Research Article - Journal of Clinical Nephrology and Therapeutics (2018) Volume 2, Issue 1
Predictors of intradialytic hypertension in chronic end stage renal dialysis patients in a tertiary government hospital in Davao city.
Kirbe Aparis Labarcon*, Ma.Theresa Lorenzo Bad-angSouthern Philippines Medical Center, Davao City, Philippines.
- Corresponding Author:
- Kirbe Aparis Labarcon
Southern Philippines Medical Center Davao City
Philippines
Tel: +63 917 303 4824
E-mail: klabarcon@yahoo.com
Accepted date: 3rd November, 2018
Citation: Labarcon KA, Bad-ang MTL. Predictors of intradialytic hypertension in chronic end stage renal dialysis patients in a tertiary government hospital in Davao city. J Clin Nephrol Ther. 2018;1(1):15-27
DOI: 10.35841/clinical-nephrology.2.1.14-26
Visit for more related articles at Journal of Clinical Nephrology and TherapeuticsAbstract
Patients on chronic maintenance dialysis have an alarmingly high mortality of approximately 15-20%. This rate is mainly due to cardiovascular comorbidity and, secondarily in part to the increasing prevalence of changes in blood pressure. These blood pressure changes are the most common complications that occur in these patients, most commonly hypotension, with Intradialytic Hypertension (IDH) occurring second. This single center, hospital based prospective observational cohort, research study investigated on (a) the prevalence of IDH and (b) determine the demographic, clinical profile and modifiable factors which can predict the occurrence of IDH. Three hundred thirty two (332) patients enrolled at the center were included in the study, with only 307 giving consent. Percentage prevalence of intradialytic hypertension and a higher odds ratio will depict the highest risk in determining IDH. Incidence of IDH in the study was at 37% which was higher as compared to the worldwide rate of 5-15% as well as to studies made in India, Nigeria and South Africa at 34.51%, 31% and 28% prevalence respectively. Males are significantly greater than women in the prevalence of IDH. Presence of hypertension as a comorbid is significantly higher in the IDH than in the non IDH group. Among the modifiable factors, serum albumin levels, ultra filtrate volumes, mean heart rate and arterial pressures showed significant difference between the WOH and WIH. Results from the bio impedance monitor likewise showed that the volumes of total fluid, extracellular water and intracellular water, levels of urea content and masses of adipose tissue and lean tissue were significantly higher in those with IDH. Using a logistic regression analysis, results revealed that those with the highest odds ratio in predicting the onset of IDH were ultra-filtrate volumes, serum albumin levels and intradialytic hypotension at 14.75 (3.782-57.534), 8.635 (3.603-20.696) and 1.167 (0.411-3.315) respectively. The high incidence of IDH should serve as an alarm to the institution. Measures should be taken to reduce its incidence by modifying certain practice that are already used to reduce its presence in hemodialysis patients and preventing more morbid complications like death.
Keywords
Intradialytic Hypertension, Chronic Kidney Disease, Dialysis.
Introduction
Background of the Study
Patients on chronic maintenance dialysis have an alarmingly high mortality of approximately 15%-20%. This rate is mainly due to cardiovascular comorbidity and, secondarily in part to the increasing prevalence of changes in blood pressure. These blood pressure changes are the most common complications that occur in these patients, most commonly hypotension, with Intradialytic Hypertension (IDH) occurring second.
Intradialytic hypertension, which is defined as any of the following: (a) an increase in Mean Arterial Pressure (MAP) of at least 15 mmHg within or after dialysis, (b) a corresponding increase of 10 mmHg in the systolic blood pressure before and after dialysis session, (c) hypertension during the session’s 2nd or 3rd hour, and (d) blood pressure resistant to ultrafiltration occurs in approximately 5%-15% of chronic hemodialysis patients [1,2]. Moreover, a unifying description by Inrig et al., [3] defined IDH as presence of an increase of at least 10 mmHg in the Systolic Blood Pressure (SBP) from pre to post hemodialysis in at least 4 out of 6 treatment sessions. The range of morbidity and mortality rates of patients with IDH has been significant. In multiple studies, it has been shown, that those whose systolic blood pressure rose or failed to lower with dialysis was directly correlated to an increased odds of hospitalization or death at 6 months. Further-more, there is an associated 22% increased risk for death with every 10 mmHg increase in SBP in patients with IDH. Currently there have been various hypotheses or proposed mechanisms which are said to be multifactorial on why such phenomenon is occurring, and these would include: (a) Renin-angiotensin sys-tem activation, (b) Sympathetic over activity, (c) Fluid overload, (d) Increased cardiac output; and (e) Removal of antihypertensive drugs during treatment.
Although several studies have been made on the different hypotheses and various applications, no study yet in the international and local setting has been made on the predictively of various individual factors on the occurrence of IDH on chronic hemodialysis patients. It is thus the aim of this study to predict and identify which subset of patients are likely to be having intradialytic hypertension, its correlation with each other and to serve as base reference for future IDH studies especially in the locality.
Review of Related Literature
Hypertension and chronic kidney disease
Hypertension is an important risk factor for a declining renal function. Specifically, arterial hypertension and proteinuria have been listed as two of the most indicative factors associated with progression of both diabetic and nondiabetic kidney diseases [3]. It has been observed that there exists a significant positive correlation between the decline in the kidney’s function with the mean arterial pressure among hypertensive and diabetic patients [4]. Among 1,125 individuals it was reported that hypertension is a primary risk factor in the development of early end stage renal disease as well as a pundit for its progression [5].
