Research Article - Biomedical Research (2016) Volume 27, Issue 4
ln vitro antioxidant and anti-herpes activities of Cuminum cyminum seeds extract
Hamed A. El-Serehy1,2*, Fahad A. Al-Misned2, Rizwan Irshad3 and Mohamed M. Ismail1
1Department of Marine Science, Faculty of Science, Port Said University, Port Said, Egypt
2Zoology Department, College of Science, King Saud University, Riyadh, Saudi Arabia
3College of Science, King Saud University, Riyadh, Saudi Arabia
Accepted date: April 14, 2016
Abstract
The curing of herpes simplex viruses 1 (HSV-1), and 2 (HSV-2) infections with the currently available synthetic therapies as Acyclovir often leads to the problems such as drug resistance and latency duration of the virus. However, studies based on alternative medicines showed that some of the natural products including medicinal plants have a therapeutic application and can be used as an antiviral agent. Thus, the current experiment aims to evaluate the antiretroviral activity of Cuminum cyminum seeds extract. In addition, the cytotoxicity and the antioxidant property of the extract of this plant using the DPPH free-radical assay were performed. The present study revealed that the seeds of C. cyminum contained a total phenolic content of 22.08 μg/mg (gallic acid equivalent dried extract) whereas, the flavonoid concentration was found to be 15.81 μg/mg (quercetin equivalents of flavonoids/mg dried extract). Furthermore, Cuminum cyminum seeds methanolic extract at 4 μg/ml provided 61% inhibition of plaque of HSV-1 and 49% inhibition of plaque of HSV-2. In addition, the results indicated that 137.4 μg/ml of the extract expressed 50% DPPH radical scavenging activity, thence suggesting the potential use of this seeds for treatment of the infection by these two viruses.
Keywords
Antiretroviral, Antioxidant, Cuminum cyminum, Herpes simplex virus.
Introduction
Natural products provide researchers with an effective substitute for the designing of new therapeutic agents against a variety of ailments including viral infections since the very early human history. A large number of crude extracts and pure compounds have been reported for their potent effect on human health. The antiviral activity for some of the medicinal plants has been established and the researchers attribute the virus-inhibitory activity to the presence of polyphenol compounds, apart from others. Herpes simplex viruses (HSVs) are highly contagious human pathogens belong to family Herpesviridae, Subfamily Alphaherpesvirinae, genera of Simplexvirus [1] categorized into two types: herpes type 1 (HSV-1, mainly causing oral herpes and more widespread in human infection) and herpes type 2 (HSV-2, responsible for, apart from others, genital herpes). A recent review gives an insight into the pathogenesis and life cycle and other parameters of HSVs infection. The infection by HSV-1, might lead to fever blisters, and the first symptoms of this virus infection are flu-like with fever, accompanied with itching and finally painful papules [2]. The real problem with this virus infection is not about the painful papules, it is about the ability of this virus to remain latent in the nervous tissue for a potential; recurrent infection after the pathogen gets activated from the sensory neurons. This recurrent infection, or virus reactivation, is usually triggered by local and systemic stimuli like exposure to radiation, and other related factors such as sunlight, oxidative stress and menstruation [3]. HSV-2 infection is accountable for serious neurological morbidity, and probably more than many other viruses [4]. According to some studies, around 85% to 90% of patients suffering from HSV-2 are unrecognized and therefore remain undiagnosed [4-5]. Consequently, patients who carried the virus can spread the disease without even knowing it. The virus (type 1 and 2) is prevalent in ~90 human populations causing oral and genital herpes, eye infections and infections of mucocutaneous zone. To add to the severity of the disease, the viruses infect the human population across geographic regions, the races, gender and age groups (even prevalence of neonatal herpes is very significant). A variety of ways the virus is communicated poses serious threats to human subjects especially in developing countries with a much higher burden of type-2 infections. Though not a cure, acyclovir, famciclovir and valacyclovir have been the routine treatment for relief and the patient compliance however; resistant viruses in immunocompromised subjects are reported in some studies [2,6]. These factors, together with the prolonged viral shedding and more severe disease recurrences suggest a need of alternate therapy with wide acceptance of the patients. Parallel with the viral infections and other ailments, the oxidative species have a significant role in health and pathophysiological conditions and hence justify the antioxidant research. The scavenging activity of an antioxidant moiety is measure of the potency of defense against these reactive species. Avoiding expensive, time consuming and equipment dependent direct quantification of radical scavenging activity is achieved by employing DPPH, a proven radical and scavenger for other radicals.
