Research Article - Biomedical Research (2017) Volume 28, Issue 1
Modification of blood pressure and vascular reactivity to angiotensin II in the perfused heart of hypertensive rats treated with hyperbaric oxygenation
Emilio M. Lopez-Calderon1, Gustavo Guevara-Balcazar1,2, Horacio Osorio-Alonso3, Eleazar Lara-Padilla1,2,3, Enrique Hong-Chong4, Israel Ramirez-Sanchez1 and Maria C. Castillo-Hernandez1,2*1Instituto Politecnico Nacional, Escuela Superior de Medicina, Seccion de Estudios de Posgrado e Investigacion, Plan de San Luis y Diaz Miron s/n, Col Casco Sto Tomas, Mexico
2Latin American College for Hyperbaric and Subaquatic Medicine, Mexico City
3Instituto Nacional de Cardiologia, Calle Juan Badiano 1, Tlalpan, Belisario Dominguez Sección XVI, 14080 Ciudad de México
4CINVESTAV-IPN, Calz. de los Tenorios No. 235, Col. Granjas Coapa, México
- *Corresponding Author:
- Maria C Castillo-Hernandez
Instituto Politecnico Nacional. Escuela Superior de Medicina
Seccion de Estudios de Posgrado e Investigacion, Mexico
Accepted date: May 05, 2016
Abstract
Introduction: Systemic hypertension (SHT) leads to high morbidity and mortality. Since the reninangiotensin system (RAS) represents one mechanism of regulating blood pressure, some first-line drugs target it to treat SHT. However, even with drug treatment some functional and structural changes continue to occur, leading to further complications. Hence, new coadjuvant treatments are needed that can result in more favorable outcomes. HBO (administration of 100% oxygen) at a pressure of 2 absolute atmospheres improves smooth vascular muscle relaxation and favors neovascularization. The aim of the present study was to analyze whether HBO can modify the response to angiotensin II at the cardiovascular level in hypertensive rats.
Materials and methods: male Wistar rats (340 ± 20 g) maintained under standard lab conditions, SHT was induced with surgery. Beginning on day 8 post-surgery, HBO therapy was given for 20 sessions (one/ day). Rats were then sacrificed and the heart extracted to analyze the vascular and cardiac response to angiotensin II (1 × 10-9 to 1 × 10-5 M) and determine the expression of AT1R and AT2R in the different groups.
Results: The systolic BP was 150 ± 10 mmHg in hypertensive rats without HBO as well we have an increase in vascular response in vascular reactivity, while in those with HBO therapy a reduction was found in this parameter as well as in vascular reactivity to angiotensin II in the heart and aorta.
Conclusions: HBO therapy reduced vascular reactivity to angiotensin II in both aortic segments and in coronary arteries of hypertensive rats.
Keywords
Hypertension, Hyperbaric oxygen, Heart, Thoracic aorta, Angiotensin II, Vascular reactivity.
Abbreviations
BP: Blood Pressure; HBO: Hyperbaric Oxygen; ANG II: Angiotensin II; RAS: Renin-Angiotensin System; HT: Hypertension; receptor AT1R and AT2R
Introduction
Due to its prevalence, physiopathology and mortality [1], hypertension (HT) is one of the most important chronic conditions. It is the main risk factor for the development of strokes, cardiac ischemia, renal failure and heart failure [1,2].
The physiopathology of HT is complex and implies modifications of multiple organs and systems, within which the renal and cardiac systems are of utmost importance. Modifications of the latter two systems lead to changes in the secretion of systemic amines and mineral corticoids, as well as in the stimulation of the synthesis of pro-hypertensive substances such as those produced by the renin-angiotensin system (RAS) [3].
Control of cardiac activity by the RAS is mainly exercised by AT1 receptors (AT1R), which participate in the regulation of physiological and structural processes in the heart [3,4]. In SHT the deregulation of the RAS results in several complications, including a rise in blood pressure (BP) and hypertrophy of the left ventricle, the latter accompanied by necrotic zones of myocytes leading to myocytolysis. Additionally, there are histological and anatomical changes [3], such as the proliferation of fibroblasts and an increase in α- actin, β-BHC, ANF, fibronectin, TGF–β and collagen I and III, which cause hyperplasia of the left ventricle and hardening of heart muscle tissue [1,5-8].