In multiple studies, presence of hypertension among hemodialysis patients is at 60% to 90% [6-8] and has been a big factor or incriminated in the developmental pathology being observed in cardiovascular morbidity and mortality [8,9]. Foley et al. [4] on the other hand has reported that in Caucasian hemodialysis patients, hypertension was associated with echocardiographic evidence of an increased left ventricular mass index and an increased risk for developing de novo cardiac failure by 36%. Furthermore, Mazzuchi et al. [10] reported that a systolic blood pressure of more than 160 mmHg attributed the excellent survival that was observed in Tassin's Centre de Rein Artificiel to improved blood pressure control. In another study, results of the Multiple Risk Factor Intervention Trial (MRFIT) showed that in the general population, hypertension is a strong independent factor for having end stage renal disease. It showed that in patients classified as stage 4 hypertension or those with SBP >210, there is a 20 fold higher risk as compared to those in the normal population [10-12]. Moreover, in a study by Vupputri et al. [12] among patients with early kidney function decline or (Glomerulus Filtration Rate) GFR of >60, results revealed that there is a greater decline in the estimated GFR in patients with higher blood pressure at 2.67, with a corresponding greater risk in developing decline in kidney function at 5.21 fold. Furthermore, this study concluded, that a better control of blood pressure can prevent the onset of chronic kidney disease in already hypertensive individuals. In another study, wherein incidence in declining renal function and baseline blood pressure was compared, results revealed that systolic blood pressure was a better predictor for chronic kidney disease progression as well as a 2.4:1.3 relative risk comparison among higher and lower values of blood pressure [13].
Intradialytic hypertension
Intradialytic hypertension or IDH has multiple definitions but none yet has become a standard. Moreover, it is the increase in the blood pressure during sessions of dialysis or an increase from the baseline to the end of the session [1,2]. Hypertension has a high prevalence in patients undergoing chronic hemodialysis. IDH per say, has a prevalence of 5% to 15% in the target patients. Even with this occurrence and alarmingly increased data, targets of blood pressure in these types of patients are very hard to establish, mainly because its relationship with that of mortality has not yet been calculated nor has it been reported even with the use of the blood pressure measurements [14]. What is known is that, both IDH and ambulatory blood pressure are in sync with both morbidity and mortality. However, the burden of increasing blood pressure between sessions among deemed patient with IDH is not yet known [14,15]. There have been a lot of factors owing to the pathogenesis or occurrence of IDH. First is the presence of an overload of the extracellular fluid, which is said to be the main promoter of such event [15-17]. This increase would subsequently increase both the cardiac output and the stroke volume promulgating an increase in the blood pressure. Majority of the patients that have been included in the studies returned back to a euvolemic state after a series of active and higher ultrafiltration, which may take weeks to months [16- 18]. Other factors for IDH would include excess of endothelin levels [19], low serum albumin [20], activation of the (Renin Angiotensin Aldosterone System) RAAS, presence or usage of sodium and calcium concentrated dialysates and stimulants of erythropoietin could lead to vasoconstriction and would further stimulate the sympathetic portion of the nervous system [21-23]. There have been several studies relating the effect on mortality or morbidity in dialysis patients with that of IDH. In the CLIMB study, results showed that there was a double in the risk for probable hospitalization or death with a presence of intradialytic hypertension [24]. Furthermore, the USRDS (United States Renal Disease System) trial showed that there was a significant 6% increase of same morbidity and mortality in every 10 mmHG increase in blood pressure when as compared to the baseline [25]. Also, in a study by Agarwal et al. [17] in 2009 results revealed that, a normal blood pressure or a low dry weight would lead to a normal ambulatory blood pressure, thus saying that controlling the volume or pre-venting overload would lessen the risk of morbidity or mortality among individuals undergoing dialysis. Park et al [26] also agreed with these results showing that in a retrospective cohort study in 113,525 patients, there is a higher risk or hazard for mortality with increasing blood pressure.
Bioimpedance guided monitor studies
Bioimpedance provides a noninvasive and a reliable and a simple bedside technology for diagnosing subclinical fluid accumulation, utilizing the electrical properties of body tissues–i.e. specifically relying on conductance (ionic) and reactance (tissue properties) to measure water. This technique has been introduced in different forms during the last 15 years but recently gained momentum on the basis of new solid evidence from clinical studies on fluid status assessment [26,27]. It has also been used successfully to guide HD patients towards normal hydration and better BP control. Despite this increasingly large body of evidence, clinicians are reluctant to adopt bio impedance based technologies mainly because of the lack of a definitive randomized controlled trial with hard end points and adequate follow-up demonstrating the superiority of Bioimpedance Analysis (BIA) to usual clinical best practice.
In a study by Onofriescu et al. [27] in 2014 there is a significant difference in survival, (Pulse Wave Velocity) PWV, (Blood Pressure) BP, and fluid overload between the bioimpedance group and the clinical methods (control) group after a 2.5- year intervention period. In addition, 1 year after the end of the intervention, stopping bioimpedance guided fluid [28- 30] management led to loss of the initial improvement in Pulse Wave Velocity (PWV) and a decrease in the difference in arterial stiffness between study groups. Furthermore, Wabel et al. [27] in 2009 analyzed 500 HD (Hemodialysis) patients to describe and compare different profiles of BP and hydration status. The results showed that 25% of the patients were overhydrated hypertensive patients, with a significant proportion (25%) of normotensive or even hypotensive, but overhydrated, patients; these findings suggests the need for different therapeutic approaches such as the bio composite monitor. In a subsequent prospective study, using the same technique, Wizemann et al. [27] measured baseline over hydration in 269 HD patients who were followed up for 3.5 years. The extracellular water to total-body water ratio was shown to be an independent predictor of mortality, with a Heart Rate (HR) for all-cause mortality of 2.1, second only to that related to diabetes (HR, 2.7). Similarly, extracellular to intracellular water ratio was identified as the only significant predictor for patient survival (relative risk, 1.37 for every 0.1-unit increase in extracellular to intracellular water ratio) in dialysis patients. These results were then backed up by a study of Moissl et al. [27] in 2013 showed that an active fluid management using the said device was improved in both the patient’s fluid status and blood pressure, wherein using the Bio Composite Monitoring (BCM), there was an observed decrease of at least 9.9 mm/Hg in systolic pressure with 1 L decrease in fluid.
Research question
What are the different factors that may be predictors of intradialytic hypertension among end stage kidney disease of any etiology undergoing chronic hemodialysis?
Objectives
General objective
The study was conducted to determine the predictors of intradialytic hypertension among end stage kidney disease of any etiology undergoing chronic hemodialysis in a tertiary hospital in Davao City.
