Research Article - Current Pediatric Research (2017) Volume 21, Issue 1
Preventive effect of pleuran (β-glucan from Pleurotus ostreatus) in children with recurrent respiratory tract infections-open-label prospective study.
Jaroslaw Pasnik1, Agnieszka Ślemp2, Agnieszka Cywinska-Bernas1, Krzysztof Zeman1 and Milos Jesenak31Department of Pediatrics, Prevention Cardiology and Clinical Immunology, Medical University, Lodz, Poland.
2Department of Pediatrics and Clinical Immunology, Institute of Polish Mother’s Health Hospital, Lodz, Poland.
3Department of Pediatrics, Centre for Diagnosis and Treatment of Primary Immunodeficiencies, Jessenius Faculty of Medicine, Comenius University in Bratislava, Martin, Slovakia.
- *Corresponding Author:
- Milos Jesenak
Department of Pediatrics
Jessenius Faculty of Medicine
Comenius University in Bratislava
Kollarova 2, 036 59 Martin, Slovakia.
Tel: +421-43-4203-959
Fax: +421-43-4222-678
E-mail: jesenak@gmail.com
Accepted date: January 06, 2017
Abstract
Background: Recurrent respiratory tract infections (RRTIs) are one of the most common problems in pediatric practice. Several natural preparations are used in management of RRTIs, but the scientific for their efficacy is sometimes insufficient. On the other hand, preventive effect of some natural preparation (e.g. β-glucans) was confirmed by relevant clinical studies. Methods: In our prospective open label study we enrolled 194 children suffering from RRTIs. We aimed to analyse the effect of Imunoglukan P4H® syrup containing pleuran (insoluble β-glucan isolated from Pleurotus ostreatus) on general respiratory morbidity comparing previous year with the same season during the study. Results: Supplementation of Imunoglukan P4H® syrup significantly decreased the total number of respiratory tract infections during the treatment and subsequent follow-up period compared to the same period of the previous year (4.18 ± 2.132 vs. 8.71 ± 1.89; p<0.001). In detail, the number of various types of respiratory tract infections (otitis, laryngitis, bronchitis and flu) was also significantly reduced (p<0.01 for all subtypes of infections). Moreover, a reduction in the number of day-off in kindergarten and school was also noticed. The syrup was well tolerated and no serious adverse effects were observed. Conclusion: Our study supports the use of pleuran in the complementary treatment and preventions of RRTIs in children. β-glucans seem to be an effective and safe tool in the management of RRTIs.
Keywords
β-glucan, Natural immunomodulator, Pleuran, Recurrent respiratory tract infections.
Introduction
Recurrent Respiratory Tract Infections (RRTIs) are one of the most commonly occurring diseases and are typically associated with early childhood [1]. According to epidemiological studies, approximately 6% of children between 2−6 years suffer from RRTIs [2,3]. Due to their high incidence, RRTIs have an important socio-economic impact (affecting quality of life, causing absences from school and loss of parental working days, requiring repeated medical examinations, hospital admissions and also antibiotic prescriptions) [4,5]. Most of the children with RRTIs do not have any serious underlying disease and the high frequency of respiratory tract infections is the consequence of the combination of various minor factors (immune system immaturity, high exposure to infectious agents in family or children collective, environmental factors such as pollutants, chronic focal infections such as adenoid hypertrophy, etc.) [6]. Moreover, allergic inflammation in the airways can also contribute to the higher susceptibility to respiratory infections [7].
The management of RRTIs consists not only from the treatment of acute attacks but should be aimed especially on their prevention. Since most of the children with RRTIs do not have any serious underlying immunological or non-immunological pathology, the therapeutic and preventive strategy should be aimed on the support of the resistance of mucosal surfaces, e.g. by immunomodulation. Several preparations of natural origin have been used for prevention of RRTIs, but only few of them have scientific evidence for real clinical efficacy. Biologically active polysaccharides (BAPs) – e.g. β-glucans – are one of the most studied substances of natural origin with proven pleiotropic biological activities. Nowadays they represent and effective tool of complementary therapy in various diseases and pathological conditions. They are a mixture of non-cellulose polymers of glucose units connected by the linear β (1→3) and lateral β (1→6) glycosidic linkages. There are several known natural sources of BAPs, but it was shown by many studies, that the most effective BAPs are originated from fungi. Their branched structure is essential for effective interaction between the BAPs molecules and receptors on the surface of the immune cells. Moreover, BAPs can also interact with non-immune cells (e.g. fibroblasts, keratinocytes) what can expand their clinical application [8,9].
