Editorial - Journal of Cardiovascular Medicine and Therapeutics (2017) Volume 1, Issue 1
Novel Anticoagulants following Biological valve implantations: A new Hope with lacking evidence.
- *Corresponding Author:
- Mahmoud Sabbah
Department of Cardiology Faculty of Medicine, Suez Canal University Ismailia-Egypt
Tel: +2-064-3229810
Fax: +2-064-3229810
E-mail: dr1sabbah@yahoo.com
Accepted date: February 02, 2017
Citation: Sabbah M, Abdellah AT. Novel Anticoagulants following Biological valve implantations: A new Hope with lacking evidence. J Cardiovasc Med Ther. 2017;1(1):1-3.
Introduction
Atrial fibrillation (AF) is the most common arrhythmia and is associated with a 3 to 5 fold increased risk of stroke [1]. Degenerative valvular heart disease (DVHD) and AF frequently coexist as both share multiple common risk factors, such as age and hypertension. Degenerative aortic valve stenosis itself is associated with higher rate of AF because of pressure overload and diastolic dysfunction [2]. Similarly, both mitral regurgitation (MR) and AF is common [3], particularly in the elderly [3], who are also at high risk for AF [4] and because MR tends to cause diastolic dysfunction and left atrial (LA) enlargement, a possible precursor of AF [5]. Pre-existing AF was high across TAVI studies and widely ranges from 16% up to 51.1% [6]. Moreover, AF is a common problem in patients with mitral regurgitation referred for percutaneous intervention. In the EVEREST II study, AF was present in 27% of the study population at baseline [7]. These patients are usually older, have advanced valvular disease [7] and non-cardiac comorbidities [8]; hence, they require long-term anticoagulation as a prophylaxis against thromboembolism. Moreover, percutaneous mitral valve (MV) repair and its consequences; blood exposure to rough surface and turbulence flow through the valve can lead to blood stasis in the left atrium which may increase the risk of thrombus formation and mandates appropriate anticoagulation, especially if AF coexists.
Transcatheter valve therapies (Transcatheter Aortic Valve Implantation [TAVI] and MitraClip) has evolved as a promising new technique for the patients with DVHD deemed ineligible for surgery [9-11]. In general, the use of anti-platelet and anticoagulants before, during, and after the transcatheter valve procedures is not well defined and left to the discretion of the operating physician. Furthermore, the appropriate anticoagulation strategy in non-valvular AF patients undergoing percutaneous valve procedures and the choice of proper anticoagulant agent remain a matter of controversy with no sufficient data guiding the therapeutic strategy.
New oral anticoagulants (NOACs) have emerged as good alternatives to the usual vitamin-K antagonists with considerable efficacy and safety that was consistent across a wide range of patients with non-valvular AF [12]. Use of NOACs was associated with similar or even lower rates of both ischemic stroke and major bleeding compared to adjusted dose warfarin (INR of 2.0 to 3.0) [12].
However, patients with Valvular AF, defined as having mitral stenosis or artificial heart valves, have been systematically excluded from the pivotal randomized clinical trials testing the NOACs [13-16]. The pathophysiological reason for excluding these patients was that the mechanism of thrombus formation in this particular cohort may be substantially different from the usual AF patients. Nevertheless, the exclusion criteria for concomitant valve disease varied slightly in these trials, with exclusion of most valvular disease patients implemented in some studies [13,14] while others included some patients with nonrheumatic valvular disease, valve repair or bio prostheses [16].