One of the most common drug strategies used to treat SHT is the modulation of the RAS through enzymatic inhibitors and blockers of At1Rs. [3]. In spite of the multiple advantages of such treatment, 30% of patients continue to suffer from chronic complications of SHT. For this reason, coadjuvant treatments have been sought for the control of this pathology and its complications.
Hyperbaric oxygen (HBO) therapy has recently been included in the treatment of SHT. This therapy is given inside a chamber with 100% oxygen supplied above atmospheric pressure, which produces a mechanical effect (due to the increased pressure to which the organism is exposed) and a volumetric effect (caused by the increase in the partial pressure of oxygen in tissues) [9].
The modifications in the posterior circulatory system caused by HBO therapy include a 10-15% decrease in heart rate, apparently secondary to an increase in parasympathetic muscular tone and in the oxygenation of tissues. Consequently, there is a reversal of hypoxia in the brain, heart, kidneys and other organs as well as the stimulation of both angiogenesis and the production of nitric oxide (NO) [10-18].
Although HBO has shown a therapeutic effect when used for various pathologies, its mechanism of action is not completely clear. To provide more information in this respect, the aim of the present study was to determine the participation of the RAS in the cardiovascular response to HBO treatment (a total of 20 sessions) using a rat model of SHT induced by surgery.
Materials and Methods
Animals
Male Wistar rats (280-320 g, 16 weeks old) were obtained from the bioterium of the Escuela Superior de Medicina, Instituto Politécnico Nacional and maintained under standard lab conditions of temperature and circadian rhythm with free access to food and water. Six groups (n=6) were formed: the healthy control with and without HBO, the sham control with and without HBO, and hypertensive rats with and without HBO.
The model of hypertension
The PAGE technique was used to produce hypertension in rats. This technique is based on provoking perirenal fibrosis by using a physical irritant, which is placed in the animal through surgery. Abdominal surgery involved the removal of the parietal and visceral peritoneum to expose the kidneys. Each kidney was wrapped with a 4 × 4 cm sheet of cellophane, which was held in place by cotton thread. Then the abdominal wall was closed and the skin stitched. Seven days post-surgery, a pressure transducer was placed on the tail of the animals in order to detect the pulse of the caudal artery and therefore determine systolic BP. The irritation caused by the cellophane leads to an inflammatory reaction around the kidneys, which generates a fibrin capsule that covers these organs. This layer puts pressure on the kidneys and thus provokes an increase in their peripheral resistance [19,20]. Sham-operated animals only had manipulation of the kidneys.
Exposure to hyperbaric oxygenation
Three groups were exposed to hyperbaric oxygenation (healthy, sham and hypertensive rats) during 20 one-hour sessions at a pressure of 2 ATA in an experimental hyperbaric chamber (MISSA).
Langendorff method
Rats were sacrificed by decapitation after being anesthetized with 60 mg/kg of sodium pentobarbital administered ip. A thoracotomy was performed to expose and isolate the heart, and a cannula was mounted 3 mm from where the aorta is attached. The cannula was connected to a system with a constant flow (at 12 ml/min) of Krebs solution (in g/L): NaCl 6.9 g/L, KCl 0.35 g/L, KH2PO4 0.16 g/L, MgSO4 0.3 g/L, CaCl2 0.37 g/L, Na2HCO2 2.1 g/L, dextrose 2.11 g/L, and calcium disodium EDTA 0.01 g/L. The solutions was maintained at a constant temperature of 37°C and a pH of 7.4 and bubbled with a mixture of 95% O2 and 5% CO2. A pair of stimulating electrodes, made of small stainless, steel wire, were placed 2 mm apart in the right atrium. Pacing was achieved by applying electrical threshold at a rate of 4.5 ± 0.3 hz. A Biopac system TSD104A pressure transducer was used to measure changes in flow. With this procedure, measurements were made of BP as well as perfusion in the coronary arteries. Once the heart was stabilized in this system, a dose-response curve was established to determine the effect of angiotensin (ANG) II (1 × 10-9 to 1 × 10-5 M), each dose injected 30 μl/min for 5 minutes.
