Research Article - Biomedical Research (2018) Volume 29, Issue 7
Preparation of two oligopeptides from corn protein and their protective effect on acute alcohol intoxication in mice
Naxin Sun1, Tongcheng Xu2, Yuehui Liu1, Chunjiang Ye1, Zhuqing Jiang2, Fangling Du2 and Yuanxiu Wang1*
1College of Biological Science and Technology, University of Jinan, Jinan, PR China
2Institute of Agro-food Science and Technology, Shandong Academy of Agricultural Science, Jinan, PR China
- *Corresponding Author:
- Yuanxiu Wang
College of Biological Science and Technology
University of Jinan
Jinan, PR China
Accepted date: December 28, 2017
DOI: 10.4066/biomedicalresearch.29-17-3647
Visit for more related articles at Biomedical ResearchAbstract
The objective of the study was to isolate the hydrolysates of corn gluten meal and to investigate the role of the prepared peptides in anti-inebriation treatment. CP1 and CP2 (corn peptides, CPs) were prepared by proteolysis with the combined alcalase, neutral protease and papain and then by ultrafiltration using membranes with molecular weight cut-off of 30, 10, and 3 kDa, through sephadex G-15 and HPLC. The amino acid sequences of them were also analysed by LC-MS/MS, as CP1 and CP2 were dipeptide (Val- Leu (Ile)) and tripeptide (Gly-Met-Leu (Ile)), respectively. The CPs were delivered by gavage, and alcohol was delivered subsequent to the final treatment. The effects of CPs on the blood alcohol concentrations, alanine aminotransferase levels and aspartate aminotransferase levels were measured. The activities of alcohol dehydrogenase, aldehyde dehydrogenase, superoxide dismutase and catalase, as well as malonaldehyde levels, were also recorded. The results demonstrated that the mixture of dipeptide and tripeptide prepared from GCM showed significant effects on the prevention of acute alcohol intoxication, accelerating the metabolism of alcohol in the liver and reducing the oxidative damage caused by acute alcoholism.
Keywords
Oligopeptide, Branched-chain amino acid, Alcoholic intoxication, Liver protection
Introduction
At present, alcohol abuse is of serious physical and socioeconomic problems throughout the world. The toxicity of alcohol is mainly associated with increased generation of free radicals and the development of oxidative stress. It is known that liver is one of the major target organs of ethanol actions [1]. Upon consumption, alcohol is rapidly oxidized into acetaldehyde by alcohol dehydrogenase (ADH) and then into acetate by acetaldehyde dehydrogenase (ALDH) in the liver, which is accompanied by free radical generation [2]. In addition, ethanol promotes the formation of reactive oxygen species (ROS) within the mitochondria and decreases mitochondria GSH content, thus making these organelles more susceptible to oxidative damage [3]. Consequently, the content of malondialdehyde (MDA), a product of lipid peroxidation, has been usually used as an indicator of hepatic injury. On the other hand, hepatocytes have antioxidation enzymes, such as superoxide dismutase (SOD) and catalase (CAT), to eliminate ROS for maintaining homeostasis. Once this balance is destroyed by alcohol, excessive ROS peroxide the unsaturated lipids, disrupting the important structural and protective functions associated with biomembrane, causing cell damage and disease. And certain pathological events in vivo result from this oxidation [4]. Therefore, excessive alcohol ingestion has been directly related to a number of biochemical changes and disorders in the liver and other organs [5].
