In addition, miRBase analysis (http//microrna.sanger.ac.uk) revealed that IGF-1 was a potential target of miR-206. miRNA is known to control gene expression via mRNA, post-transcription. In other words, miRNA is thought to affect expression at the protein level; the results of the present study reinforce this hypothesis. That is, there were no significant differences in IGF-1 mRNA expression in the myocardium of the hypothyroidism rat models compared to that of the normal control group. However, IGF-1 protein levels showed a significant decrease, with the miR-206 effectively restraining IGF-1 activity. Thus, TH appears to adjust IGF-1 expression via miR-206 activity.
Overall, based on the results obtained here and in the previous studies, we deduced that α-MHC, β-MHC, IGF-1, and miR-206 were abnormally expressed in the myocardium of the hypothyroidism rat models. This in turn indicated that TH hyposecretion resulted in an increase in miR-206 expression in the myocardium, which accompanied restrained IGF-1 expression and synthesis. In addition, there was a reduction in myocardial cell synthesis, with the cells exhibiting slow growth. We also observed a reduction in MHC synthesis, and Ca2+ and ATP enzyme activity promotion. This caused declined contractility in the myocardial cells, along with reduced cardiac output, myocardial ischemia, and hypoxia. This ultimately damaged the myocardial cells and led to their death. Reduced IGF-1 expression resulted in a reduction in preventive functions, which prevented the myocardial cells from dying. Under conditions of hypothyroidism, TH is involved in the process of damaging the myocardial functions by controlling the restraining effect of miR-206 on IGF-1. However, this process needs to be studied further in order to determine the specific mechanism of action.
Acknowledgement
The study was supported by The Natural Science Foundation of Liaoning Province (Grant No. 2013023023) and The Dalian Science and Technology Project (Grant No. 2010E15SF171).
Declaration of Conflict of Interest
None
References
- Vargas-Uricoechea H, Bonelo-Perdomo A, Sierra-Torres CH. Effects of thyroid hormones on the heart. Clin Investig Arterioscler 2014; 26: 296-309.
- Vargas-Uricoechea H, Sierra-Torres CH. Thyroid hormones and the heart. Horm Mol Biol Clin Investig 2014; 18: 15-26.
- Colegrave M, Peckham M. Structural implications of beta-cardiac myosin heavy chain mutations in human disease. Anat Rec (Hoboken) 2014; 297: 1670-1680.
- Michael JJ, Gollapudi SK, Chandra M. Effects of pseudo-phosphorylated rat cardiac troponin T are differently modulated by alpha- and beta-myosin heavy chain isoforms. Basic Res Cardiol 2014; 109: 442.
- Hutvagner G, Zamore PD. A microRNA in a multiple-turnover RNAi enzyme complex. Science 2002; 297: 2056-2060.
- Harel L, Gefen N, Carmi O, Orbach P, Einat P, Abitbol G. Novel expression vectors enabling induction of gene expression by small-interfering RNAs and microRNAs. PLoS One 2014; 9: 115327.
- Boon RA, Dimmeler S. MicroRNAs in myocardial infarction. Nat Rev Cardiol 2015; 12: 135-142.
- Lee Y, Ahn C, Han J, Choi H, Kim J. The nuclear RNase III Drosha initiates microRNA processing. Nature 2003; 425: 415-419.
- Dong H, Paquette M, Williams A, Zoeller RT, Wade M. Thyroid hormone may regulate mRNA abundance in liver by acting on microRNAs. PLoS One 2010; 5: 12136.
- Leme JA, Silveira RF, Gomes RJ, Moura RF, Sibuya CA. Long-term physical training increases liver IGF-I in diabetic rats. Growth Horm IGF Res 2009; 19: 262-266.
- Katz LE, Gralewski KA, Abrams P, Brar PC, Gallagher PR, Lipman TH, Brooks LJ, Koren D. Insulin-like growth factor-I and insulin-like growth factor binding protein-1 are related to cardiovascular disease biomarkers in obese adolescents. Pediatr Diabetes 2016; 17: 77-86.
- Halldin M, Brismar K, Fahlstadius P, Vikstrom M, de Faire U, Hellenius ML. The metabolic syndrome and ECG detected left ventricular hypertrophy-influences from IGF-1 and IGF-binding protein-1. PLoS One 2014; 9: 108872.