Isolated organ chamber with thoracic and abdominal aortic rings
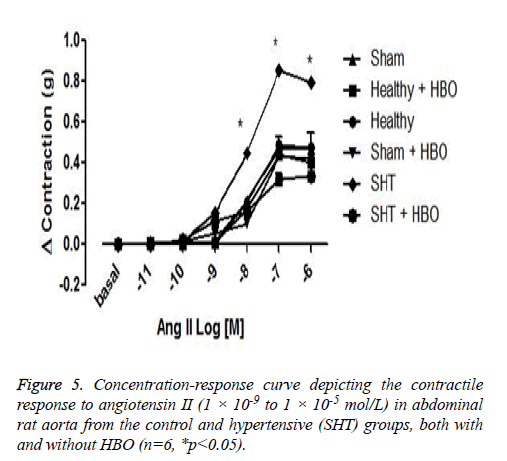
After sacrificing animals by decapitation (previously anaesthetized with pentobarbital at 60 mg/kg), the thoracic and abdominal portions of the aorta were extracted. The thoracic aorta was excised from the diaphragm to the aortic arch, and the abdominal aorta from the diaphragm to the iliac artery. Aortic segments were immediately submerged in cold Krebs solution to remove all adjacent connective tissue. The thoracic and abdominal segments were cut into aortic rings (4-5 cm long), each mounted on two stainless steel hooks in an isolated organ chamber. One of the hooks was fixed to the bottom of the chamber and the other to a transductor linked to a Biopac System apparatus for registering changes in tension. The isolated organ chamber contained 10 ml of Krebs bicarbonate solution with the following composition (in g/L): NaCl 6.9 g/L, KCl 0.35 g/L, KH2PO4 0.16 g/L, MgSO4 0.3 g/L, CaCl2 0.37 g/L, Na2HCO2 2.1 g/L, dextrose 2.11 g/L, and calcium disodium EDTA 0.01 g/L. The chamber was maintained at a constant temperature of 37°C and pH of 7.4, and was continuously bubbled with a mixture of 95% O2 and 5% CO2. The rings were given an initial tension of 2 g and then left to stabilize for 2 h, during which time viability tests were conducted with phenylephrine (10-6 mol/L) to determine the contractile response of the tissue to acetylcholine (10-6 mol/L) and thus assure the integrity of the endothelium. Afterwards, dose-response curves were constructed for ANG II (1 × 10-11 to 1 × 10-7 mol/L) by measuring the response in grams of force.
Western blot
Approximately 20 mg of thoracic or abdominal aorta were homogenized with a polytron device (PT 1200 E, Kinematica Technology, INC, NY, USA) in 200 μl of denaturing lysis buffer (1% triton X-100, 20 mM Tris, 140 mM NaCl, 2 mM EDTA, and 0.1% SDS) with protease and phosphatase inhibitor cocktails (P2714 and P2850, Sigma-Aldrich, St. Louis, Missouri) supplemented with 0.15 mM PMSF, 5 mM Na3VO4 and 3 mM NaF. Homogenates were sonicated for 30 min at 4°C and centrifuged (12,000 g) for 15 min. The total protein content was measured in the supernatant using the Bradford method. A total of 40 μg of protein was loaded into a denaturing 4%-15% TGX precast polyacrylamide gel (SDSPAGE) (Bio-rad), electrotransferred into a polyvinylidene fluoride membrane (PVDF Immobilon-P, Millipore) at 12 V, 50 minutes, using a Trans-Blot Semidry system from Bio-rad. Then, incubated for 1 h in blocking solution (5% nonfat dry milk in TBS plus 0.1% Tween 20 [TBS-T]), followed by overnight incubation at 4°C with primary antibodies. Rabbit polyclonal anti- AT1 (Abcam cat. Ab18801; 41kDa), rabbit polyclonal anti-AT2 (Abcam cat. Ab9244; 40kDa) and anti-β- actin rabbit polyclonal primary (Lab Novus Biological cat. NB 110-55433) antibodies were diluted in TBS-T plus 5% nonfat dry milk. Membranes were washed (3X for 5 min) in TBS-T and incubated 1 h at room temperature in the presence of HRPconjugated secondary antibodies (Anti-rabbit IgG, Novus Biological Lab, Cat. NB7156) HRP-linked antibodies Cell Signaling diluted 1:10,000 in blocking solution. Membranes were again washed 3 times in TBS-T, and the immunoblots were developed using an ECL Plus detection kit (Amersham- GE). The band intensities were digitally quantified using ImageJ software (http://www.nih.gov).
Statistical analysis
All values represent the mean ± SEM of the rats in each group (n=6). To obtain the maximum effect (Emax), a non-linear regression adjustment was made for the concentration-response curves. The comparison between groups with and without HBO was made with two-way ANOVA, with the post-hoc Bonferroni test employed to determine statistical significance. In all cases, statistical significance was considered with p<0.05.