In our prospective open label study we aimed to investigate the preventive effect and safety of Imunoglukan P4H® syrup (containing pleuran – β-glucan isolated from Pleurotus ostreatus) on the frequency and selected characteristics of RRTIs in children.
Methods
Patients
This prospective open study enrolled 194 children (aged 3.8 ± 7.0 years) with a history of RRTIs during the previous season (from October 2011 to March 2012), which were recruited at Department of Pediatrics, Prevention Cardiology and Clinical Immunology in Lodz (Poland). From the initially enrolled 194 children, 3 were excluded during the study due to refusal of the syrup due to its taste, so the final number of the children participating and completing the study was 191 (boys–120, 62.8%; girls–71, 37.2%). The general demographic characteristics of the studied subjects are summarized in Table 1. The enrollment of the children was based on the adapted definition of RRTIs by De Martino et al. [1]: for children younger than 4 years ≥ 6 respiratory infections and for children older than 5 years ≥ 3 respiratory infections during the period from October to March in the previous year. Children suffering from any chronic severe disease (e.g. primary immunodeficiency syndromes, primary ciliary dyskinesia, cystic fibrosis, bronchial asthma, bronchopulmonary dysplasia, chronic diarrhea) were excluded from the study. Children who were treated with other immunomodulators (e.g. inosine pranobex, bacterial lysates, thymic hormones, homeopathy, montelukast and corticosteroids) or with antibiotics 14 days prior to enrollment were also excluded. During the study period, none of the children was vaccinated against influenza.
Study Design
This open label study consisted from 3-months period of supplementation of Imunoglukan P4H® syrup and follow-up period of 3 months. The whole study lasted 6 months from September 2012 to March 2013 with three clinical visits (at the beginning, after 3 months of treatment and after 3 months of follow-up). The children that met inclusion criteria were required to take 1 mL of Imunoglukan P4H® syrup (10 mg of pleuran and 10 mg of vitamin C in 1 mL of syrup) per 5 kg of body weight every morning on empty stomach during three months. The active substance of the administered natural product was extracted by unique and patented technology from the Pleurotus ostreatus. The active substance within this natural product was previously isolated, identified and chemically characterized by Karacsonyi and Kuniak [10]. This natural immunomodulator is registered and distributed in 20 European and non-European countries and is endotoxin free. The testing for toxicity was performed by the Institute of Preventive and Clinical Medicine of Slovak Medical University (Final Report No. 5-51/04) and the tests were performed in compliance with the criteria of the Directive of Good Laboratory Practice and Directive 2004/10/EC of the European parliament and the Council of 11th February 2004.
The children were regularly checked by pediatricians during the whole study. The protocol of the study was accepted by the local Ethics Committee for Scientific Research (Lodz, Poland; No. RNN/849/12/KB) and written consent from the patients’ parents was obtained. The pediatricians filled out a questionnaire containing the data from personal, family, epidemiologic and socioeconomic history. During the treatment and follow-up period following data were recorded: the number of infections during the study, accompanying symptoms, the need for antibiotic therapy and admission to hospital. During the whole study, children did not use any other immunodulating treatment. Moreover, the tolerability of the study product and the appearance of the adverse side effects were also recorded.
| Characteristics | Mean | min | max |
|---|---|---|---|
| Age [years] | 3.7 | 1.1 | 10.7 |
| Gestational age [weeks] | 37.8 | 34 | 41 |
| Birth weight [kg] | 3.371 | 2.05 | 4.35 |
| Breastfeeding to 6th month of life [% of subjects] | 74 | - | - |
| Older siblings [% of subjects] | 49 | - | - |
| Younger siblings [% of subjects] | 34 | - | - |
| Weight [kg] | 13.8 | 8.0 | 42.6 |
| Height [cm] | 90.2 | 77 | 141 |
| First year of Kindergarten [% of subjects] | 26.9 | - | - |
| Second year of Kindergarten [% of subjects] | 24.1 | - | - |
| Mother smoker [% of subjects] | 17 | - | - |
| Father smoker [% of subjects] | 45 | - | - |
Table 1. General characteristics of the studied population
Statistical Analysis
The results are presented as mean values ± standard deviation (SD). Data were analyzed with the software package STATISTICA for Windows version 7.1 (StatSoft Inc., USA). The categorical variables were analyzed by means of Chi-squared tests and Fisher’s exact tests. The numerical variables were analyzed for the same population at different times by the Student’s t-test when the distributions were normal, or by Wilcoxon nonparametric tests. The comparison of continuous variables was performed by means of the Student’s test and the Mann-Whitney U-test.