- Peng J, Fu J, Deng SZ, Wang RG, Liu L, Sun DM, Xia K. Changes in serum insulin-like growth factor-1 and insulin-like growth factor-binding protein-3, and their significance in children with left-to-right shunt congenital heart disease associated with heart failure. Zhongguo Dang Dai Er Ke Za Zhi 2013; 15: 277-280.
- Lawlor DA, Ebrahim S, Smith GD, Cherry L, Watt P, Sattar N. The association of insulin-like-growth factor 1 (IGF-1) with incident coronary heart disease in women: findings from the prospective British Womens Heart and Health Study. Atherosclerosis 2008; 201: 198-204.
- McMullen JR. Role of insulin-like growth factor 1 and phosphoinositide 3-kinase in a setting of heart disease. Clin Exp Pharmacol Physiol 2008; 35: 349-354.
- Gonzalez AB, Young L, Doll JA, Morgan GM, Crawford SE, Plunkett BA. Elevated neonatal insulin-like growth factor I is associated with fetal hypertrophic cardiomyopathy in diabetic women. Am J Obstet Gynecol 2014; 211: 290-297.
- Van Treeck BJ, Masoud AG. Hypothyroid cardiomyopathy complicated by a left ventricular laminar thrombus. Mo Med 2014; 111: 444-446.
- Ramos CF, Zamoner A. Thyroid hormone and leptin in the testis. Front Endocrinol (Lausanne) 2014; 5: 198.
- Jabbar A, Razvi S. Thyroid disease and vascular risk. Clin Med (Lond) 2014; 14: 29-32.
- Fraczek MM, Lacka K. Thyroid hormone and the cardiovascular system. Pol Merkur Lekarski 2014; 37: 170-174.
- Lv H, Lipes MA. Role of impaired central tolerance to alpha-myosin in inflammatory heart disease. Trends Cardiovasc Med 2012; 22: 113-117.
- Danzi S, Klein I. Cardiac specific effects of thyroid hormone analogues. Horm Metab Res 2011; 43: 737-742.
- Haddad F, Jiang W, Bodell PW, Qin AX, Baldwin KM. Cardiac myosin heavy chain gene regulation by thyroid hormone involves altered histone modifications. Am J Physiol Heart Circ Physiol 2010; 299: 1968-1980.
- Zhong WW, Withers KW, Hoh JF. Effects of hypothyroidism on myosin heavy chain composition and fibre types of fast skeletal muscles in a small marsupial, Antechinus flavipes. J Comp Physiol B 2010; 180: 531-544.
- Patel M, Mishra V, Pawar V, Ranvir R, Sundar R, Dabhi R. Evaluation of acute physiological and molecular alterations in surgically developed hypothyroid Wistar rats. J Pharmacol Pharmacother 2013; 4: 110-115.
- Walsh R, Rutland C, Thomas R, Loughna S. Cardiomyopathy: a systematic review of disease-causing mutations in myosin heavy chain 7 and their phenotypic manifestations. Cardiology 2010; 115: 49-60.
- Pandorf CE, Jiang W, Qin AX, Bodell PW, Baldwin KM, Haddad F. Regulation of an antisense RNA with the transition of neonatal to IIb myosin heavy chain during postnatal development and hypothyroidism in rat skeletal muscle. Am J Physiol Regul Integr Comp Physiol 2012; 302: 854-867.
- Ock S, Ahn J, Lee SH, Kang H, Offermanns S, Ahn HY, Jo YS, Shong M, Cho BY, Jo D, Abel ED, Lee TJ, Park WJ, Lee IK, Kim J. IGF-1 receptor deficiency in thyrocytes impairs thyroid hormone secretion and completely inhibits TSH-stimulated goiter. FASEB J 2013; 27: 4899-4908.
- Miell JP, Zini M, Quin JD, Jones J, Portioli I, Valcavi R. Reversible effects of cessation and recommencement of thyroxine treatment on insulin-like growth factors (IGFs) and IGF-binding proteins in patients with total thyroidectomy. J Clin Endocrinol Metab 1994; 79: 1507-1512.
- Harakawa S, Yamashita S, Tobinaga T, Matsuo K, Hirayu H, Izumi M, Nagataki S, Melmed S. In vivo regulation of hepatic insulin-like growth factor-1 messenger ribonucleic acids with thyroid hormone. Endocrinol Jpn 1990; 37: 205-211.