Natural products have been considered traditionally as a rich source of remedies with various chemical structures and bioactivities against a number of diseases, including viral infection as well as to cater the presence of highly reactive oxidant species. There is a growing acceptance for use of herbal medicines to meet the primary health care needs across the world [6]. Therefore, exploring newer plant derived antivirus agents with antioxidants potentiality may contribute to manage the increasing problem of viral infection management with fewer drug resistance and toxicity. In this regard, cumin (C. cyminum L.) may show promising activity in different health issues. The aromatic compounds of cumin have attracted the attention of researchers across the world to validate and quantify its medicinal uses. Johri has taken the stock of available literature highlighting the use and practice of Cumin in various indigenous healing systems.
The cumin plant (C. cyminum) belongs to the family Apaiaceae, and is considered one of the most widely cultivated aromatic plants in Afro-Eurasia. Naturally, it is native to south west asia and Middle East growing to about 18 inch height, with branched stem pinnate leaves and flowers arranged in a flat umbel. It is considered as one of the oldest herbs cultivated by man across the world. The major active ingredients observed in cumin seeds are cuminaldehyde (4- isopropylbenzaldehyde; C10H12O), limonene (C10H16), α- and β-pinene (C10H16), 1,8-cineole (C10H18O), o- and p-cymene (C10H14), α- and γ-terpinene (C10H16), safranal (C10H14O) and linalool (C10H18O) [7]. Cumin has shown significant antioxidant property in several test methods including 1,1- diphenyl-2-picrylhydrazyl (DPPH) radicals and lipid peroxidation scavenging methods [8-9]. In folk medicine, the cumin seeds are in prescriptions to treat a wide variety of pathogenic and nonpathogenic diseases and other disorders including but not limited to epilepsy, tooth pain, stomach disorders, diarrhea, prevents bloating etc [10-13]. Furthermore, the essential oils extracted from cumin seeds showed a potential antibacterial activity [14]. Therefore, the present work was initiated to examine the anti-Herpes Simplex (Types 1 and 2) activity of C. cyminum seeds extract.
Materials and Methods
Preparation of seeds for methanol extract
One hundred grams of C. cyminum seeds were collected randomly from local market in Cairo, Egypt, collected in sterilized polyethylene bags and stored until use. The sample was ground into fine powder. The obtained powder was percolated with 100% methanol (500 ml) and kept in a shaking incubator (120 rpm) for 48 h at 40°C followed by centrifugation at 10000 rpm for 10 minutes. The supernatants separated using filter paper and allowed the filtrate to evaporate for complete dryness using a rotary evaporator. The percentage of phenolic compounds and flavonoids were determined in the C. cyminum seeds extract using standard methods as described by Abdel Moneim and El-Khadragy [15].
DPPH radical scavenging assay
The present study evaluated the antioxidant activity of C. cyminum seeds extract (CcSE) by employing Diphenylpicrylhydrazyl (DPPH) radical scavenging assay according to the methods described by Abdel Moneim [16]. The DPPH was prepared by dissolving 2.5 mg of DPPH in 100 ml of pure methanol and an aliquot of 100 μl of the extract at the concentrations of 25, 50, 100, 150, 200 and 250 μg/ml were added in 3 ml of the DPPH solution, separately. The contents were incubated for 20 minutes at room temperature in dark room and later, the absorbance was recorded at 517 nm. To calculate the percentage DPPH decolourisation of the sample, the following equation was used:
% DPPH scavenging=[(A control – A sample)/A control] -100
Where A refers to the absorbance.
Cell culture
Considering the ease of growth and manipulation, the Human Embryonic Kidney cells (HEK293. Source: ATCC, Manassas, Virginia, USA) were used. The HEK293 maintained in modified cell culture medium (Dulbecco's modified Eagle's growth medium) were supplemented with 10% fetal bovine serum and added with penicillin (100 U/ml)/streptomycin (100 mg/ml) (Invitrogen) according to Rasooly et al. [17]. Cell lines were cultured in a humidified incubator at temperature of 37°C, with 5% CO2 to use for cytotoxicity and antiviral assays with approximately 80% confluent.
Cytotoxicity assay
Cell cytotoxicity was determined employing a 3-(4,5- dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay based on colorimetric parameter. The HEK293 cells were seeded in a microtiter 96-well plate at a density of 1 × 104 cells per well and treated with different concentrations of CcSE (0.01–100 μg/ml) or vehicle for 48 h. Later, the cells were maintained with 0.5 mg/ml MTT (Sigma) at 37°C for 4 h. The purple colored insoluble formazan precipitate produced by viable cells from MTT were extracted and dissolved using the solvent isopropanol. The absorbance intensity was measured at 562 nm. The data (percentage of viable cells) was compared with the control.