Results
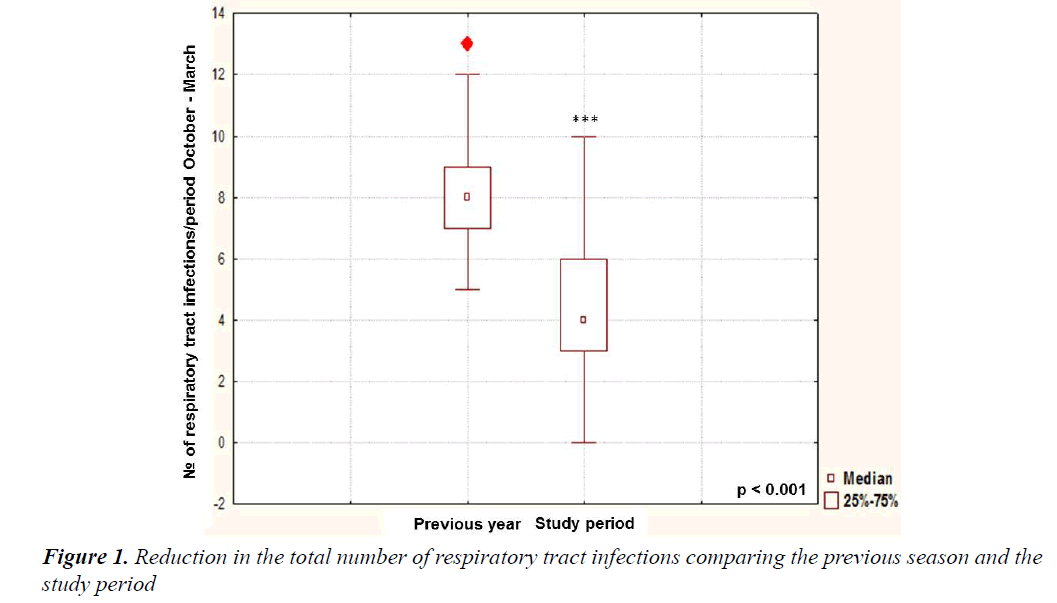
The demographic and anthropometric characteristics of 191 enrolled children are presented in Table 1. The supplementation of Imunoglukan P4H® led to a significant reduction of the total number of respiratory infections during the study period compared with the same period of the previous year (4.18 ± 2.132 vs. 8.71 ± 1.89; p<0.001) (Figure 1). Comparable significant decline of total number of RRTIs was found in both age groups: in children younger than 5 years (5.14 ± 1.93 vs. 9.11 ± 2.56; p<0.001) and in children ≥ 5 years as well (3.12 ± 1.98 vs. 7.87 ± 2.32; p<0.001). Regarding the frequency of the particular types of respiratory tract infections, otitis media was decreased by 64.2% (OR [95%Cl]: 3.76; p<0.001), laryngitis by 33.02% (OR [95%Cl]: 3.99; p<0.001), bronchitis by 30.75% (OR [95%Cl]: 3.45; p<0.001), and flu by 27.82% (OR [95%Cl]: 4.56; p<0.001). We did not note any significant reduction of pneumonia and tonsillitis incidence (Table 2). Regarding the secondary outcomes, the reduction of the number of days-off from kindergarten or school compared to the same period of the previous year was noticed (19.3 ± 12.1 days vs. 31.8 ± 10.5 days; p<0.01).
We did not observe any significant adverse effects attributable to the study product in the whole population and the syrup was well tolerated (except from three children who refused to use it due to its taste and who were excluded from the study). The majority of the subjects (89.96%) reported good or very good acceptance of the product.
