Research Article - Journal of Parasitic Diseases: Diagnosis and Therapy (2016) Volume 1, Issue 1
Morphological and Histopathological Studies of Trichuris Ovis in Goat Intestine
Tahmina Zainab and Wajihullah Khan*Tahmina Zainab, Department of Zoology, Aligarh Muslim University, Aligarh, India
Wajihullah Khan, Department of Zoology, Aligarh Muslim University, Aligarh, India
- *Corresponding Author:
- Wajihullah Khan
Department of Zoology, Aligarh Muslim University, Aligarh-202002, India
Tel: 9045493671
E-mail: wajihullahkhan@yahoo.co.in
Received Date: October 24, 2016; Accepted Date: October 26, 2016; Published Date: November 04, 2016
Abstract
Morphological and histopathological study of large intestine of goats infected with Trichuris ovis revealed the embedded attenuated anterior end in mucosa, surrounded with fibroblast and leucocytes in histopathological sections. There was no sign of fibrosis and necrosis. Crypts of Leiberkuhn, muscularis mucosa, submucosa and muscularis externa were clearly differentiated with slight infiltration of inflammatory cells like mononuclear cells, leucocytes, goblet cells along with epithelial changes and overall mucosal architecture in vicinity of worm penetration.
Keywords
Trichuris ovis; pathology; intestine; caecum; goat
Introduction
India’s livestock sector is one of the largest in the world and accounting for 26.40% goats and plays an important role in economy (Anon, 2012). Trichuriasis is a neglected tropical disease causing significant animal and human health problems as well as considerable socio-economic consequences worldwide. Trichuris is a widespread gastrointestinal parasite that can be found in a broad range of hosts. Its life cycle is direct where orally ingested embryonated eggs hatch in the small intestine and the released larvae burrow into the intestinal wall of the caecum and proximal colon where they develop to mature worms (Jenkins, 1970., Beer, 1973). The anterior end of the worm forms syncytial tunnels of epithelial cells around itself as it burrows through the mucosa (Panesar, 1980., Croll, 1980., Cliffe, 2004., Grencis, 2004). The posterior end protrudes into the lumen facilitating copulation and egg release. Trichuris ovis is a whipworm infecting the caecum and colon of sheep, goats, cattle and other ruminants in all parts of the world but is relatively harmless. However, clinical diseases due to T. ovis have been reported in sheep and cattle (Kuchai et al. 2013). Heavy infection may be observed in very young lambs. Animals may suffer from bloody colitis and diphtheritic caecitis which may cause ulcerative and necrotic lesions on the mucosa. In animals with high worm burden severe anaemia and dehydration and jaundice may lead to the death of the animals (Soulsby, 1982., Bowman, 2002., Taylor et al. 2007). After a century of research on the biology and control, this parasite continues to be an important constraint on ruminant production. Novel developments for the management of nematode parasites such as vaccines, biological anthelmintics, genetic markers, and selective breeding may, in the future, provide additional or alternative means of parasite control. However, such alternative control methods are likely to be more dependent on sound understanding of the species, life cycle and their pathogenicity (Kuchai et al. 2013). Keeping these facts in view the present study was aimed at morphological and histopathological observation in goats infected with T. ovis.
Materials and Methods
The large intestine of goat was obtained from the local abattoir and Trichuris ovis were collected from caecum of slaughtered goat which was incised in the laboratory. Both males and females recovered from the caecal mucosa were collected, washed and stretched in normal hot saline. These worms were preserved in 70% alcohol, dehydrated in glycerine-alcohol (10 parts glycerine and 90 parts 70% alcohol) and then placed in desiccator at room temperature for 3-4 weeks for clearing. Dehydrated nematodes were then mounted on glass slides in anhydrous glycerine. The slides were observed under the compound microscope and photographs were taken under Nikon Research Microscope (Eclipse 600) at X40, X100 and X400.
For histopathological studies, affected tissue pieces of intestinal caecum containing the embedded parasites were taken out and washed thoroughly in Hank’s Balanced Salt Solution to remove the debris. Washed specimens were fixed in Bouin’s fixative for 42 h. Fixed pieces of tissues were again washed thoroughly and dehydrated through ascending alcoholic grades, cleared in xylene and embedded in paraffin wax. The block was then trimmed and affixed to a holder which is inserted in the microtome to perform microtomy. The blocks were cut at 5 μ and were then affixed to slides and stretched on stretching box. Sections were dewaxed by putting slides in xylene and then sections were hydrated by passing through descending grades of alcohol. These slides were stained with haematoxylin and eosin and washed with water followed by treatment with 1% acid alcohol. The histopathological changes associated within tissues were examined under the compound microscope (Nikon E-600) and photographs were taken by the attached Nikon digital camera (DN 100).