The interaction of reagents with ethanol metabolism has been examined for the modulation of alcohol toxicity. Both natural resources like flavonoids extracts from various fruits [6-9] and synthetic drugs like quetiapine [10] or traditional Chinese medicine [11], have been reported to possess potential alcohol intoxication protective activities. And more effective and safe reagents have been investigated for the potential use in preventing and avoiding the harm of alcohol. Corn gluten meal (GCM), a main by-product in corn starch producing, contained approximate 60% protein [12]. However, it is usually used for animal feed rather than human food mainly due to its insolubility in water, and the hydrolysate of GCM has been studied for its further use. Compared with GCM, the value of its hydrolysate was greatly increased not only by its solubility in water but also by its various physiological activities. For example, it has been documented that the oligopeptides prepared from CGM exhibited an obvious protection from mitochondria against oxidative damage [13], and other bioactivities, such as anti-fatigue [14], anti-hypertension [15], inhibition of lipid absorption in intestine and increment [16] and hepatoprotective activity against D-galactosamine-induced liver injury [17] have also been extensively studied. It is noted that corn protein has high amount of branched-chain amino acids (BCAA), especially of leucine, exerting a variety of beneficial effects in experimental animals and humans by increased mitochondrial biogenesis and by up-regulated ROS defense system. A potential role of CGM and its hydrolysate in the alleviation of acute alcohol intoxication makes this an interesting area for further investigation [12].
In our previous studies, the hydrolysates of CGM exerted protective effects on the drunken behaviors and some biochemical parameters in an acute alcoholic intoxication model of mice (unpublished data). In this study, to clarify the structure of its main components, the hydrolysate mixture was further isolated and purified by Sephadex G-15 and reversedphase High Performance Liquid Chromatography (RP-HPLC). The amino acid sequences of the oligopeptides were also identified by liquid chromatography-mass spectrometry (LCMS). In addition, their hepatoprotective activity was evaluated using ethanol model in mice, and the possible mechanism was investigated.
Materials and Methods
CGM was kindly supplied by Luzhou Group of China. Protex6L (Bacillus licheniformis), protex7L (Bacillus amyloliquefaciens) and papain were purchased from Genencor Int (Rochester, NY). Sephadex G-15 was purchased from Sigma. The ethanol, solvents and other chemicals were procured from reputed manufacturers.
Preparation of CGM Hydrolysates Using Composite Enzymes: CGM was dispersed in distilled water (112 g/L) and incubated at 55 for 2 h while stirring continuously. The pH of CGM suspension was adjusted to 11.0 with 1M NaOH. The reaction was initiated by the addition of the combinations of three of proteases (protex6L, protex7L and papain, 4:1:1/g), and the addition of the total enzyme to yield a final enzyme-tosubstrate ratio of 1:50/g. After this hydrolysis, the mixture was heated at 100 for 5 min to inactivate the enzyme and centrifuged at 4000 r/min for 10 min to separate the hydrolysates. In order to absorb free aromatic amino acids (AAAs) or peptides terminated with AAAs as soon as possible, the supernatant was adjusted to pH 2, and powdered activated carbon was added as the solid-liquid ratio of 1:9 and incubated at room temperature for 3 h while stirring continuously. The collected solution was then separated through Sephadex G-50 gel filtration to determine the molecular weight (MW) distribution profile of peptide/oligopeptide components.
Isolation and Purification Oligopeptides Rich in BCAA: The solution was ultrafiltrated successively by Molecular Weight Cut Off (MWCO) 30000, 10000 and 3000 grade, and anion exchange resin and cation exchange resin were used to remove ions. The filtrate after MWCO 3000 was collected and lyophilized for further analysis. The hydrolysate was separated by Sephadex G-15 gel filtration. Three fractions were obtained and designed as P1, P2 and P3. As the main component in the filtrtion, P3 was then separated by reversed-phase high performance liquid chromatography (RP-HPLC). The samples were diluted in ultrapure water and centrifuged at 12000 rpm for 10 min to retain the supernatant. The HPLC conditions were as described for the HPLC-U 3000 system: column: Zorbax SB-C18 (4.6 × 250 μm); injection volume: 10 μL; equilibration buffer: 5% acetonitrile; elution buffer: 5% acetonitrile containing; flow rate: 0.6 mL/min; detection: 220 nm. In final, the P3 component was isolated in two components: CP1 and CP2.