The ROCKET-AF trial, evaluating rivaroxaban against warfarin, excluded only haemodynamically significant mitral valve stenosis and prosthetic heart valves, but permitted the inclusion of patients with other diseases in native valves, as well as patients treated with annuloplasty, commissurotomy or valvuloplasty [16]. Moreover, dabigatran was not inferior to warfarin in preventing intracardiac thrombus formation in patients with bioprosthesis [17]. Therefore, there might be a place for NOACs in AF patients undergoing percutaneous valve procedures and or biological valve implantations and particularly, rivaroxaban may have a potential benefit over warfarin in AF patients undergoing percutaneous valve implantations However, there is no data directly comparing the safety and effectiveness of NOACs versus warfarin in this particular cohort. Therefore, a sufficiently powered randomized clinical trial comparing the safety and effectiveness of NOACs in AF patients undergoing percutaneous or surgical valve implantations is required. In fact, several randomized clinical trials are currently ongoing in order to answer the question of the best antithrombotic regime in AF patients undergoing TAVI procedures (ClinicalTrials.gov Identifier: NCT02664649, NCT02556203 and, NCT02247128). Similar trials in MR patients undergoing percutaneous mitral valve repair are needed, simplified proposed protocol for such a trial is attached.
Patient Selection
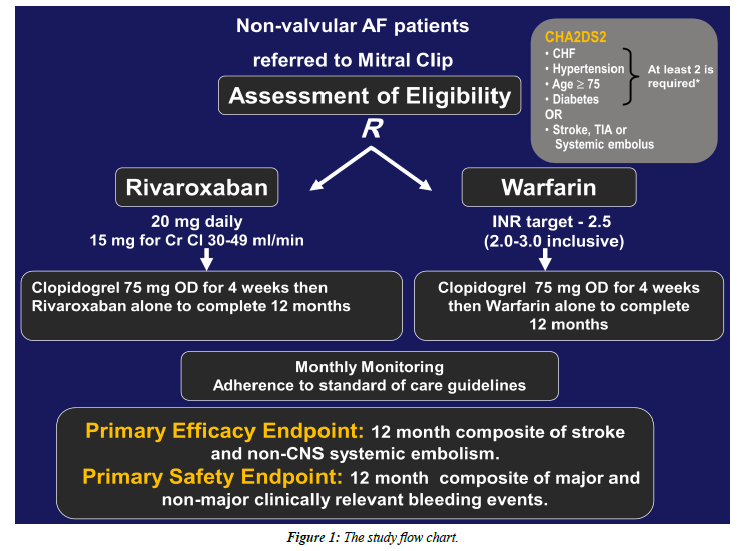
All patients referred for percutaneous MV repair will be screened and the eligible non-valvular AF patients with moderate-to-high risk for stroke based on CHA2DS2-Score calculation, regardless of the mitral regurgitation etiology, will be enrolled after giving written informed consent.
Eligibility criteria
All patients with non-valvular atrial fibrillation (paroxysmal, persistent or permanent) and CHA2DS2-Score of 2 or more referred to percutaneous mitral valve edge-to-edge repair will be included.
Non-valvular AF, defined as paroxysmal, persistent or permanent AF in patients without prosthetic valve or hemodynamically significant mitral valve stenosis.
The study arms and intervention: Attached, the study flow chart (Figure 1).
• Eligible patients will be randomly allocated to each study arm prior to the Mitral Clip procedure, to receive either:
a) Adjusted-dose warfarin + Clopidogrel (75 mg/d).
- Warfarin therapy will be initiated and adjusted before the Mitral Clip procedure and Clopidogrel will be started immediately after the procedure and both therapies will be continued for 4 weeks, then warfarin alone to complete 12 months.
- Peri-procedural withdrawal of warfarin with or without bridging anticoagulation will be left to the operator indiscretion.
b) Rivaroxaban [20 mg daily or 15 mg daily for those with estimated creatinine clearance (eCrCl) 30–49 mL/min] + Clopidogrel (75 mg/d).
- Both Rivaroxaban and Clopidogrel will be initiated after Mitral Clip procedure and continued together for 4 weeks then Rivaroxaban alone to complete 12 months.
• Peri-procedural anticoagulation in both study arms will be according to the usual practice standards.
• Patients in both groups will be evaluated for the occurrence of primary and secondary endpoints at 1, 6 and 12-months follow-up.
Study End-points
The primary efficacy end point: The composite of stroke (ischemic or hemorrhagic) and systemic embolism.
The primary safety end point: The composite of major and nonmajor clinically relevant bleeding events.