Results
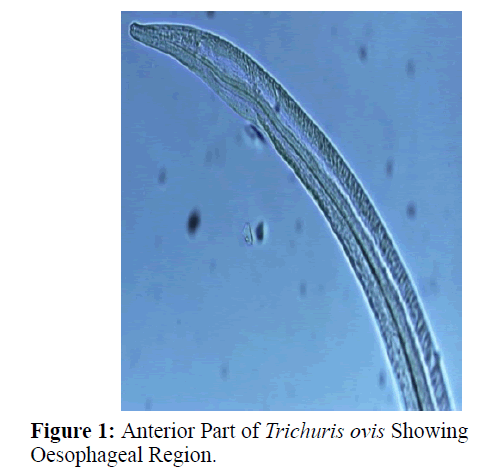
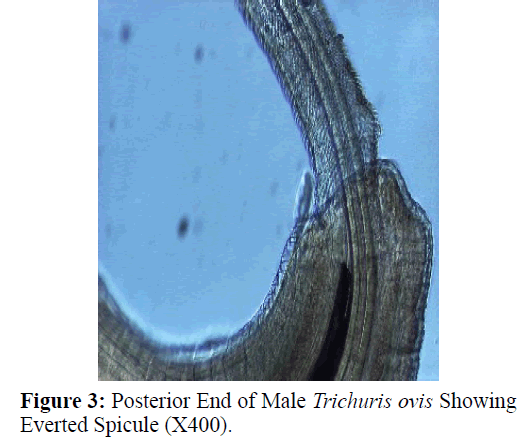
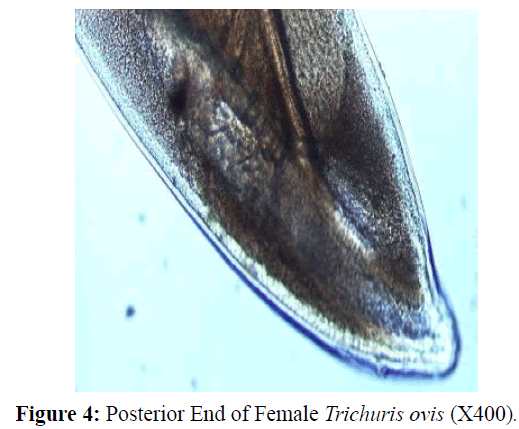
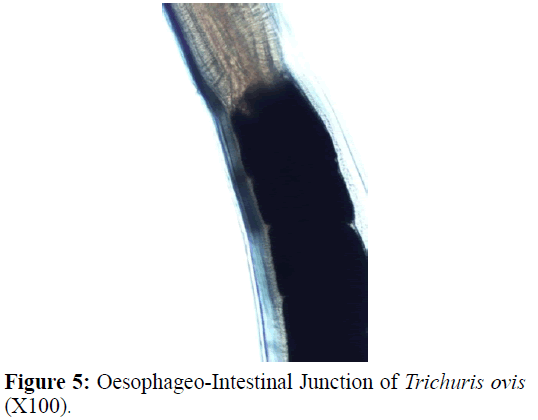
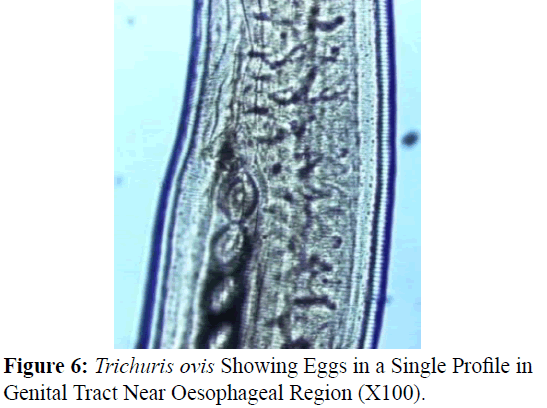
The adult males were 31.25-68.50 mm long and 0.28-0.60 mm in width; with a slender filiform anterior end comprising about three-fourths of the entire body length. Spicule was 4.8-6.0 mm long along with spicular sheath measuring 1.45 mm, covered with spines. The adult females were 51.00-70.50 mm long and 0.32- 0.65 mm in width. Eggs were lemon shaped with plug on both the poles. Buccal cavity was provided with spear like structure, but devoid of lips. The cuticle was transversally striated with wide longitudinal bacillary band. Oesophagus consists of a thin-walled tube surrounded by large unicellular glands, the stichocytes (Figures 1 and 2). In males, the posterior end which was spirally coiled with a single spicule, having slightly expanded proximal and a pointed distal end. Spicular sheath had a slightly stretched globular expansion at its distal end and was covered with closely set spines (Figure 3). In case of female, posterior extremity was slightly curved (Figure 4), but the vulvar opening was situated near the oesophageo-intestinal junction (Figure 5) with genital tract bearing fully developed eggs in a single profile (Figure 6).