Analysis of Amino Acid Sequences by LC-MS/MS: The component CP1 and CP2 were determined by MS for molecular mass determination and the characterization of peptides. MS conditions were designed as follows: detection method: positive ions; mass scan range: m/z 250-1500; ion source voltage: 5.5 V; auxiliary gas: 20 units; air curtain air: 25 units; cluster solution voltage: 40 V; collision gas: N2; injection pump injection speed: 20 μL/min. Resulting analytes [M+H]+ after excimer ion peak, and then use ion scan mode [M+H]+ performed MS/MS analysis.
Experimental design: Kunming mice (8 weeks old, males, SPF, 18-22 g body weight) were purchased from the Shandong Laboratory Animal Center (Jinan, China), and were housed in stainless steel wire-bottomed cages with free access to water and feed at 22 ± 2 with a 12-h/12-h light/dark. All animals were treated humanely, and the studies reported here have been carried out in accordance with the principles for the Humane Treatment of Animals set by the Association of Laboratory Animal Sciences at College of Biological Science and Technology, Jinan University. A total of 50 Kunming male mice were randomly divided into four groups with 10 mice in each group as follows: A, the normal control group without alcohol and treatment (Control); B, an acute alcoholism model group without treatment (Model); C, (CP1 and CP2, CPs) oligopeptides recipe group in low-dose (0.1 mg/g body weight (BW)) (Low); D, group in middle-dose (0.3 mg/g BW) (Med) and E, group in high-dose (0.5 mg/g BW) (High). All mice were fasted on water for 12 h before the experiment. The treatment groups were administrated the CPs by gavage. The Model group received the equal volumes of normal saline instead. 30 min later, mice in each group, except for the control group, were dosed with 0.014 mL/g BW 56° Red Star Erguotou by gavage to establish a mouse model of acute alcoholism. At the end of the experiment, all mice were sacrificed under the ether anaesthesia 6 h after treatment. The blood samples were collected, precipitated by centrifuge at 3000×g for 10 min at 4. The livers were collected for biochemical analysis.
Assay of the Metabolism of Ethanol in the Serum: Blood samples were centrifuged at 6000×g for 10 min at 4 to obtain serum. The serum alcohol concentration was measured using an ethanol assay kit (BioVision, Mountain View, CA) per manufacturer’s instructions. The activities of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) in the serum were determined using commercial detection kits according to the manufacturer’s instructions (Biosino Biotechnology Co. Ltd., China).
Assay of the metabolism of ethanol in the liver: Liver tissue samples were prepared by homogenization with ice-cold physiological saline to yield a 10% (w/v) tissue homogenate. The homogenate was centrifuged at 3000×g for 15 min, and the resulting supernatant was stored at -80 for superoxide dismutase (SOD), malondialdehyde (MDA) and catalase (CAT) determination. The SOD activity was measured by means of pyrogallol autoxidation, and the MDA content was measured by the presence of thiobarbituric acid reactive substances (TBARS) according to Buege [18]. The activities of CAT were determined by ultraviolet spectrophotometry. The activities of alcohol dehydrogenase (ADH) and aldehyde dehydrogenase (ADLH) were determined by using commercial detection kits according to the manufacturer’s instructions (Biosino Biotechnology Co. Ltd., China).
Statistical analysis
In this study, statistical analysis was performed by SPSS software package version 17.0. Data were expressed as the means ± standard deviation (SD). The significance of the differences between groups was determined using analyses of variance (ANOVA). A value of P<0.05 (2-sided) was considered statistically significant.
Results and Discussions
Isolation and identification of oligopeptides rich in BCAA
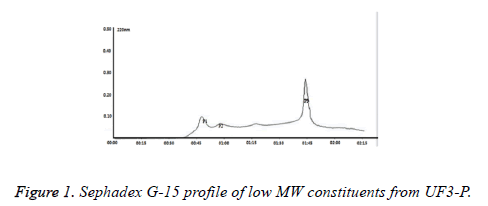
The hydrolysates of GCM, obtained by the combined action of Alcalase, Neutral protease and Papain, were extensively hydrolysed with a final DH value of 29.78%. Then the hydrolysate was fractioned consecutively through ultrafiltration (UF) membranes having MWCO of 30, 10 and 3 kDa. As the molecular weight distribution profile of the peptide/oligopeptide components in the hydrolysates through Sephadex G-50 was shown that the hydrolysate contained ~76% of oligopeptides (<1 kDa) (data not shown), only the permeate, mainly composed of peptides <3 kDa, was collected for further analysis. The ultrafiltrate was then fractioned using Sephedex G-15, which yielded three different hydrolysate products: P1, P2 and P3 (Figure 1). According to the results, P3 alone was selected for further analysis, for it not only accounted for the most proportion of the hydrolysate (~80%), but also had the lowest MW among them, which in most cases implied an better bioactivity and absorption than large peptides.