Results
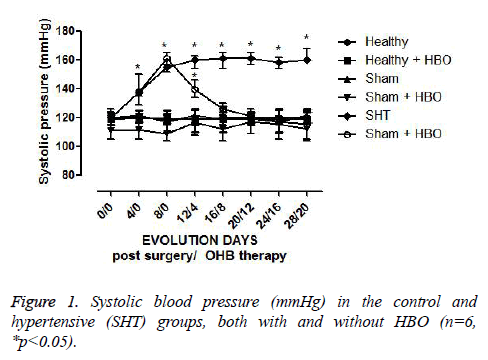
The rats in the healthy and sham groups without HBO therapy did not show any change in systolic BP (120 mmHg ± 7 mmHg) (Figure 1). There was no significant difference in this parameter between healthy rats with and without HBO therapy. During the period (one week) of recovery from surgery, hypertensive rats developed a systolic BP of 150 mmHg ± 5 mmHg, which with posterior HBO therapy decreased until becoming normal. This decrease began with the first HBO session and was observed until the eighth session, at which time there was a systolic BP of 120 ± 5 mmHg, which remained stable until the end of the study. Hence, from the eighth to the twentieth HBO session, there was no significant difference between the systolic BP level in hypertensive rats with HBO and that observed in the healthy control group without surgery or HBO (Figure 1).
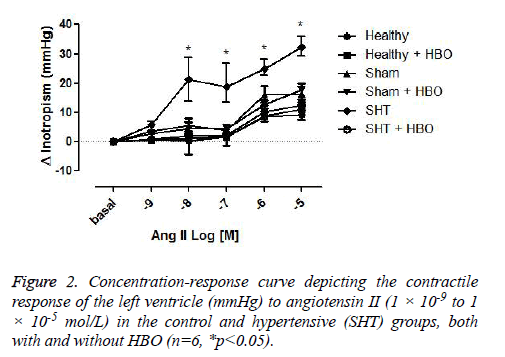
The contractile force of the left ventricle in response to ANG II (1 × 10-9 to 1 × 10-5 M) was similar in the healthy control and sham groups with and without HBO. However, this parameter was significantly greater in the group of hypertensive rats without HBO compared to the healthy control (p>0.001) (Figure 2).
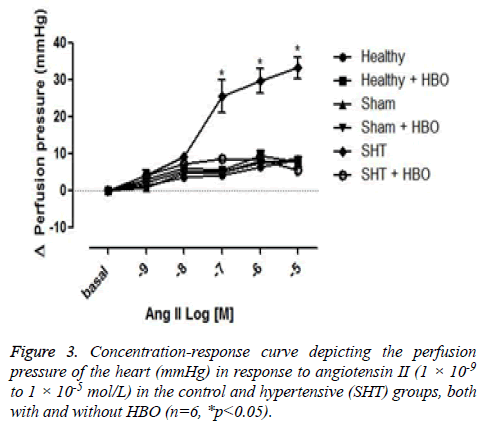
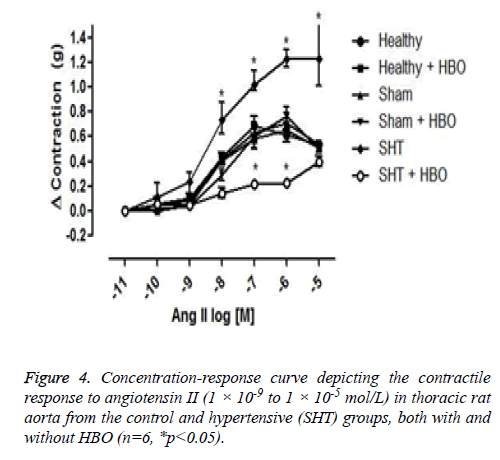
No significant difference was observed between the coronary perfusion pressures of five of the groups: the healthy control group with and without HBO, the sham group with and without HBO, and the hypertensive group with HBO. However, the hypertensive group without HBO showed a significant increase in perfusion pressure (32 ± 7 mmHg) compared to the other groups (p>0.001) (Figure 3). On the other hand we could observe differences in the contractile response to angiotensin II in the thoracic rat aorta from the group SHT + HBO vs. Control and SHT (Figure 4). However abdominal aorta exhibits an effect similar like the coronary perfusion pressure (Figure 5).