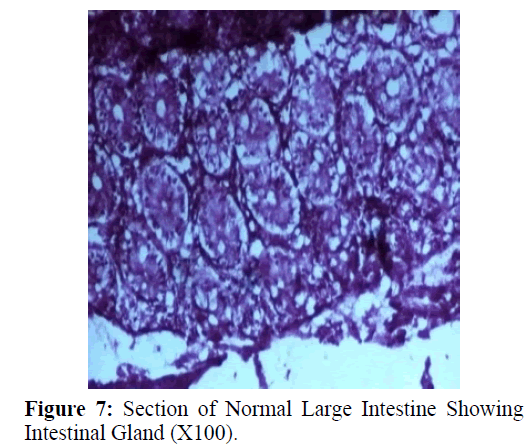
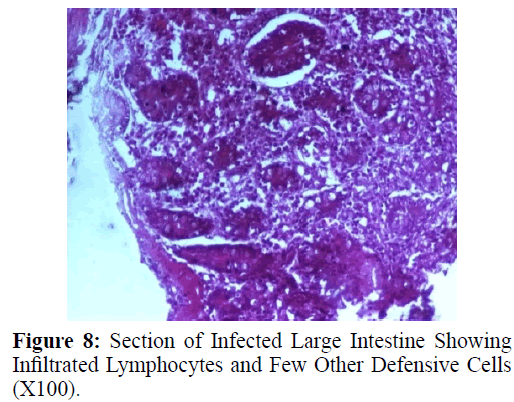
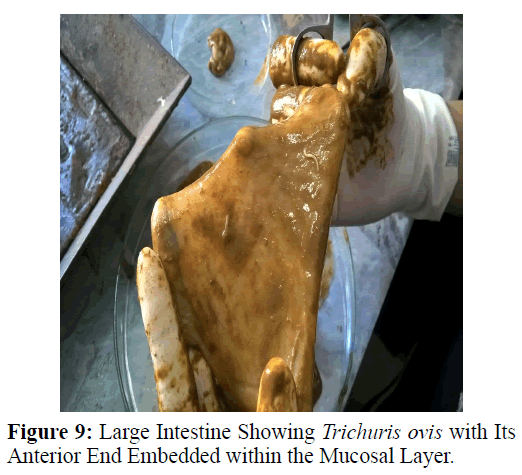
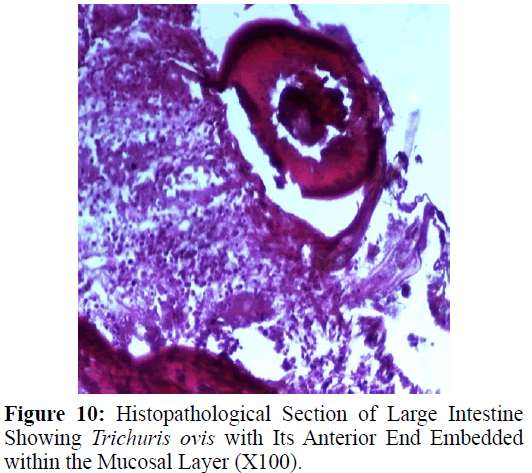
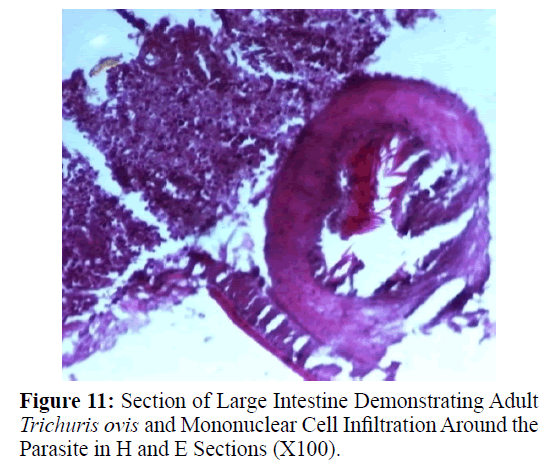
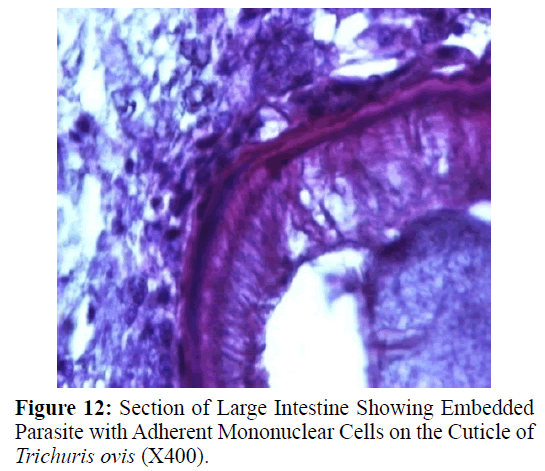
Haematoxylin and eosin stained sections showed intestinal glands (Crypts of Leiberkuhn) and lamina propria (Figures 7 and 8). In the vicinity of worm penetration moderate leucocyte infiltration was also visible. Sections revealed the embedded attenuated anterior end of Trichuris ovis surrounded with fibroblast and leucocytes (Figures 9 and 10). It revealed a cellular infiltration around the embedded worm (Figures 10-12). Worms were neither encapsulated nor calcified as parasite penetrates its anterior end under the mucosa to receive nutrition and withdraw it. But sometimes when it remains embedded for longer duration, cell infiltration increases. Macrophage, neutrophils and lymphocytes were the predominant cell types surrounding the worm. A few adherent leucocytes were also visible on and around the cuticle of T. ovis (Figures 10-12).
Discussion
The pathology is a consequence of the host immune response, which is assumed to be generated to eliminate invading pathogens. Lesions may also be caused by the direct damage from worm attachment or the indirect damage from soluble products released by the worms (Abner et al. 2002). Bacterial invasion takes place at the site of tissue damage which may elicit immune response against the bacteria and aggravate the lesion by enhanced cellular infiltrations (Mansfield, 1996., Urban, 1996). The foremost expression of the host against the parasite invasion reflected by the changes in the formed elements of blood. The leucocyte response vary from host to host against a particular infection, which may also vary with age and frequency of exposure, being more severe in non-specific host while it is feeble in the specific host as an adjustment is made between the host and parasite. Sometimes, it is specific and characteristic and may be taken in conjunction with other findings in diagnosis of a particular species.