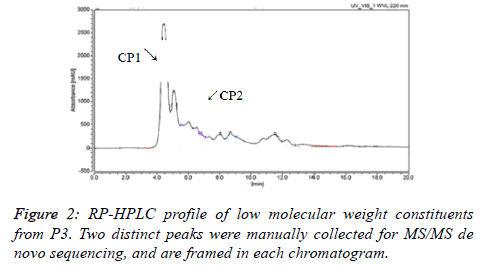
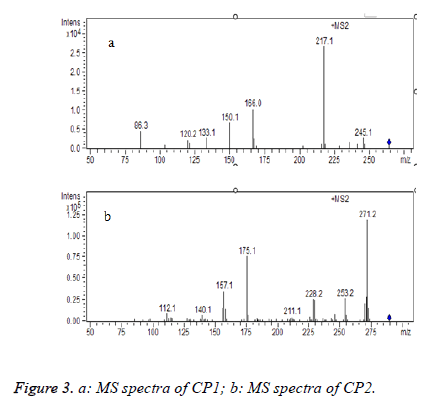
After RP-HPLC separation of P3 (Figure 2), P3 was fractionated into two major polar fractions CP1 and CP2. The adjacent peaks might interpret the result that they failed to separate on Sephadex G-15 gel. CP1 and CP2 were further analysed by the HPLC-MS/MS method, and the MS/MS spectra of a single-charged ion of the two components was shown in Figures 3a and 3b. The analysis of MS showed that the two major fractions were dipeptide and tripeptide, respectively (Table 1). It should be noted that both CP1 and CP2 are rich in BCAAs. Supplements of BCAAs are advocated and widely used in relation to performance, for they serve not only as an essential substrate in the synthesis of body proteins, but also as an important regulator of protein turnover [19]. In addition, BCAAs have already been used as a supplemental therapy to improve malnutrition in patients with liver cirrhosis or other liver disease [20]. So, the protective effect of the CP1 and CP2 (CPs) on the liver damage were further investigated on an acute alcohol intoxication model in mice.
| Code | m/z | qa | Observed mwb | Calculated mwb |
|---|---|---|---|---|
| CP1 | 289.48 | 1 | 288.47 | 287.36 |
| CP2 | 264.17 | 1 | 263.16 | 262.35 |
Table 1. Oligopeptides identified by HPLC-MS/MS method.
Dealcoholic properties of CPs
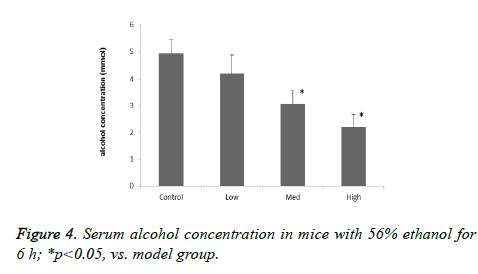
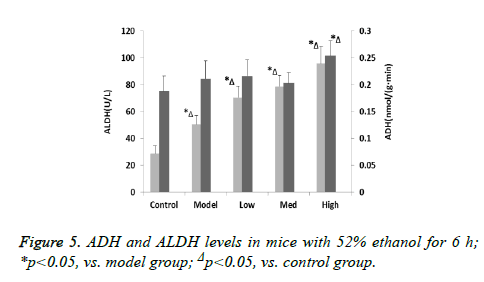
To examine the effect of CPs on acute alcohol-induced hangovers, the serum ethanol levels of mice was measured at 6 h after oral administration of 56% alcohol. The blood alcohol concentrations were significantly reduced in the Med and High dosage of treatment groups after 6 h alcohol administration, compared with the model group (Figures 4 and 5). In addition, the ethanol content in mice blood decreased as the oligopeptides concentration increased, which demonstrated that they might have dose related effect on ethanol reduction. ADH and ALDH, oxidizing alcohol to acetaldehyde and acetate, are directly related with alcohol metabolism. The activities of ADH of model group in the liver were shown to be no significant difference compared with control group (p>0.05), and the increase in ADH activity of CPs-pretreated mice was not remarkable in the liver, either; only in the High group ADH activity of the mice was significantly increased compared with the model group (p<0.05). On the other hand, acute alcohol administered to mice induced greatly incremented ALDH activity in the liver (p<0.05), and there were significant differences between the model group and all treatment groups for ALDH activity (p<0.05). This implied that CPs might exert different effect on the two alcoholic metabolic enzymes.