Discussion
Recurrent respiratory tract infections represent an important problem in daily clinical pediatric praxis and appropriate approach in their management is crucial in decreasing all the health and socio-economic impacts in children and their families’ life. In our prospective study we evaluated the potential preventive effect of the natural preparation containing highly-purified mixture of biologically active polysaccharides on the frequency and particular type of respiratory infections in a population of 191 children. The study was performed in a typical period for high incidence of respiratory infections between October to March. Comparing the previous season, Imunoglukan P4H® was effective in a significant reduction of the total number of respiratory infections in more than 50% of enrolled children. In detail, active treatment reduced the number of episodes of various types of respiratory infections and this effect was mostly pronounced in the case of otitis media and laryngitis. Due to decline of RRTIs, we observed also the improvement of secondary study end points – decrease of the number of days-off from kindergarten or school. The product was well tolerated and no serious side effects were reported.
| Previous year [%] |
Study period [%] |
Decline [%] |
OR [95% CI] | p | |
|---|---|---|---|---|---|
| Flu | 96.5 | 69.5 | 27.82 | 4.56 | <0.001 |
| Otitis media | 24.6 | 9.3 | 62.2 | 3.76 | <0.001 |
| Laryngitis | 95.4 | 63.9 | 33.02 | 3.99 | <0.001 |
| Tonsilitis | 65.5 | 41.2 | 37.1 | 2.3 | N.S. |
| Bronchitis | 68.3 | 47.3 | 30.75 | 3.45 | <0.001 |
| Pneumonia | 23.4 | 18.6 | 20.52 | 2.03 | N.S. |
N.S: Non-Significant.
Table 2. The changes of the frequency of particular form of respiratory tract infections
Several studies evaluated the clinical efficacy of various biologically active polysaccharides in the prevention of recurrent respiratory tract infections. Regarding the fact, that various sources of beta-glucans can vary in the purity, immunomodulating capacity or in general in the complex biological activities, it is important to perform the studies with the particular type of beta-glucans. We tested a natural immunomodulator containing highly purified mixture of BAPs extracted by patented technology from Pleurotus ostreatus. In our study, we confirmed the beneficial preventive effect of Imunoglukan P4H® syrup on the frequency of various forms of respiratory infections. Regarding this particular product, there are several clinical studies which evaluated its immunomodulating and other adjuvant biological activities and the preventive potential in the management of RRTIs and yielded comparable results to our observations. In an open-label study performed in a group of 215 children with the similar design like presented study, the application of this natural substance yielded a positive therapeutic response (more than 50% reduction of RRTIs frequency) in 71.2% of the children and the total number of RRTIs dropped to 3.6 episodes/ year compared to 8.9 respiratory infections/year during the previous season before the study [11]. Similar efficacy was also observed in another prospective, observational, multicenter study in Spain. In a group of 151 children with the history of RRTIs, active treatment led to the reduction of general respiratory morbidity (4.27 episodes/ year vs. 8.88 episodes/year, p<0.001) and significantly decreased the number of pneumonia, bronchitis, laryngitis, tonsillopharyngitis, otitis media and common cold. Moreover, the reduction of the number of emergency department visits, use of other pharmacotherapy and missed school days was also achieved. The majority of the parents (85.7 %) reported the improvement of clinical status after the treatment with Imunoglukan P4H® [12]. The efficacy and capacity of Imunoglukan P4H® in the prevention of RRTIs was confirmed also in double-blind placebo-controlled multicenter randomized study in a group of 175 children aged 5.65 years. In the active group, 36% of children did not suffer from any respiratory tract infections throughout the treatment period compared to 21% in the placebo arm. Moreover, active treatment decreased also the incidence of flu and flu-like diseases as well as the frequency of lower respiratory tract infections. Authors used a vitamin C as an active placebo just to show that the immunomodulation effect clinically expressed by the decrease of infections should be attributed especially to the active substance – pleuran – and not to the vitamin C, which is also contained in Imunoglukan P4H® [13]. The same product was able to decrease also the frequency of intercurrent infections in a group of patients with Crohn’s diseases compared to placebo [14]. In two placebocontrolled studies with elite athletes pleuran prevented the post-exercise immune suppression and decreased the number of respiratory tract infections [15,16]. Positive effect on respiratory symptoms or respiratory tract infections was shown in some other studies also with the supplementation of β-glucans from other sources, e.g. yeast or cereals [17-23]. Interestingly, feeding the followup formula enriched with yeast β-glucan was shown to be associated with fewer episodes and shorter duration of acute respiratory infections, as well as less antibiotic use in 3-4 year old children [24].