Specific objectives
Specifically, the study aimed to:
1. Determine the prevalence of intradialytic hypertension among patients on chronic hemodialysis in the institution.
2. Describe the demographic and clinical profile of patients undergoing intradialytic hypertension in the institution;
3. Determine which of the following factors can predict occurrence of intradialytic hypertension among patients on chronic hemodialysis in a univariate and multivariate model.
a. Age
b. Gender
c. Comorbid Illnesses
1. Diabetes Mellitus
2. Hypertension
3. Heart Disease
d. Duration of Dialysis
e. Mode of Dialysis
1. Conventional hemodialysis
2. Slow Low Efficiency Hemodialysis (SLED)
3. Online Demodiafiltration (HDF)
f. Longevity of the dialysis
g. Etiology of kidney failure
h. Average ultra-filtrate Volume
1. > 2 L
2. < 2 L
i. Medications
1. ACE (Angiotensin Converting Enzyme) Inhibitors (Yes/ No)
2. Calcium Channel Blockers (Yes/No)
3. Beta Blockers (Yes/No)
4. Angiotensin Receptor Blockers (Yes/No)
j. Serum Albumin
k. Baseline Mean Arterial Pressure (mmHg)
l. Baseline heart rate (min)
m. Use of Erythropoietin
n. Total Fluid Overload (Liters)
o. Total Urea Content
p. Extracellular Water (Liters)
q. Intracellular Water (Liters)
r. Lean Tissue Mass (kg)
s. Adipose Tissue Mass (kg)
Conceptual framework
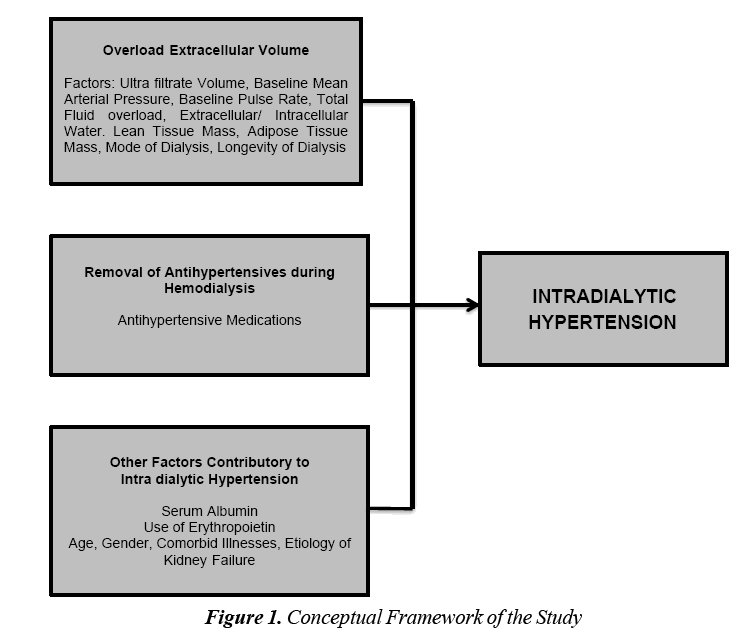
Various hypotheses have tried to explain the occurrence of IDH in patients on hemodialysis especially those on the long term setting. Figure 1 shows the conceptual framework of the study. It can be seen that the various variables included in the study were divided among the different hypotheses that explains IDH (Figure 1).
Significance of the study
In Southern Philippines Medical Center, tertiary government referral hospitals in Davao City, there are currently a total of 420 regular out-patients on maintenance hemodialysis. This was a 20% to 25% increase from the monthly averages of 256 and 343 End Stage Renal Disease (ESRD) patients being catered for hemodialysis at the renal dialysis center in 2012 and 2013 respectively. The unit also accommodated an average of 25 new in-patients and 15 new out-patients every month for the year 2016 (C. Manguray, personal communication, March 2017). Mortality and morbidity rates, has been alarming mainly due to several factors, with cardiovascular causes and hypertension being the leading cause of demise and deterioration. This most specifically would occur among patients with high pre and intradialytic blood pressure which resulted from factors such as chronic subclinical volume overload, among others. This is in turn directly associated with arterial stiff-ness, left ventricular hypertrophy, heart failure and eventually leading to mortality.
This study is deemed important because it will likely benefit the chronic hemodialysis patients, in their management as to the medications and to the set-up of their day to day dialysis sessions. Furthermore, the study would also benefit the future researchers by using the data as a reference in conducting a study about the varied causes of such occurrence among chronic hemodialysis patients in Davao City.
Definition of terms
End Stage Kidney Disease–represents the stage of chronic kidney dis-ease where patients may require renal replacement therapy including hemodialysis
Intradialytic Hypertension-This is defined as ≥10 mmHg rise in the SBP between pre- and post-dialysis in at least four of six consecutive dialysis sessions.
Pre-dialytic Blood Pressure-This is defined as blood pressure taken 5 min prior the start of each dialysis session
Post-dialytic Blood Pressure-This is defined as blood pressure taken 5 min at the end of each dialysis session.
Bioimpedance Monitor - This is a noninvasive tool that is used in diagnosing subclinical fluid accumulation which utilizes the electrical properties of body tissues to measure water and will be placed on patients prior the start of the study.
Methodology
Research design
The study employed a single center; hospital based prospective observational cohort design.
Research setting
The study was done at the Outpatient Department of the Mindanao Dialysis Center of the Southern Philippines Medical Center (SPMC) after the approval of the Ethics and research Committee from January 2017 to March 2017.
Research population
All patients undergoing hemodialysis in the outpatient department of the Mindanao Dialysis Center of SPMC were included in the study.
Inclusion criteria:
1. Age 19 years old and above.
2. All patients on maintenance hemodialysis at least 2X a week for more than or equal to 6 months.
3. All patients who are able to give consent.
4. All patients on hemodialysis on various modes which include a conventional hemodialysis, SLED, online HDF.
Exclusion criteria:
1. All admitted patients undergoing hemodialysis.
2. All patients with end stage kidney disease of any etiology; newly-diagnosed case and has been on maintenance hemodialysis for less than 6 months.
3. All patients with acute kidney injury of any etiology requiring hemodialysis.
4. All patients on hemodialysis with any of the following contraindications to a Body Composition Measurement (BCM) which includes amputees, patients with preexisting implanted cardiac devices such as pacemakers or those whose blood pressure could not be measured routinely in the upper limbs.