Protective effect of CPs in liver function
The data of ALT and AST activities of the serum was presented in Table 2. The serum ALT and AST levels were both statistically increased in model group compared with those in the control group (p<0.05). The increased activities of liver enzymes such as ALT and AST in the serum have usually been detected in alcohol-administered mice, owing to the damage of both the hepatic cellular and mitochondrial membranes induced by the ethanol metabolism [21]. In our study, the levels of AST were decreased by pre-administration of CPs 6 h after 56% ethanol oral-administration (p<0.05), and the serum ALT levels were also slightly reduced in the High CPs group compared to the model group, although there was no statistical significance (p>0.05). However, some findings pay more attention on the changes of the AST/ALT ratio rather than AST or ALT alone in human as a useful biochemical marker of liver injury due to alcohol [22]. Gurung demonstrated that the rising value of AST/ALT ratio might indicate not only the diagnosis of alcoholic related liver injury, but also more severe damage of liver due to alcohol based on a retrospective data [23]. In accordance with the observation, the value of AST/ALT ration in the model group was significantly increased compared to the normal mice, and oral pretreatment of CPs in mice made an effective reduction of the value (p<0.05). These result indicated that CPs could induce some mechanism to preserve the membrane structural integrity of liver cells and/or hepatic mitochondria from the adverse effect of alcohol.
| Groups | ALT activity (U/L) | AST activity (U/L) | AST/ALT |
|---|---|---|---|
| Control | 8.31 ± 1.03 | 12.96 ± 3.42 | 1.56 |
| Model | 12.67 ± 2.29Δ | 40.49 ± 2.94Δ | 3.20Δ |
| Low-dose | 12.39 ± 0.45Δ | 38.19 ± 0.31Δ | 3.08Δ |
| Medium-dose | 12.42 ± 0.37Δ | 33.11 ± 0.31*Δ | 2.67*Δ |
| High-dose | 11.29 ± 0.67Δ | 26.87 ± 0.56*Δ | 2.38*Δ |
Table 2. The effect of CPs on AST and ALT in serum of acute alcoholism mice.
Effect of CPs on MDA content and SOD, CAT activity in liver tissue
The acute alcoholic intoxication was characterized by oxidative stress [2]. The data of oxidation in liver tissue obtained from measurements of MDA content, SOD and CAT activity was shown in Table 3. In contrast with the induction model group, concentration of MDA was lower in the supplemented groups (p<0.05). It is also showed both the activities of SOD and CAT in the CPs treatment were significantly decreased when compared with the induction group (p<0.05). However, levels of MDA in the treatment groups were rather high relative to the control group, which might indicate that CPs could to some extent reduce the elevation of MDA caused by ethanol administration in mice. These results agreed with to that obtained by Li [12], in which the hydrolysate mixture prepared from corn peptides was capable of abating hepatic superoxidative stress. The antioxidative effect of CPs should be related to the amino acid composition of the two oligopeptides, esp. the BCAAs. Recent research have been administrated that BCAAs, mainly Leu, can be functioned as transferring signals when they are in the cytosol, which could play a key role on ROS scavenging systems and cell energy metabolism [20]. Studies in patients with liver disease indicated that BCAAs effectively decrease oxidative stress and positively affect protein synthesis [24]. Further experimental studies are required to clarify the more precise mechanism of hepatoprotection of dipeptide and tripeptide, such as applying the synthetic dipeptide/tripeptide to test its bioactivity, or examining the effect of CPs and their synthesis on removing ROS in vitro.