Secondary efficacy end points a composite of stroke, systemic embolism, death from cardiovascular causes, or myocardial infarction; and individual components of the composite end points.
Outcome definitions: All endpoint definitions will be according to the recent Consensus Document from the Mitral Valve Academic Research Consortium [18].
We think that this innovative idea might have a clinical impact which can solve the controversy at this point, and More importantly, the results of these studies can be used as a framework for further studies to assess safety and effectiveness of rivaroxaban in patients undergoing biological valve or percutaneous bio-prosthetic implantation if anticoagulation is required.
Conflicts of Interest
Authors have nothing to declare.
References
- Lloyd-Jones DM, Wang TJ, Leip EP, et al. Lifetime risk for development of atrial fibrillation: The Framingham Heart Study. Circulation. 2004;110:1042-46.
- Steine K, Rossebo AB, Stugaard M, et al. Left ventricular systolic and diastolic function in asymptomatic patients with moderate aortic stenosis. Am J Cardiol. 2008;102:897-901.
- Singh JP, Evans JC, Levy D, et al. Prevalence and clinical determinants of mitral, tricuspid, and aortic regurgitation (the Framingham Heart Study). Am J Cardiol. 1999;83:897-902.
- Benjamin EJ, Levy D, Vaziri SM, et al. Independent risk factors for atrial fibrillation in a population-based cohort. The Framingham Heart Study. JAMA. 1994;271:840-44.
- Vasan RS, Larson MG, Levy D, et al. Distribution and categorization of echocardiographic measurements in relation to reference limits: the Framingham Heart Study: Formulation of a height- and sex-specific classification and its prospective validation. Circulation. 1997;96:1863-73.
- Sannino A, Gargiulo G, Schiattarella GG, etal. A meta-analysis of the impact of pre-existing and new-onset atrial fibrillation on clinical outcomes in patients undergoing transcatheter aortic valve implantation. EuroIntervention: Journal of EuroPCR in collaboration with the Working Group on Interventional Cardiology of the European Society of Cardiology 2016;12:e1047-e1056.
- Herrmann HC, Gertz ZM, Silvestry FE, et al. Effects of atrial fibrillation on treatment of mitral regurgitation in the EVEREST II (Endovascular Valve Edge-to-Edge Repair Study) randomized trial. J Am CollCardiol. 2012;59:1312-9.
- Zuern CS, Bauer A, Lubos E, et al. Influence of non-cardiac comorbidities on outcome after percutaneous mitral valve repair: results from the German transcatheter mitral valve interventions (TRAMI) registry. Clinical research in cardiology: Official Journal of the German Cardiac Society 2015;104:1044-53.
- Puls M, Lubos E, Boekstegers P, et al. One-year outcomes and predictors of mortality after MitraClip therapy in contemporary clinical practice: Results from the German transcatheter mitral valve interventions registry. Eur Heart J 2016;37:703-12.
- Downs EA, Lim DS, Saji M, et al. Current state of transcatheter mitral valve repair with the MitraClip. Ann Thorac Surg.2015;4:335-40.
- Leon MB, Smith CR, Mack M, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363:1597-607.
- Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: A meta-analysis of randomised trials. Lancet (London, England) 2014;383:955-62.
- Connolly SJ, Eikelboom J, Joyner C, et al. Apixaban in patients with atrial fibrillation.N Engl J Med.2011;364:806-17.
- 14.Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med.2009;361:1139-51.
- Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981-92.
- Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med.2011; 365:883-891.
- Duraes AR, De Souza Roriz P, De Almeida-Nunes B, et al. Dabigatranversuswarfarin after bioprosthesis valve replacement for the management of atrial fibrillation post-operatively: DAWA Pilot Study. Drugs in R&D 2016;16:149-54.
- Stone GW, Adams DH, Abraham WT, et al. Clinical trial design principles and endpoint definitions for transcatheter mitral valve repair and replacement: Part 2: Endpoint definitions: A consensus document from the Mitral Valve Academic Research Consortium. Am J Cardiol.2015;66:308-21.