Sample size computation
Ideal sample size was calculated to compute for odds ratio with 95% confidence interval of having intradialytic hypertension for selected exposures based on the assumptions that (1) the ratio of unexposed to exposed is 2 (2) The outcome occurs in 18% of the participants in the unexposed group (3) The out-come occurs in 39% of the participants in the exposed group. The odds ratio to be detected as significant is at 0.12. A sample size of 87 will have 80% power of rejecting the null hypothesis (no significant increase or decrease in the odds ratio of having the outcome) if the alternative holds.
Sampling procedure
All adult patients undergoing maintenance hemodialysis for at least 2x a week for at least 6 months who were able to meet all other inclusion criteria during the given time frame were included. Demographic and clinical data were reviewed as part of the profiling.
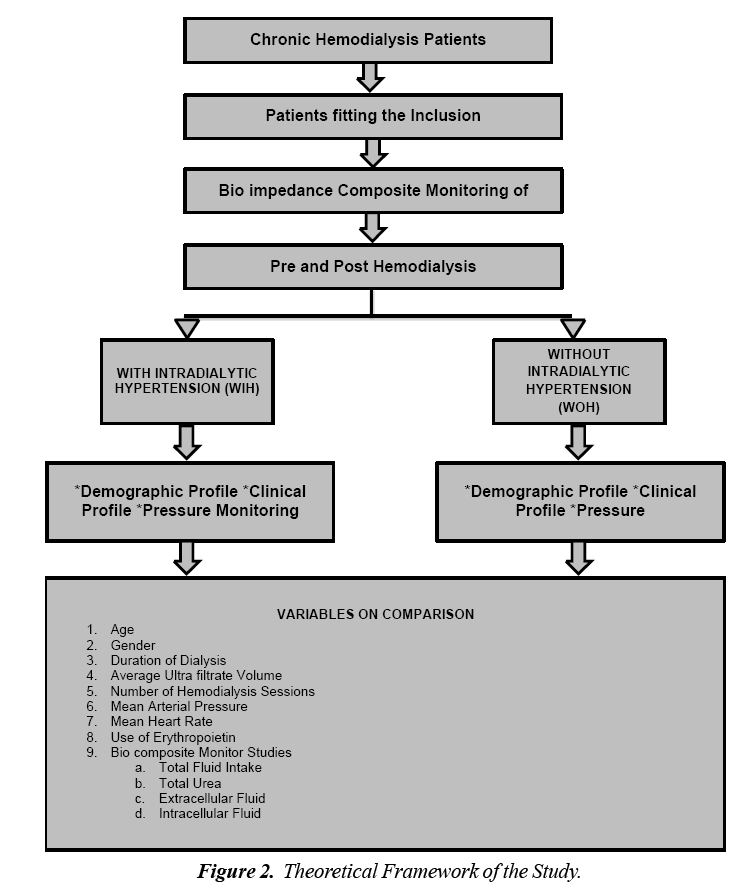
Patients on antihypertensive agents were allowed to continue taking their medications. All subjects were then hooked to a bioimpedance monitor machine to determine the level of fluid, urea, and body mass and fat prior the start of the first out of six hemodialysis sessions (Figure 2).
Blood pressures were recorded using a sphygmomanometer. Pre-hemodialysis blood pressures was measured in the nonaccess arm after a 5 min rest with the patient seated and both feet on the floor before the actual needle insertion. The same procedure was made after every dialysis procedure for each of the next 6 sessions. These then represented the post hemodialysis blood pressure monitoring of the study.
IDH for this study was defined as ≥ 10 mmHg rise in the SBP between pre- and post-dialysis in at least four of six consecutive dialysis sessions [1,2]. The subjects were then distributed into two groups: with and without IDH. Laboratory results and other significant information were gathered from the charts of the subjects that were taken from the records of the dialysis unit. During the research period, in any case the patient underwent any adverse complications or reactions; the medical team which was led by the junior consultant on duty was the first one to be called to assess the patient and his/ her condition. The principal investigator had nothing to do on the management and decisions that will arise from the patient’s current status.
Randomization
Not Applicable
Independent variables
Independent variables identified in the study include age, gender, presence of Diabetes Mellitus hypertension and heart disease, duration of hemodialysis, presence and etiology of kidney disease, medications, malnourishment, presence of other illnesses, mean arterial pressure, mean heart rate at the baseline as well as the parameters that can be taken from the bioimpedance monitor (fluid overload, extra/intracellular fluid levels, urea level, lean and adipose tissue masses).
Dependent variables
Dependent variable noted in the study was the occurrence of intradialytic hypertension.
Data handling, management and analysis
Continuous variables were expressed as mean ± SD (Standard Deviation) while categorical variables were noted as frequency and percentage. The paired t-test was then applied for all continuous variables such as age, gender, duration of dialysis, average ultra-filtrate volume, Mean Arterial Pressure, Mean Heart Rate, Use of Erythropoietin, Total Fluid Overload and Co-morbid illness) , while the chisquared test was used for categorical variables to assess the association. These were then analyzed using a univariate analysis. The significant factors were then further analyzed by using the multivariate analysis where the continuous variables were dichotomously categorized. Values of P < 0.05 will be considered statistically significant.
Results
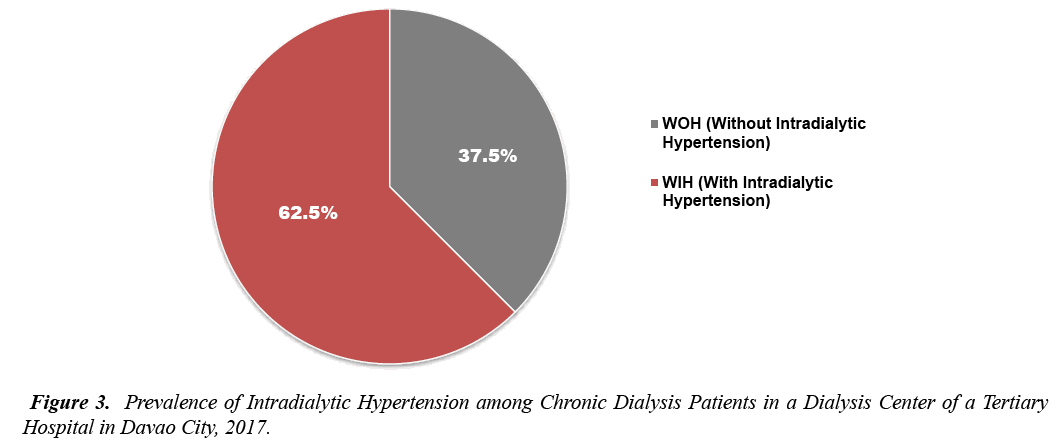
A total of 332 patients were enrolled at the Mindanao Dialysis Center during the time of conduction of study who were able to meet the set inclusion criteria were included. Of the 332, only 309 patients gave consent with a dropout rate of 7.5%. Figure 3 shows that one hundred seventeen or 37.5% of the total number of subjects comprised those with intradialytic hypertension. The remaining 193 of the cases were those with no presence of intradialytic hypertension (Figure 3).