| Groups | MDA (µmol/g) | SOD (U/g) | CAT (U/g) |
|---|---|---|---|
| Control | 1.005 ± 0.164 | 161.514 ± 6.082 | 8.004 ± 0.649 |
| Model | 2.109 ± 0.121Δ | 132.514 ± 16.082Δ | 7.042 ± 0.387Δ |
| Low-dose | 1.843 ± 0.116*Δ | 146.712 ± 13.375*Δ | 7.202 ± 0.265*Δ |
| Medium-dose | 1.228 ± 0.141*Δ | 159.808 ± 20.037* | 7.741 ± 0.309* |
| High-dose | 1.122 ± 0.121*Δ | 177.113 ± 21.063*Δ | 8.661 ± 0.266*Δ |
Table 3. The effect of CPs on MDA, SOD and CAT in liver tissue of alcoholism mice.
Conclusion
In the present study, the mixture hydrolysate prepared via enzymatic hydrolysis of corn proteins was fractioned through UF and Sephadex G-15, and P3 was obtained and further isolated by RP-HPLC to get two oligopeptides, CP1 and CP2. The amino acid sequence of CP1 and CP2 were identified by LC-MS/MS as a dipeptide (Val-Leu (Ile)) and a tripeptide (Gly-Met-Leu (Ile)), respectively. The activity of CPs to facilitate alcohol metabolism was evaluated in vivo. The results suggested that pre-oral administration of CPs was significantly effective in preventing acute alcohol intoxication. CPs accelerated the metabolism of alcohol in the liver and alleviated the oxidative damage caused by acute alcoholism. These effects are thought to be mediated by interactions of BCAAs, which could protect anti-oxidative system form excessive ROS, and enhance the detoxification by increasing the activities of enzymes related to the anti-oxidation. Our results suggest the possibility that the CPs prepared from the corn gluten meal may be useful as a preventive agent in acute alcohol intoxication.
Acknowledgements
The authors gratefully thank the Bureau of Science and Technology of Jinan Municipality (TNK0908) for financial support of this research.
References
- Feher J, Lang I, Deak G, Csomos G, Gergely P. Effect of free radical scavengers in alcoholic liver disease. J Hepatol 1987; S124.
- Albano E. Alcohol, oxidative stress and free radical damage. Proc Nutr Soc 2006; 27: 277-284.
- Fernandez-Checa JC, Kaplowitz N. Hepatic mitochondrial glutathione: transport and role in disease and toxicity. Toxicol Appl Pharmacol 2005; 204: 263-273.
- Wagner BA, Buettner GR, Burns CP. Free radical-mediated lipid peroxidation in cells: oxidizability is a function of cell lipid bis-allylic hydrogen content. Biochem 1994; 15: 4449-4453.
- Ward RJ, Lallemand F, De WP. Biochemical and neurotransmitter changes implicated in alcohol-induced brain damage in chronic or 'binge drinking' alcohol abuse. Alcohol Alcohol 2009; 44: 128-135.
- de Freitas V, da Silva Porto P, Assunção M, Cadete-Leite A, Andrade JP, Paula-Barbosa MM. Flavonoids from grape seeds prevent increased alcohol-induced neuronal lipofuscin formation. Alcohol Alcohol 2004; 39: 303-311.
- Park HY, Choi HD, Eom H, Choi I. Enzymatic modification enhances the protective activity of citrus flavonoids against alcohol-induced liver disease. Food Chem 2013; 139: 231-240.