In the present study, examination of histological sections revealed anterior region of T. ovis inserted in the lining of the large intestine (caecum) of the host. The nematode was found exclusively under the mucosal layer covered by the apical surface of mucosal epithelium, forming a tunnel. The crypts of Leiberkuhn, submucosa, muscularis mucosae and muscularis externa were clearly differentiated with slight infiltration of mononuclear cells as earlier observed by (Afrin et al. 2016). Infection of Trichuris in large intestine caused thickening of mucosa which was observed during autopsy. Grossly, large intestine showed congestion, haemorrhagic spots, ulcer formation and nodule formation with thickening of the caecal valve. Similar observations were made by earlier workers in different host infected with their respective species of genus Trichuris. It was earlier observed that in trichuriasis, lymphoid nodules on the lamina propria was enlarged from which the parasite may produce some chemical mediators that cause lymphoid proliferation locally (Mohanta et al. 2007). Acute or chronic inflammation at the mucosa of the caecum and sometimes colon was observed in dogs infected with Trichuris vulpis (Soulsby, 1982., Bowman, 2002., Taylor et al. 2007). Trichuris lacerate the tissues with an oral stylet projected through the oral opening within the tunnel under mucosal layer. Anterior end of these whipworms move underneath the epithelium looking for blood and fluids by inserting the stylet into the blood vessels to create blood pools which are ingested.