The mean age of 46.59 +/- 14.212 years in the WIH group was comparable to that in the WOH whose average age was 44.09 +/- 12.021 years. The two groups did not differ when it comes to their body mass indexes. In terms of frequency of their comorbidities, it can be seen that those without intradialytic hypertension is significantly greater in those with hypertension (p<0.05) and diabetes mellitus (p<0.05) and is less than with urate (p<0.05) compared to those with intradialytic hypertension. Interestingly enough, the two groups significantly varied (p=0.024) in terms of sex; with more patients being in the male population in both groups. Results also of the study revealed that the duration of hemodialysis is an important predictor of IDH. It can be seen that the longer the time of dialysis the lower the chances of having IDH (3.661 ± 2476 years vs 2.828 ± 2.231, p=0.003) (Table 1).
| Parameters | WHO (n=193) | WIH (n=116) | p-value <0.05 |
|---|---|---|---|
| Age, mean ± SD, years | 44.09 +/- 12 | 46.59 +/- 14.2 | .101ns |
| Sex | |||
| Male, frequency (%) | 117 (60.6%) | 61 (53.5%) | 0.024s |
| Female, frequency (%) | 74 (38.4%) | 55 (48.2%) | |
| No of Months/ Years on Hemodialysis | 3.7 +/- 2.48 | 2.83 +/- 2.23 | 0.003s |
| Body mass index (BMI), mean ± SD, kg/m2 | |||
| Underweight | 8 (4.15%) | 0 (0%) | |
| Normal | 99 (51.3%) | 57 (49.1%) | 0.058ns |
| Overweight | 84 (43.5%) | 59 (50.9%) | |
| Hypertension (%) | 31 (16.1%) | 47 (40.5%) | 0.000s |
| Diabetes Mellitus (%) | 60 (31.1%) | 39 (33.6%) | 0.205ns |
| Cardiovascular Diseases (%) | 15 (7.78%) | 3 (2.6%) | 0.000s |
| Urate Nephropathy (%) | 25 (12.95%) | 4 (3.4%) | 0.000s |
| Other Causes | 81 (41.96%) | 37 (31.9%) | 0 |
Table 1: Comparison of the baseline characteristics of patients with and without intradialytic hypertension including the demographic profiles and risk factors of both groups in a dialysis center of a tertiary hospital in Davao city, 2017 [*WHO: without IDH; WIH: With IDH; ns: -Not Significant; s: Significant]
Table 2 showed that majority of patients in both groups had calcium channel blockers as their main antihypertensive medication with beta blockers coming second. Furthermore, it can be seen that there are more patients without intradialytic hypertension who uses ARBS (Angiotensin II Receptor Blockers) and CCB (Calcium Channel Blocker) as antihypertensive medication. Average ultra-filtrate volume per session is also a significant factor in the absence or presence of IDH with majority of those with greater than 3 L coming from those without IDH (p=0.044). Serum albumin levels (p=0.000) are significantly greater in patients in the WOH over WIH group at 37.69±5.395 and 32.2±5.623 respectively. On the other hand, mean arterial blood pressure and mean heart rate values differed significantly (p=0.000) with lower values at 98.75 ±11.544 and 86.38 ± 11.258 in the WOH than the WIH with a mean of 107.34 ± 10.059 and 96.02 ± 18.395 respectively. Lastly, the presence of episodes of intradialytic hypotension is not significant in the presence or absence of intradialytic hypertension (Table 2).
| Parameters | WOH (n=193) | WIH (n=116) | p-value <0.05 |
|---|---|---|---|
| Medications | |||
| ACE Inhibitors | 16 (8.4%) | 11 (9.5%) | 0.248ns |
| Angiotensin Receptor | 63 (33%) | 44 (37.9%) | 0.036s |
| Blockers | |||
| Beta Blockers | 85 (44.5%) | 49 (42.2%) | 0.208s |
| Calcium Channel Blockers | 147 (77%) | 71 (61.2%) | 0.000s |
| None | 2 (1%) | 9 (7.8 %) | 0.000s |
| Intradialytic Hypotension | |||
| With IDHypotension | 45 (23.3%) | 31 (26.7%) | 0.104ns |
| Without IDHypotension | 146 (75.6%) | 85 (73.3%) | 0.104ns |
| Mode of Dialysis, Frequency (%) | |||
| Conventional Dialysis | 180 (93.26%) | 113 (97.4%) | 0.004s |
| SLED | 0 | 0 | - |
| HDF | 11 (5.74%) | 3 (2.6%) | 0.004s |
| Ultrafiltrate Volume | |||
| < 3 Liters | 122 (63.2%) | 87 (75%) | |
| > 3 Liters | 69 (35.7%) | 29 (25%) | |
| Serum Albumin | 37.69+/-5.395 | 32.2+/-5.623 | 0.000s |
| Mean Arterial Pressure | 98.75 +/- 11.544 | 107.34 +/- 10.059 | 0.000s |
| Mean Heart Rate | 86.38 +/- 11.258 | 96.02 +/- 18.396 | 0.000s |
Table 2: Comparison of the Modifiable Factors in Chronic Kidney Disease Patients of Both Groups in a Dialysis Center of a Tertiary Hospital in Davao City, 2017 [*WHO: without IDH; WIH: With IDH; ns: -Not Significant; s: Significant]
A bioimpedance monitor was used among all subjects in the study. Table 3 shows the comparison among the level of water, urea, fat and muscle mass of the two groups. It can be noted that the amount of fluid is significantly different among those with and without intradialytic hypertension in the tested subjects. The greater the amounts of total fluid intake (p=0.000), extracellular water (p=0.000) and intracellular water levels (p=0.000), corresponds to a higher occurrence of intradialytic hypertension. Lean mass and adipose tissue have varying results. There was significant difference in the lean tissue mass of both groups, with patients having intradialytic hypertension having a greater mass than those without at 43.13 ± -10.169 kg and 29.14 ± -6.324, respectively. On the other hand, there was no significant difference among groups in the patient’s adipose tissue content. Lastly, urea showed a significant difference among groups with p value=0.000, with patients having intradialytic hypertension bearing higher levels of urea (Table 3).