The observed clinical preventive efficacy of pleuran could be explained by the mode of action of this natural modifier of biological responses. Several authors confirmed the complex immunomodulatory activity of various β-glucans, especially those isolated from fungi and yeast, in generally healthy subjects but also in patients with different chronic diseases [9,25]. It was shown that pleuran supports the development of antibody production, activates immune cellular response and functions and is able to improve postvaccination response [13,16,26]. In the children with RRTIs, pleuran was capable to suppress the non-specific markers of allergic inflammation [27]. This observation is important in expanding and clarifying the efficacy of pleuran in children with RRTIs, since many of them suffers from allergic inflammation in the airways [7]. The immunomodulation activity is in insoluble β-glucans mediated through the interaction with immune cells in intestinal Peyer’s patches without any absorption into the blood, what is consistent with the principles of safe immunomodulation [28]. β-glucans represent an important group of natural modifiers of biological responses and are in generally well tolerated and no serious side effects were reported [11,13-16]. The good or very good tolerability observed in our study was also reported in the other studies with pleuran in children with RRTIs [11-13].
One of the weaknesses of our study is its prospective open-label design. The similar design was used in Spain and also these authors confirmed the significant decrease of respiratory infections [12]. Another point is that with increasing age the respiratory tract infections frequency could decrease also spontaneously. The majority of the studied children were younger than 5 years and according to the epidemiological studies, the most significant decline of the frequency of recurrent respiratory tract infections can be observed during the school age [29]. Therefore, besides the spontaneous decline of infections rate within the increasing age, the observed decrease of respiratory morbidity can be attributed also to the active studied natural substance. Its efficacy was also confirmed in placebo-controlled study [13].
β-glucans are one of the mostly studied natural immunomodulators with many proven and potential indications in the daily clinical practice. Our study provided further evidence supporting the use of pleuran in the management of recurrent respiratory tract infections. Evidence is stressed by similar and uniform results of the studies performed with the same product in different populations and geographical settings. Moreover, good tolerability and excellent safety profile are other advantages. Therefore we can conclude, that pleuran (insoluble β-glucan isolated from Pleurotus ostreatus) could be used as an effective and safe tool for prevention of RRTIs in children. Moreover, among the other β-glucans, pleuran possesses the highest evidence for this indication based on the results of the published studies.
Conclusion
Our study supports the use of pleuran in the complementary treatment and preventions of RRTIs in children. β-glucans seem to be an effective and safe tool in the management of RRTIs.
References
- De Martino M, Balloti S. The child with recurrent respiratory infections: Normal or not? Pediatr Allergy Immunol 2007; 18: 13-18.
- Belanti JA. Recurrent respiratory tract infection in paediatric patients. Drugs 1997; 54: 1-4.
- Bush A. Recurrent respiratory infections. Pediatric Clinics of North America 2009; 56: 67-100.
- Woroniecka M, Ballow M. Office evaluation of children with recurrent infection. Pediatric Clinics of North America 2000; 47: 1211?1224.
- Owayed AF, Campbell DM, Weng EE. Underlying causes of recurrent pneumonia in children. Archives of Pediatric and Adolescent Medicine 2000; 154: 190?194.
- Jesenak M, Ciljakova M, Rennerova Z, et al. Recurrent respiratory infections in children ? Definition, diagnostic approach, treatment and prevention. In: Martin-Loeches I, eds. Bronchitis, 1st Edn. Rijeka: InTech 2011; 119-148.
- Ciprandi G, Tosca MA, Fasce L, et al. Allergic children have more numerous and severe respiratory infections than non-allergic children. Pediatr Allergy Immunol 2006; 17: 389-391.
- Vetvicka V, Vashishta A, Saraswat-Ohri S, et al. Immunological effects of yeast- and mushroom-derived Ã-glucans. J Med Food 2008; 11: 615-622.
- Rop O, Mlcek J, Jurikova T. Beta-glucans in higher fungi and their health effects. Nutr Rev 2009; 67: 624-631.
- Karacsonyi S, Kuniak L. Polysaccharides of Pleurotus ostreatus: Isolation and structure of pleuran, an alkali-insoluble Ã-D-glucan. Carbohydr Polym 1994; 24: 107-111.