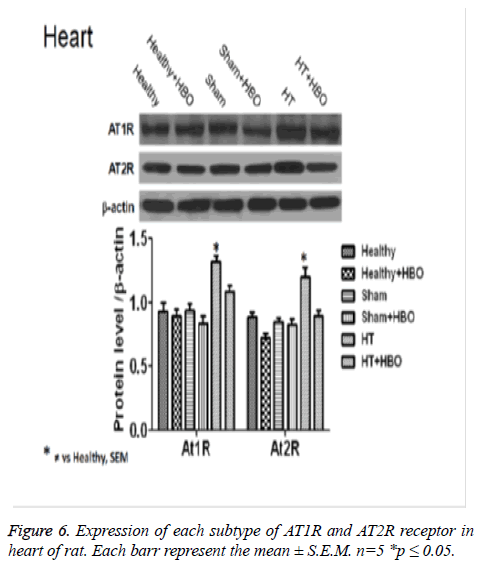
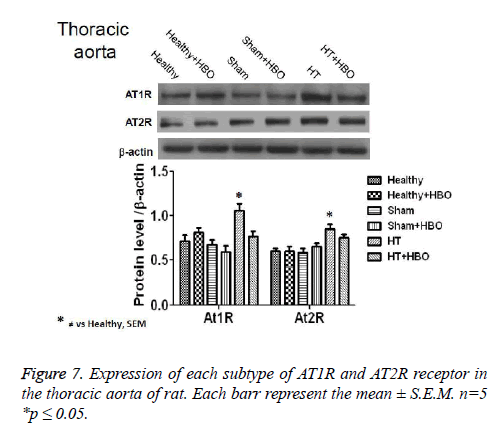
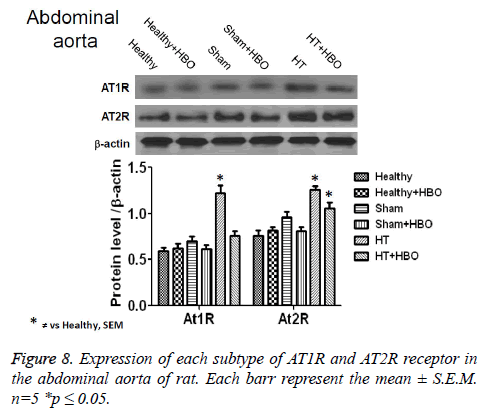
Finally, we observe an increase in the expression of AT1R and AT2R receptor in heart (Figure 6), thoracic aorta (Figure 7) and abdominal aorta (Figure 8) of rat in the group with SHT and a decrease in the expression of this receptors after the HBO therapy.
Discussion
The aim of the present study was to determine whether the RAS participates in the changes produced by HBO therapy in a rat model of SHT. The BP of hypertensive rats measured 7 days post-surgery was 150 mmHg. However, HBO therapy without the use of drugs proved to modulate the BP of hypertensive rats. These results are in accordance with Nagamoto et al. [21], who reported that SHT rats treated with HBO showed a reduction in systolic and diastolic BP. Other authors, including Thomensen (2002) and Thom (2009) attributed this effect to an increase in the non-enzymatic production of NO, which could explain part of the therapeutic effects of HBO [22,23].
Contrarily, Demenchenko et al. reported that there was no improvement in BP with HBO therapy. However, this study was carried out under different conditions [24]. Only one HBO session was used, this at a pressure of up to 3 ATA. It is worth noting that different associations of hyperbaric medicine around the world have recommended a minimum of 10 sessions for the optimum use of HBO therapy. This has been corroborated in our previous lab studies, in which we demonstrated that at least 15 sessions of HBO are necessary to be able to obtain an optimal concentration of O2 in tissues and thus to have an effect on the cardiovascular apparatus.
In the present study, the contractile response to stimulation with ANG II was significantly greater in the hypertensive rats without HBO compared to the healthy group without HBO. Some authors have found this same effect, attributing it to an increase in the number of active AT1R during hypertension [5,22-28]. On the other hand, among hypertensive rats the contractile response to ANG II was lesser in those with than without HBO. This could be due to a lower reactivity to ANG II, probably as a result of a reduction in the number of AT1Rs. However, to date there are no studies that refer to the effects of HBO on the RAS. Only one report, to our knowledge, has any relation to this question. Gregorovich et al. found that HBO therapy improved the general contractile function of skeletal muscle. This effect was attributed to better oxygenation of muscle tissue and a generalized vasodilation [29].