| Parameters | WOH (n=193) | WIH (n=116) | p-value <0.05 |
|---|---|---|---|
| Total Fluid Intake (%) | 26.96 ± -4.40 | 40.54 ± -6.657 | 0.000s |
| Extracellular Water (%) | 13.45 ± -2.57 | 19.29 ± -4.657 | 0.000s |
| Intracellular Water (%) | 13.79 ± -2.52 | 22.46 ± -4.432 | 0.000s |
| Adipose Tissue (%) | 14.1 ± -8.72 | 16.68 ± -9.158 | 0.150ns |
| Lean Tissue Mass (%) | 29.14 ± -6.324 | 43.13 ± -10.169 | 0.000s |
| Urea Content (%) | 24.89 ± -4.76 | 35.59 ± -6.911 | 0.000s |
Table 3: Comparison of bioimpedance monitor readings (water, urea, fat and muscle mass) of CKD patients of both groups in a dialysis center of a tertiary hospital in Davao city, 2017 [*WHO: without IDH; WIH: With IDH; ns: -Not Significant; s: Significant]
Table 4, shows a univariate analysis correlating the various factors on the presence of intradialytic hypertension among chronic kidney patients on a regular hemodialysis in Davao City. Results reveal that various etiologies of kidney failure such as presence of diabetes mellitus, urate nephropathy and other causes are significant in the presence of IDH with p-values of 0.007, 0.000 and 0.003 respectively. Furthermore, among the hypertensive medications used in the study, only the use of ace inhibitors showed a significant effect in occurrence of IDH. As hypothesized, those with higher ultrafiltrate volume (p=0.000) showed significant effects towards IDH. Other variables that showed significance include presence of intradialytic hypotension and levels of serum albumin with p-values of 0.013 and 0.01 respectively. Based on gathered results from the use of a bioimpedance monitor both total fluid intake and levels of intracellular water showed significance when compared to presence of IDH with both having p-values of 0.000. Sex, age, diabetes mellitus, duration and mode of dialysis as well as levels of urate, lean and adipose tissue masses and extracellular water were not significant when compared with intradialytic hypertension or p-values greater than 0.05 (Table 4).
| Significant Factors | 95% Confidence Interval | p-value | |
|---|---|---|---|
| Sex | -0.019 | -0.017 | 0.140ns |
| Age | -0.125 | 0.043 | 0.336s |
| Hypertension | -0.085 | 0.192 | 0.445ns |
| Diabetes Mellitus | -0.406 | -0.066 | 0.007s |
| Cardiovascular Disease | -0.099 | 0.34 | 0.281ns |
| Urate Nephropathy | -0.645 | -0.194 | 0.000s |
| Other Causes | -0.501 | -0.101 | 0.000s |
| Duration of Dialysis | -0.023 | 0.01 | 0.424ns |
| Body Mass Index | -0.048 | -0.089 | 0.556ns |
| Use of ACE Inhibitors | -0.391 | -0.063 | 0.007s |
| Use of ARBs | -0.125 | 0.039 | 0.301ns |
| Use of Beta Blockers | -0.019 | 0.126 | 0.150ns |
| Use of Calcium Channel Blocker | -0.088 | 0.134 | 0.680ns |
| No antihypertensive Medications | -0.21 | 0.191 | 0.013ns |
| Intradialytic Hypotension | -0.202 | -0.024 | 0.013s |
| Mode of Dialysis | -0.459 | 0.191 | 0.417ns |
| Ultrafiltrate Volume | 0.083 | 0.245 | 0.000s |
| Albumin Level (mg/dl) | -0.014 | -0.004 | 0.001s |
| Mean Heart Rate | -0.003 | 0.005 | 0.522ns |
| Mean Arterial Pressure | -0.001 | 0.006 | 0.104 |
Table 4: Univariate analysis of significant risk factors of patients with intradialytic hypertension in a dialysis center of a tertiary hospital in Davao city, 2017 [*WHO: without IDH; WIH: With IDH; ns: Not Significant; s: Significant]
To test the possible interaction between the noted significant factors on developing intradialytic hypertension, a multivariate analysis was then performed and seen in Table 5. Among all the factors, the presence of diabetes, mellitus, urate nephropathy and other comorbidities; the use of ACE inhibitors; level of ultra-filtrate volume; serum albumin and intracellular water levels showed significance in predicting the presence of intradialytic hypertension among patients on chronic hemodialysis. Furthermore, using Logistic Regression, the amount of ultra-filtrate volume showed the highest risk in predicting IDH at 14.75 (3.782-57.534), followed by a non-normal serum albumin, which gave an odds of 8.635 (3.603-20.696) and third was the presence of intradialytic hypotension at 1. 167 (0.411-3.315). The use of ACE Inhibitors showed a negative predictive effect with an odds ratio of 0.034 (0.006-0.208). The other 3 etiological risk factors namely diabetes mellitus, urate nephropathy and other causes are no modifiable in nature hence does not have further significance (Table 5).