- Jesenak M, Sanislo L, Kuniakova R, et al. Imunoglukan P4H® in the prevention of recurrent respiratory infections in childhood. Cesko-slovenska Pediatrie 2010; 65: 639-647.
- Sapena Grau J, Pico Sirvent L, Morera Ingles M, et al. Beta-glucan from Pleurotus ostreatus for prevention of recurrent respiratory tract infections. Acta Pediatrica Espanola 2015; 73: 186-193.
- Jesenak M, Majtan J, Rennerova Z, et al. Immunomodulatory effect of pleuran (Ã-glucan from Pleurotus ostreatus) in children with recurrent respiratory tract infections. Int Immunopharmacol 2013; 15: 395-399.
- Batovsky M, Zamborsky T, Radwan K, et al. Beta-(1,3/1,6)-D-glucan helps to decrease opportunistic infections in Crohn?s disease patients treated with biological therapy. Archives of Clinical Gastroenterology 2015; 1: 005-008.
- Bergendiova K, Tibenska E, Majtan J. Pleuran (Ã-glucan from Pleurotus ostreatus) supplementation, cellular immune response and respiratory tract infections in athletes. Eur J Appl Physiol 2011; 111: 2033-2040.
- Bobovcak M, Kuniakova R, Gabriz J, et al. Effect of pleuran (Ã-glucan from Pleurotus ostreatus) supplementation on cellular immune response after intensive exercise in elite athletes. Applied Physiology, Nutrition and Metabolism 2010; 35: 755-762.
- Auinger A, Riede L, Bothe G, et al. Yeast (1,3)-(1,6)-beta-glucan helps to maintain the body?s defense against pathogens: A double-blind, randomized, placebo-controlled, multicentric study in healthy subjects. Eur J Nutr 2013; 52: 1913-1918.
- Graubaum HJ, Busch R, Stier H, et al. A double-blind, randomized, placebo-controlled nutritional study using an insoluble yeast beta-glucan to improve the immune system. Food and Nutrition Sciences 2012; 3: 738-746.
- McFarlin B, Carpenter KC, Davidson T, et al. Baker?s yeast beta glucan supplementation increases salivary IgA and decreases cold/flu symptomatic days after intense exercise. J Diet Suppl 2013; 10: 171-183.
- Talbott S, Talbott J. Effect of Beta 1,3/1,6 GLUCAN on upper respiratory tract infection symptoms and mood state in marathon athletes. J Sports Sci Med 2009; 8: 509-515.
- Talbott S, Talbott JA. Baker?s yeast beta-glucan supplement reduces upper respiratory symptoms and improves mood state in stressed women. J Am Coll Nutr 2012; 31: 295-300.
- Vetvicka V, Richter J, Svozil V, et al. Placebo-driven clinical trials of yeast-derived Ã-(1,3) glucan in children with chronic respiratory problems. Ann Transl Med 2013; 1: 26.
- Nieman DC, Henson DA, McMahon M, et al. Ã-glucan, immune function and upper respiratory tract infections in athletes. Medicine & Science in Sports & Exercise 2008; 40: 1463-1471.
- Li F, Jin X, Liu B, et al. Follow-up formula consummation in 3 to 4 year olds and respiratory infections: an RCT. Pediatrics 201; 133: e1533-e1540.
- Oloke JK, Adebayo EA. Effectiveness of immunotherapy from oyster mushroom (Pleurotus ostreatus) in the management of immunocompromised patients. International Journal of Immunology 2015; 3: 8-20.
- Haladova E, Mojzisova J, Smrco P, et al. Immunomodulatory effect of pleuran on specific and non-specific immunity after vaccination in puppies. Acta Veterinaria Hungarica 2011; 59: 77-86.
- Jesenak M, Hrubisko M, Majtan J, et al. Anti-allergic effect of pleuran (Ã-glucan from Pleurotus ostreatus) in children with recurrent respiratory tract infections. Phytother Res 2014; 28: 471-474.
- Spriet I, Desmet S, Willems L, et al. No interference of the 1,3-Ã-D-glucan containing nutritional supplement ImunixX with the 1,3-Ã-D-glucan serum test. Mycoses 2011; 54: e352-e353.
- Heikkinen T, Jarvinen A. The common cold. Lancet 2003; 361: 51-59.