A change in the vascular reactivity of coronary arteries to different stimuli is another aspect that was analyzed in the present study. The contractile response to ANG II increased in hypertensive rats, resulting in a decrease in oxygenation. This effect caused cardiac hypertrophy, which was reversed after 20 HBO sessions. As occurred herein with cardiac hypertrophy, HBO may reverse other acute complications of SHT.
To evaluate the changes in resistance of coronary arteries, we determined the changes in perfusion pressure, observing that HBO sessions diminished reactivity to ANG II. This effect may be due to the blocking of AT1Rs, which would cause a reduction in BP. Indeed, in a study by Androwiki et al. treatment with losartan reduced BP in hypertensive rats as a result of a decrease in At1Rs [30].
After 20 HBO sessions in the present study, hypertensive rats showed a decrease in the expression of AT1Rs and AT2Rs in heart and thoracic aorta. These changes were attributed to the reduction in BP caused by modifications in vascular reactivity in the aorta as well as the coronary arteries of the heart. Such modifications, when they take place in hypertensive patients, lead to clinical improvement. However after 20 HBO sessions we do not have any chage in the expression of AT2Rs, this point is very interesting because of we have a decrease in the expression of this receptors in the heart and thoracic aorta [26,27,31].
Conclusions
Due to the high morbi-mortality of systemic hypertension (SHT), new therapies are constantly being developed to reduce complications by controlling BP. Since the renin-angiotensin system has an important role in the development of SHT, the aim of this study was to analyze whether HBO treatment can diminish SHT by modifying vascular reactivity to angiotensin II in coronary arteries. The current results lead to the conclusion that HBO therapy reduced BP and decreased vascular reactivity to angiotensin II in the coronary arteries of hypertensive rats. These changes were associated with a decrease in the expression of AT1Rs.
Acknowledgements
This work was supported by the SIP project (Escuela Superior de Medicina, IPN) and COFAA.
References
- Ushio-Fukai M, Griendling KK, Becker PL, Hilenski L, Halleran S. Epidermal growth factor receptor transactivation by angiotensin II requires reactive oxygen species in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol 200; 21: 489-495,.
- Oscar CA, Oparil S. Essential Hypertension: Part I: Definition and Etiology. Circulation 2000; 101: 329-335.
- OMS. Guidelines for the treatment of hypertension. 2006
- Takahashi H, Yoshika M, Komiyama Y, Nishimura M. The central mechanism underlying hypertension: a review of the roles of sodium ions, epithelial sodium channels, the renin–angiotensin–aldosterone system, oxidative stress and endogenous digitalis in the brain. Hypertension Research 2011; 34, 1147–1160
- Schieffer B, Schieffer E, Hilfiker-Kleiner D, Hilfiker A, Kovanen PT, et al. Expression of angiotensin II and interleukin 6 in human coronary atherosclerotic plaques: potential implications for inflammation and plaque instability. Circulation 2000; 101: 1372-1378.
- Kim S, Ohta K, Hamaguchi A. Yukimura T, Miura K. Angiotensin ii induces cardiac phenotypic modulation and remodeling in vivo in rats. Hypertension 1995; 25; 1252-1259.
- Force T, Pombo CM, Avruch JA, Bonbentre JV, Kyriakis JM. Stress-activated protein kinases in cardiovascular disease. Circ res. 78; 1996; 947-953.
- Sugden PH, Clerk A. “Stress-responsive” mitogen-activated potein kinases (c-jun n-terminal kinases and p38 mitogen-activated protein kinases) in the myocardium. Circ Res 83; 1998; 345-352.
- Wang Y, Su B, Sah VP, Brown JH, Han J. Cardiac hypertrophy induced by mitogen-activated protein kinase 7, a specific activator for c jun nh-2 terminal kinase in ventricular muscle cells. J Biol Chem 1998; 273: 5423-6426.
- Subbotina N, Camaras Hiperbaricas. Cap 1, Mecanismo de Acción del Oxigeno Hiperbárico. Altuna Impresores, 2006.
- Wattel F, Mathieu D, Coget JM, Millard V. Hyperbaric oxygen therapy in chronic vascular wound management. Angiology 1990; 41: 59-61.