| Significant Factors | Odds Ratio | 95% Confidence Interval | p-value | |
|---|---|---|---|---|
| Diabetes Mellitus | 0.022 | 0.005 | 0.097 | 0.000 |
| Urate | 0.006 | 0.001 | 0.004 | 0.000 |
| Other Co-morbidities | 0.009 | 0.002 | 0.041 | 0.000 |
| ACE Inhibitors | 0.034 | 0.006 | 0.208 | 0.000 |
| Intradialytic Hypotension | 1.167 | 0.411 | 3.315 | 0.771 |
| Ultrafiltrate Volume | 14.752 | 3.782 | 57.534 | 0.000 |
| Serum Albumin | 8.635 | 3.603 | 20.698 | 0.000 |
| Total Fluid iIntake | 0.99 | 0.356 | 2.758 | 0.985 |
Table 5: Multivariate Analysis of Significant Risk Factors of Patients With Intradialytic Hypertension in a Dialysis Center of a Tertiary Hospital in Davao City, 2017. [*WHO: Wwithout IDH; WIH: With IDH; ns: -Not Significant; s: Significant]
Discussion
Renal decline has become a very serious public health issue worldwide. Currently statistics show that over 1.4 million are on renal replacement therapy with an average of 10-16% of the population being affected [31]. Hypertension re-main to be one of the most important risk factors for a declining renal function and accounts 27% of all cases worldwide [32,33]. On the other hand, intradialytic hypertension, which is a well-known but uncommon complication, has a prevalence rate of 5 to 15% among various registered studies worldwide [1,2]. This increases the incidence of both morbidity and mortality among patients with majority having myocardial infarctions, stroke and sudden cardiac deaths [3]. Several studies have looked upon the effects of intradialytic hypertension among various individuals or has studied the different theories regarding its occurrence but none yet has tack-led its predicting factors. Hence, this study was conducted to correlate a group of factors from a subject’s demographic to laboratory and hemodialysis profiles down to bioimpedance guided readings on water and mass levels as possible predictors of intradialytic hypertension especially in the Filipino population.
Primary outcome
The IDH prevalence of 37% recorded in the study is high when compared to the recorded prevalence of 5%-15% worldwide. Some no studies that reviewed the prevalence of IDH includes a study by Van Buren et al. which showed a 21.3 per 100 prevalence and Nongnuch et al. with a prevalence of 18%. Among the Asian counterparts, a study from China revealed only a 10% prevalence of IDH among 131 individuals [34-36]. In a study among Western South African province treatment centers, which like the Philippines is a developing country a prevalence rate of 28% was recorded, which is still lower 40. This pattern also holds true among tested patients in Nigeria and India wherein 61 (31.3%) and 49 (34.51%) of the subjects had presence of IDH as a complication 41,42.
Prevalence in the studies may differ owing to variations in defining the IDH. The aforementioned studies defined IDH as ≥ 10 mmHg increase in SBP during a hemodialysis session whereas our study included that the increase must have occurred in at least four of six prior sessions [34-36]. Even with the strict benchmark, the prevalence in the study still remained high. Another factor that could have contributed to the higher prevalence observed was the patients included in the studies employed the routine use of a bioimpedance monitor every 3 months to serve as guide for fluid management [34- 36]. In our center, only a yearly routine check by the monitor at the beginning of the year is made with clinical judgment serving as tool in fluid management for the next sessions throughout. Hence, prevalence of IDH in both studies made in Africa which has the same practice as our center were at par to each other [37-39]. It is also observed that prevalence in third world countries are at the high 30 percentile which also can be pointed out to the poor health care system or patients not having easy access to healthcare hence the late diagnosis of symptoms and complications.
Demographic profiles and risk factors
The mean age in both groups did not differ significantly (44.09 (+/- 12.021, WOH vs. 46.59 +/- 14.212, p-value=0.101). The result in the study is lower than most studies wherein majority of the patients with IDH belong to the 55 and older age bracket and is significantly different from those without IDH [2-4,17,31,38]. This occurrence may be related or explained by presence of arterial stiffness in elderly individuals [39]. The trend towards detection of IDH in a relatively younger age group in this study maybe secondary to an earlier age occurrence of the complications of kidney failure in majority of patients being catered by the institution. This can be then related to the patients financial instability/incapability to detect and treat renal complications at an earlier stage. This was also reported in the mentioned study in Nigeria, wherein the mean age of those having IDH is at 41.2 years and mostly are lying close to the poverty or marginal line 40. Moreover, end stage renal disease population is getting younger due to the increasing adaptation of unhealthy diet and sedentary lifestyle more common and rampant in the younger population. Hence, measures to prevent childhood obesity should be given importance. Majority of patients in both groups were males similar to reported studies [3,18,19,37,38]. However, these results were not significantly different as compared to our study. In this study, no relation was found between the incidence of IDH and body mass index. This result is further supported by studies made by Inrig et al. [3], Eftimovska- Otovic, et al. [38], Chou et al. [26] and Park et al. [22].
No significant relationship was found between the incidence of IDH and those with previous diabetes in CKD patients. On the contrary, a previous hypertension, hyperuricemia and other causes like chronic glomerulonephritis showed a significant relationship in IDH prevalence. A proposed mechanism for this possibility is the removal of antihypertensive medications during hemodialysis [39].
Modifiable factors, bioimpedance readings and prevalence of IDH.
The use of ARB’s, Calcium channel blockers and beta blockers showed a significant relationship with the incidence of IDH. In a study by Inrig et al. [3] the class of antihypertensive agents its timing and dosing should be reviewed when patients have intradialytic hypertension. The study reported ARB’s and beta blockers being removed by dialysis hence should be immediately changed to nondialyzable ones. Furthermore, it was stated that the removal of any antihypertensive agents during HD should be considered in any patient with intradialytic hypertension, it has not been investigated whether this plays a significant role in the pathogenesis of intradialytic hypertension and a prior study demonstrated intradialytic hypertension occurred in patients off antihypertensive agents [22].