- Liu XN, Zhao YL, Gao EZ, Liu T, Xiao-Han YU, Zhi-Guo YU. Interventional effect of mungbean flavonoids on alcohol-induced liver injury mouse model. J Shenyang Pharmaceutical University 2015.
- Wu SK, Zhang N, Shen XR, Mei WW, He Y, Ge WH. Preparation of total flavonoids from loquat flower and its protective effect on acute alcohol-induced liver injury in mice. J Food Drug Anal 2015; 23: 136-143.
- Martinotti G, Andreoli S, Di NM, Di GM, Sarchiapone M, Janiri L. Quetiapine decreases alcohol consumption, craving, and psychiatric symptoms in dually diagnosed alcoholics. Hum Psychopharmacol 2008; 23: 417-424.
- Chen C, Wen DC, Gao SD, Hu XY, Yi C. The Protective Effects of Buzui on Acute Alcoholism in Mice. Evid Based Complement Alternat Med 2016; 2016: 1-8.
- Li HM, Guo P, Hu X, Xu L, Zhang XZ. Preparation of corn (Zea mays) peptides and their protective effect against alcohol-induced acute hepatic injury in NH mice. Biotechnol Appl Biochem 2007; 47: 169-174.
- Xu L, Li X, Huang Y, Wu X, Hou R, Wang H, Wang N, Zhang X. Protection of small molecule corn peptide Leu-Asp-Tyr-Glu from mitochondria against oxidative damage. Chemical Research in Chinese Universities 2004; 25: 1073-1075.
- Zheng H. Anti-fatigue Effect of Corn Peptide. Chinese Cereals & Oils Association 2005; 20: 33-35.
- Dayton E, Henriksen K. Study on the anti-hypertension effect of corn peptide and its mechanism. Acta Nutrimenta Sinica 2007; 29: 186-188.
- Lee HM (2001) Effect of Corn Peptide on the Lipid Metabolism in Rats. Korean J Dietary Culture 2001; 16: 416-422.
- Sui Y, Sun J, He H, Guo H, Zhang S. Hepatoprotective Effects of Corn Peptides Against D-galactosamine-Induced Liver Injury in Mice. J Ethnopharmacol 2008; 117: 415-419.
- Buege JA. Biomembranes, Part C: biological oxidations. 1978; 52.
- Millward DJ. Knowledge gained from studies of leucine consumption in animals and humans. J Nutr 2012; 142: 2212S-2219S.
- Iwasa M, Kobayashi Y, Mifujimoroka R, Hara N, Miyachi H, Sugimoto R, Tanaka H, Fujita N, Gabazza EC, Takei Y. Branched-Chain Amino Acid Supplementation Reduces Oxidative Stress and Prolongs Survival in Rats with Advanced Liver Cirrhosis. PLoS One 2013; 8: 1219-1220.
- Rajagopal SK, Manickam P, Periyasamy V, Namasivayam N. Activity of Cassia auriculata leaf extract in rats with alcoholic liver injury. J Nutr Biochem 2003; 14: 452-458.
- Liangpunsakul S, Qi R, Crabb DW, Witzmann F. Relationship between alcohol drinking and aspartate aminotransferase:alanine aminotransferase (AST:ALT) ratio, mean corpuscular volume (MCV), gamma-glutamyl transpeptidase (GGT), and apolipoprotein A1 and B in the U.S. population. J Stud Alcohol Drugs 2010; 71: 249-252.
- Gurung RB, Purbe B, Gyawali P, Risal P. The ratio of aspartate aminotransferase to alanine aminotransferase (AST/ALT): the correlation of value with underlying severity of alcoholic liver disease. Kathmandu Univ Med J (KUMJ) 2013; 11: 233-236.
- Ohno T, Tanaka Y, Sugauchi F, Orito E, Hasegawa I, Nukaya H, Kato A, Matunaga S, Endo M, Tanaka Y, Sakakibara K, Mizokami M. Suppressive effect of oral administration of branched-chain amino acid granules on oxidative stress and inflammation in HCV-positive patients with liver cirrhosis. Hepatol Res 2008; 38: 683-688.