- Arzumanian V, Stankevicius E, Laukevictene A, Kevelatis E. Mechanism of nitric oxide regulation of superoxide and peroxynitrite-nitrogen-containing oxidized lipid derivatives. J Biol Chem 1994; 269: 26066-26075.
- Thom SR, Bhopale VM, Velázquez OC, Goldstein LJ, Thom LH. Stem cell mobilization by hyperbaric oxygen. Am J Physiol heart Circ Physiol 2005; 290: H 1378-1386.
- Xenos ES, Stevens SL, Freeman MB, Cassada DC, Goldman MH. Nitric oxide mediates the effect of fluvastatin on intercellular adhesion molecule-1 and platelet endothelial cell adhesion molecule-1 expression on human endothelial cell. Ann Vasc Surg 2005; 19: 386-363.
- Gonzalez F, Lorente Balanza JA. Orientación tisular y sepsis. Med Intensiva 2005; 29: 178-184.
- Allen BW, Stamler JS, Piantadosi CA. Hemoglobin, nitric oxide and molecular mechanisms of hypoxic vasodilation. J molmed 2009; 15: 452-460.
- Hodges A N H, Delaney S, Lecomte JM, Lacroix VJ, Montgomery DL. Effect of hyperbaric oxygen on oxygen uptake and measurements in the blood and tissues in a normobaric environment. Br J Sports Med 2003; 37: 516-552.
- Thom SR. Effects of hyperoxia on neutrophil adhesion. Undersea hyperb Med 2004; 31: 123-131.
- Page IH. The production of persistent arterial hypertension by cellophane perinephritis. J Am Med Ass 1939; 113: 2046-2048.
- Vanegas V, Ferrebuz A, Quiroz Y, Rodríguez-Iturbe B. Hypertension in Page (cellophane-wrapped) kidney is due to interstitial nephritis. Kidney Int 2005; 68: 1161-1170.
- Nagatomo F, Fujino H, Takeda I, Ishihara A. Effects of hyperbaric oxygenation on blood pressure levels of spontaneously hypertensive rats. Clin Exp Hypertens 2010; 32: 193-197.
- Thomensen K, Inger R, Lauritzen M. NO-and non NO-non-prostanoid-dependent vasodilatation in ratic sciatic nerve during maturation an developing experimental diabetic neuropathy. J of Physiol 2002; 543: 977- 993.
- Thom SR. Oxidative stress is fundamental tl hyperbaric oxygen therapy. J Appl Physiol 2009; 106: 988-995.
- Demchenko I, Zhilyaev SY, Moskvin AN. Baroreflex-mediated cardiovascular responses to hyperbaric oxygen. J Appl Physiol 2013; 115: 819-828.
- Yamamoto E, Kataoka K, Shitaku H, Yamashita T, Tokuyomi Y. Novel mechanism and role of Angiotensin II induced vascular endothelium injury in hypertensive diastolic heart failure. Aeterioscler Thromb Vasc Biol 2007; 27: 2569-2575.
- Higuchi S, Ohtsu H, Suzuki H, Shiirai H, Frank GD. Angiotensin II signal transduction through the At1 receptor: novel sinsights into mechanism and pathophysiology. Clin Science 2007; 112: 417-428.
- Mehta P, Griendling K. Angiotensin II cell signaling: physiological and pathological effects in the cardiovascular system. Am J Physiol cell Physiol 2006; 292: C82-C97.
- Satou R, Gonzalez-Villalobos R. JAK-STAT and the renin-angiotensin system. The role of the JAK-STAT pathway in blood pressure and intrarenal renin-Angiotensin System regulation. JAKSTAT 2012; 1: 250-256.
- Gregorovic P, Lynch G, Williams D. Hyperbaric oxygen imprives contráctil function of regeneration rat skeletal muscle after myotoxic injury. J Appl Physiol 2000; 89: 1477-1482.
- Androwiki, Camargo L, Sartoretto K. Ribeiro CI. Protein disulfide isomerase expression increases in resistance arteries during hypertension development. Effects on Nox1 NADPH oxidase signaling. Front Chem 2015; 27: 3:24.
- Herrera M, Sparks MA, Alfonso-Pecchio AR, Harrison-Bernard LM, Coffman TM. Lack of specificity of commercial antibodies leads to misidentification of angiotensin type 1 receptor protein. Hypertension. 2013; 61: 253-258.