Histological sections revealed leucocyte infiltration around the worm embedded under the mucosa but there was no sign of rigid encapsulation, fibrosis, and necrosis. This is probably due to the reason that the penetration of anterior end to receive its nutrition is a normal phenomenon and parasite move to new location after a brief period. But since the penetration of parasite inflame the tissues, leucocytes invasion takes place in the vicinity of the parasite in the inflamed areas which was observed in haematoxylin– eosin stained slides in the present study. Most of the infections caused by T. ovis are generally light and asymptomatic as earlier observed by (Soulsby, 1965). In some cases a large number of worms cause a diphtheritic inflammation of the caecal mucosa. T. ovis penetrates the intestinal wall with their anterior parts. Probably during the process of penetration they cause mild to moderate degree of damage in the intestinal surface, resulting in petechial haemorrhages. As the parasitic infection is a long standing encroachment on the intestinal wall, especially in untreated animals, it causes destruction of the lining epithelium where they predominantly inhabit (Soulsby, 1965). Due to this continuous irritation of the adult parasites on the intestinal wall, catarrhal inflammation occurs. That is why goblet cells were increased in numbers and size.
Earlier findings indicate similar but slightly higher accumulation of leucocytes in rats which were experimentally and naturally infected with Litomosoides carinii and Setaria cervi, respectively (Bertram, 1966., Mohan, 1973., Weiner, 1976., Soulsby, 1976., Khatoon et al. 1979). It has been observed that the animals moderately infected with Trichuris ovis cause slight catarrhal inflammation along with the anchored parasites. Petechial haemorrhages on the mucosa along with infiltrated lymphocytes, eosinophils and macrophages were observed in the caecum and colon. Proliferation of goblet cells was also noticed in the intestine of infected animals during the present study as earlier observed by (Kumar et al. 2015). Destruction and desquamation of epithelial lining of the intestine were also detected. This finding is conformed to the findings obtained in T. ovis where tissue invasion and occasional peripheral eosinophilia was observed (Kumar, 1987., Lal, 1987., Saha, 1998., Bhowmik, 1998., Patnode et al. 2014).
During the present study, goat infected with T. ovis showed active hyperaemia, haemorrhages and lymphocytic infiltrations in the mucosa. Similar findings were obtained in dogs infected with T. vulpis where diffuse lymphohistiocytic proliferation in both mucosa and submucosa were observed (Kirkova et al. 2005). After hatching from eggs, the larvae penetrate into small intestine’s mucosa and stayed for about 15 days which probably provoke desquamation of the epithelium, hyperaemia, mucoid dystrophy, eosinophilic infiltration around the larvae. The larvae and mature worm also induce mechanical damage to the mucosa of small and large intestines followed by extensive local inflammation and haemorrhages. Earlier studies indicated that T. suis infection cause pathological changes in the proximal colon which is evidenced by infiltration of eosinophils and mast cells, crypts hyperplasia, goblet cell hyperplasia and general mucosal hypertrophy (Lee et al. 1982., Betts et al. 1999., Koyama et al. 2000., Patnode et al. 2014., Kumar et al. 2015., Saminathan et al. 2015). Infections with parasitic helminths are often associated with intestinal infiltrations of eosinophils and mast cells, which are considered as typical Type 2-skewed immune response. It is generally accepted that nematodes are immunogenic and several immunological responses to T. trichiura have been demonstrated including intestinal mast cell (Cooper et al. 1991., Mishra et al. 2014), eosinophil and neutrophil infiltration (Kaur et al. 2002) and increased production of several Type 2 cytokines (MacDonald et al. 1994., Faulkner et al. 2002., Jackson et al. 2004). Resistance to infection with Trichuris spps in mice is associated with the activation of Th2 cells that produce the cytokines IL-4 and IL-13 (Kringel et al. 2006., Jackson et al. 2004., Cliffe et al. 2004). IL-4 and IL- 13 also stimulate goblet cells which seem true in our study as goblet cells showed an increase in infected tissue.