Serum albumin levels, ultra-filtrate volumes, mean heart rate and arterial pressures were all significant in the prevalence of IDH with p-values of 0.000, 0.044 and 0.000 respectively. This holds true to the various water levels utilized in the study; wherein those with IDH have significantly higher total fluid, intracellular and extracellular water levels. These factors are all related to the presence of volume overload which plays a significant role in poorly controlled BP in hemodialysis patients. Cirit and colleagues investigated 7 patients who exhibited significant cardiac dilation on echocardiography and had BP elevations with hemodialysis which were not responsive to antihypertensive medications. Following intense ultrafiltration and lowering of dry weight, the echocardiographic volume parameters improved and the BP response to hemodialysis normalized in most patients [18]. Another study of 6 patients with intradialytic hypertension noted that modest ultrafiltration resulted in increased cardiac output and elevations in MAP [19]. More aggressive ultrafiltration in these patients resulted in a lowering of cardiac index and MAP, suggesting significant volume overloaded as the cause of the initial increase in MAP with hemodialysis and ultrafiltration. Similar findings were also shown in study by Agarwal et al. [17], Van Buren et al. [34], Nongnuch et al. [35] and Nilrohit et al. [39]. It was also observed that BP may paradoxically rise with ultrafiltration, when patients are volume over-loaded. In a study by Inrig et al. it was found that the incidence of IDH was higher in patients having low serum albumin levels which is similar in our results. This may be due to a presence of reduced blood viscosity causing high cardiac output and increased peripheral vascular resistance. However, in another investigation, neither echo-specific volume overload nor cardiac dysfunction were identified in patients with intradialytic hypertension compared to controls [22]. Therefore, while select subsets of patients with hypervolemia may exhibit intradialytic hypertension, volume overload does not solely explain the pathophysiology of BP elevations with hemodialysis in all patients.
Furthermore, intradialytic hypertension can be caused by an increase in stroke volume, heart rate, systolic blood pressure and/or vasoconstriction with an inappropriate elevation in PVR during hemodialysis; therefore, it appears plausible that stimulation of the sympathetic nervous system should contribute its development. Further, it is well recognized that hemodialysis patients have excess sympathetic nervous activity as measured by micro neurography [40,41] . However, in an investigation by Chou et al. there was neither an increase in plasma epinephrine nor plasma norepinephrine during hemodialysis to explain the increase in PVR (Post Void Residual) among patients with intradialytic hypertension [22]. However, circulating levels of catecholamines do not always correlate with BP changes and differences in micro neurography among patients with and without intradialytic hypertension have not been performed. In literature, the use of Erythropoetin Stimulating Agents (ESAs) is associated with an increased blood pressure in hemodialysis patients [42,43]. Its effect in IDH prevalence has not been investigated in studies and was not widely utilized in this study as the amount and time of injection was not modified.
Univariate and multivariate analysis tests
Univariate analysis revealed presence of diabetes mellitus, urate nephropathy and other causes of ESRD, the usage of ACE inhibitors as an anti-hypertensive, intradialytic hypotension, level of ultra-filtrate volume and serum albumin and volumes of total fluid intake and intracellular water as significant factors for the development of Intradialytic Hypertension. Furthermore, a multivariate analysis was performed to test the effects of all the significant variables from the previous step on the primary outcome which is IDH.
After multivariate analysis, significant factors for developing IDH include those above mentioned except for intradialytic hypotension and levels of total flu-id intake. Using Logistic Regression analysis, those with the highest odds ratio in predicting the onset of IDH in order were ultra-filtrate volumes, serum albumin levels and intradilaytic hypotension at 14.75(3.782-57.534), 8.635(3.603-20.696) 1.167(0.411- 3.315)respectively. However, the presence of intradialytic hypotension was not significant based on p-value.
As discussed above both ultra-filtrate volumes and serum albumin levels are correlated to the presence of a volume overload and resulting high sympathetic activity [3,17,22,39]. Hence the need to focus on decreasing volume over-load by probably using the bioimpedance monitor in a regular basis for fluid management would be very helpful. On the contrary the use of ACE inhibitors showed negative predictively as compared to intradialytic hypertension. This was congruent with the studies made by Efrati et al. which showed that ACE inhibitors can reduce the incidence of mortality among chronic hemodialysis patients by significantly de-creasing the blood pressure levels of the samples. However in a metaanalysis review of 11 trials encompassing 1865 patients, results revealed that the use of ACE inhibitors may decrease the loss of residual renal function, mainly for patients with peritoneal dialysis. Furthermore, ACE-Is do not reduce cardiovascular events in dialysis patients which includes the effect on intradialytic hypertension. The study further concluded that a confirmation of more studies is needed to solidify the effect of ACE inhibitors in dialysis patients [44,45].
Limitation
The primary limitation of this this study is its non-controlled, non-randomized, and observational nature. Performing a randomized controlled study may be difficult since it is impossible to eliminate the bias resulting from the frequently changing clinical condition and practices of the dialysis patients. Furthermore, no additional intervention was made hence its limitation for randomization. The author does not have any hold in modifying any practice or medication that could be used within the duration of the study.
Summary and Conclusion
The study included 332 patients that were currently enrolled at a dialysis center in a government tertiary hospital in Davao City. Incidence of IDH in the study was at 37.5% which was higher as compared to the worldwide rate of 5%-15%. This incidence was comparable to the studies made in India, Nigeria and South Africa at 34.51%, 31% and 28% prevalence respectively. This suggests that IDH incidence is higher in developing countries aforementioned wherein majority of the population are just within the marginal income line that has a lower access to early determination of symptoms and diseases.
The two groups were similar in their baseline characteristics in terms of age, body mass index and presence of diabetes mellitus as comorbidity. Males are significantly greater than women in the prevalence of IDH. Presence of hypertension as a comorbid is significantly higher in the IDH than in the non IDH group. Among the modifiable factors, serum albumin levels, ultra-filtrate volumes, mean heart rate and arterial pressures showed significant difference between the WOH and WIH. Results from the bioimpedance monitor likewise showed that the volumes of total fluid, extracellular water and intracellular water, levels of urea content and masses of adipose tissue and lean tissue were significantly different in both groups. These results can be explained by the volume overload hypothesis for majority of patients with IDH. Using a logistic regression analysis, results revealed that those with the highest odds ratio in predicting the onset of IDH were ultra-filtrate volumes, serum albumin levels and intradialytic hypotension. The high incidence of IDH should serve as an alarm to the institution. Measures should be taken to reduce its incidence by modifying certain practice that are already used to reduce its presence in hemodialysis patients and preventing more morbid complications like death.
Ethics Review
The proponent of the study secured an approval from the Department of Health (DOH) Cluster Ethics Review Committee (CERC) and the Southern Philippines Medical Center–Institutional Review Board (SPMC-IRB) prior to the conduct of the study. Upon approval, permission was asked from the head of the Southern Philippines Medical Center- Mindanao Dialysis Center.
Privacy
A written c