Examination of histological sections where worms were embedded revealed hyperplasia of lymphoid follicles and lymphocytic infiltration in the submucosa and mucosa of the large intestine infected with T. ovis. Similar hyperplasia of lymphoid follicles and lymphocytic infiltrations were recorded in the infected tissue of rodent infected with T. thrichomysi (Torres et al. 2011). Proximal colon of pigs infected with T. suis showed infiltration and similar histological changes in the proximal colon resulting in infiltration of tunica mucosa and tela submucosa by inflammatory cells, crypt hyperplasia, goblet cell hyperplasia and a general hypertrophy of tunica mucosa (Kringel et al. 2006).
During the present study histological sections revealed leucocyte adherence to worm which were embedded under the mucosa. This is in response to the host defence to restrict the activity of the worm. Macrophages, lymphocytes and neutrophils were the predominant cell types around the embedded worm. Similar cell adhesions have been reported in the plural and peritoneal cavity of white rats and guinea pigs infected with Litomosoides carinii (Mohan, 1973) and Setaria cervi (Khatoon et al. 1979).
References
- Abner, S.R., Hill, D.E., Turner, J.R., Black, E.D., Bartlett, P., Urban, J.F, Mansfield, L.S. (2002). Response of intestinal epithelial cells to Trichuris suis excretory-secretory products and the influence on Campylobacter jejuni invasion under in vitro conditions. J Parasitol,88,738-745.
- Afrin, F., Chowdhury, M.M.R., Saha, S.S., Mohammad, A.R., Mohammad, A.A., Mohammad, K.I. (2016). Efficacy of curcumin against gastrointestinal parasites in goat. EJPMR, 3,158-165.
- Anonymous. (2012).19th Livestock census-2012. All India Report, Department of AnimalHusbandry, Dairying and Fisheries, Ministry of Agriculture, Government of India.
- Beer, R.J. (1973). Morphological descriptions of the egg and larval stages of Trichuris suis (Schrank, 1788). Parasitology, 67,263-278.
- Bertram, D.S. (1966). Dynamics of parasitic equilibrium in cotton rat’s filariasis. Adv Parasitol, 4,255-319.
- Betts, C.J., Else, K.J. (1999). Mast cells, eosinophils and antibody-mediated cellular cytotoxicity are not critical in resistance to Trichuris muris.Parasite Immunol, 21, 45-52.
- Bowman, D.D. (2002). Georgi’s Parasitology for Veterinarians. Saunders Company, 7.
- Cliffe, L.J., Grencis, R.K. (2004). The Trichuris muris system: a paradigm of resistance and susceptibility to intestinal nematode infection. Adv Parasitol,57,255-307.
- Cooper, E.S., Spencer, J., Whyte-Alleng, C.A., Cromwell, O., Whitney, P., Venugopal, S., … MacDonald, T.T. (1991). Immediate hypersensitivity in colon of children with chronic Trichuris trichiura dysentery. Lancet, 338,1104-1107.
- Faulkner, H., Turner, J., Kamgno, J., Pion, S.D., Boussinesq, M., Bradley, J.E. (2002). Age and infection intensity-dependent cytokine and antibody production in human trichuriasis: the importance of IgE. J Infect Dis, 185, 665-672.
- Jackson, J.A., Turner, J.D., Rentoul, L., Faulkner, H., Behnke, J.M., Hoyle, M.,Bradley, J.E. (2004). T helper cell type 2 responsiveness predicts future susceptibility to gastrointestinal nematodes in humans. J Infect Dis, 190, 1804-1811.
- Jenkins, T. (1970). A morphological and histochemical study of Trichuris suis (Schrank, 1788) with special reference to the host–parasite relationship. Parasitology, 61, 357-374.
- Kaur, G., Raj, S.M., Naing, N.N. (2002). Trichuriasis: localized inflammatory responses in the colon. Southeast Asian JTrop Med Public Health,33, 224-228.
- Khatoon, H., Ansari, J.A., Baqui, A. (1979). Fate of transplanted Setaria cervi worms in experimental white rats. Indian J Helminth,31, 8-14.
- Kirkowa, Z., Dinev, I. (2005). Morphological changes in the intestine of dogs experimentally infected with Trichuris vulpis. Bulgarian J Veter Med, 4, 239-243.
- Koyama, K., Ito, Y. (2000). Mucosal mast cell responses are not required for protection against infection with the murine nematode parasite Trichuris muris.Parasite Immunol, 22, 13-20.
- Kringel, H., Iburg, T., Dawson, H., Aasted, B., Roepstorff, A. (2006). A time course study of immunological responses in Trichuris suis infected pigs demonstrates induction of a local type 2 response associated with worm burden. Int J Parasitol, 36, 915-924.
- Kringel, H., Roepstorff, A. (2006).Trichuris suis population dynamics following a primary experimental infection. Vet Parasitol, 139, 132-139.
- Kuchai, J.A., Ahmad, F., Chishti, M.Z., Dar, J.A., Tak, H. (2013). On morphology and morphometry of Trichuris ovis Abildgaard 1795 recovered from ruminants of Ladakh. India. J Buffalo Sci, 2,49-52.
- Kumar, S., Jakhar, K.K., Singh, A.D. (2015). Etio-pathological investigations to study the gross and histopathological lesions affecting gastrointestinal tract of sheep. Academic Journals, 10, 356-361.
- Kumar, S., Lal, S.S. (1987). Oedema due to Trichuris ovis: a case report. Archiva Veterinaria,18, 33-36.
- Lee, T.D., Wakelin, D. (1982). The use of host strain variation to assess the significance of mucosal mast cells in the spontaneous cure response of mice to the nematode Trichuris muris. Int Arch Allergy Appl Immunol, 67,302-305.
- MacDonald, T.T., Spencer, J., Murch, S.H., Choy, M.Y., Venugopal, S., Bundy, D.A., Cooper, E.S. (1994). Immunoepidemiology of intestinal helminthic infections. 3. Mucosal macrophages and cytokine production in the colon of children with Trichuris trichiura dysentery. Trans R Soc Trop Med Hyg, 88, 265-268.
- Mansfield, L.S., Urban, J.F. (1996). The pathogenesis of necrotic proliferative colitis in swine is linked to whipworm induced suppression of mucosal immunity to resident bacteria. Vet Immunopathol, 50,1-17.
- Mishra, P.K., Palma, M., Bleich, D., Loke, P., Gause, W.C. (2014). Systemic impact of intestinal helminth infections. Mucosal Immunology,7, 753-762.
- Mohan, R.N. (1973). Pathological changes in white rats infected with Litomosoides carinii. Trans R Soc Med Hyg,67, 883-884.
- Mohanta, U.K., Anisuzzaman, Farjana T., Das, P.M., Majumder, S., Mondal, M.M.H. (2007). Prevalence, population dynamics and pathological effects of intestinal helminths in black Bengal goats. Bangl J Vet Med, 563-569.
- Panesar, T.S., Croll, N.A. (1980). The location of parasites within their hosts: site selection by Trichuris muris in the laboratory mouse. Inter J Parasitology, 10, 261-273.
- Patnode, M.L., Bando, J.K., Krummel, M.F., Locksley, R.M., Rosen, S.D. (2014). Leukotriene B4 amplifies eosinophil accumulation in response to nematodes. J Exp Med,211,1281-1288.
- Saha, S.B., Bhowmik, M.K. (1998). Pathomorphological changes in spontaneous trichuriasis in goat. Ind J Ani Health, 37, 37-38.
- Saminathan, M., Gopalakrishnan, A., Latchumikanthan, A., Milton, A.A.P., Aravind, M., Dhama, K., Singh, R. (2015). Histopathological and parasitological study of blood sucking Haemonchus contortus infection in sheep. Adv Anim Vet Sci, 3, 99-108.
- Soulsby, E.J.L. (1965). Textbook of Veterinary Clinical Parasitology. Vol. I - Helminths. Blackwell Scientific Publications Ltd., Oxford, UK p:1120.
- Soulsby, E.J.L. (1982). Helminths, Arthropods and Protozoa of Domesticated Animals(7thedn.) ELBS and Bailliere Tindall, London.
- Taylor, M.A., Coop, R.L., Wall, R.L. (2007). Veterinary Parasitology, Blackwell Publishing.
- Torres, E.J.L., Nascimento, A.P.F., de Souza, W. (2011). A new species of Trichuris from Thrichomys aeperoides (Rodentia:Echimydiae) in Brazil:Morphological and histological studies.Vet Parasitol Elsevier,176,226-235.
- Weiner, D.J., Soulsby, E.J.L. (1976). Fate of Litomosoides carinii adults transplanted into the pleural or peritoneal cavity of infected and naive multimammate rats (Mastomys natalensis),62,886-893.