Antiviral activity assay
The 96-well microtiter flat-bottomed plates containing cell monolayers (80% confluent) were preincubated for 2 h with increasing non-cytotoxic concentration of the cumin sample (CcSE). At least, six replicates were used for each CcSE concentration. Subsequently, the cell were subjected to Herpes virus (Type 1 & 2) at a multiplicity of infection (MOI) of 0.001 pfu/cell, keeping the same experimental conditions of 37°C and a humidified atmosphere of 5% CO2 in air. The sample was examined microscopically for cell cytopathic effect (CPE) on daily basis till the impact (CPE) was observed in all virus control wells. The samples (percentage of wells) with CPE were enumerated for each concentration, as described previously. Antiviral drug Acyclovir (Sigma®) was used as positive control at concentration of 0.05 to 5 μg/ml.
Viral plaque number reduction assay
Inhibition of HSV-1 and 2 replications were evaluated with plaque reduction assay. Vero cells were seeded in 24-well plates at a density of 10 × 104 cells. Phenotypic plaque reduction assay was used to determine the impact of HSV 1 and 2. The plaque reduction assay was done by subjecting the cell monolayers with the viruses at a MOI of 0.001 pfu/cell with serial dilutions of the cumin (CcSE) for 2 h at 37°C. Later, the medium was removed from the wells, the cells washed twice with the medium and again covered with a medium containing 1.2% methylcellulose (Sigma) and CcSE at the same concentration. After incubation for 24 h at the aforementioned environmental conditions, the supernatant removed, and the cells fixed and stained (0.1% crystal violet in 20% ethanol) thus enabling the enumeration of viral plaques count. The IC50 values, defined as the inhibitory concentration producing 50% reduction in plaque formation, were calculated. As performed for Antiviral activity assay. Acyclovir (Sigma®), a potent synthetic antiviral drug was utilized as a positive control (5 μg/ml).
Statistical analysis
Results are represent in the form of the mean ± standard deviations for three mutually independent experiments each performed in duplicate.
Results and Discussion
Total phenolic and flavonoid concentrations in the methanol extract of C. cyminum seeds was used to examine its antioxidant activity. The total phenolic contents in the CcSE was 22.08 ± 0.72 μg/mg gallic acid equivalent of phenols/mg dried extract whereas, the flavonoid concentration in the CcSE was 15.81 ± 0.39 μg/mg quercetin equivalents of flavonoids/mg dried extract. In accordance with the present results, Rebey et al. [18] found that Tunisia cumin seeds contained 18.60 ± 0.03 phenols and 12.37 ± 0.01 flavonoid while the Indian cumin seeds contained 14.50 ± 0.01 phenols and 8.18 ± 0.01 flavonoid. However, the higher yield of phenols and flavonoids in our data may be due to the nature of our solvent used to prepare the extract. Settharaksa et al. [19] reported that the methanolic solvent was better for catechin, epicatechin and epigallocatechin extraction (Table 1).
| Conditions | Total phenolicsa | Total flavonoidb |
|---|---|---|
| Aqueous extract | 22.08 ± 0.72 | 15.81 ± 0.39 |
| (a) As μg/mg gallic acid equivalent of phenols/mg dried extract. (b) As μg/mg quercetin equivalents of flavonoids/mg dried extract. | ||
Table 1. Total phenolics and flavonoids contents of Cuminum cyminum seeds.
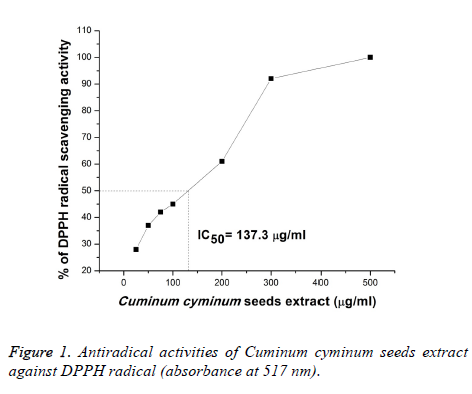
The use of the stable crystalline free radical (also an indicator and scavenger for other radicals) diphenylpicrylhydrazyl (DPPH) for estimating antioxidant activity is well established [20-21]. The antioxidant activity of cumin seeds extract was examined by measuring the radical scavenging activity by DPPH•. Contrary to the in vitro produced free radicals such as the hydroxyl radical (•OH) and superoxide anion (•O2−), using DPPH has the advantage as it remains unchanged by certain side reactions, such as metal ion chelation and enzyme inhibition. The obtained value for the DPPH• radical scavenging activity are illustrated in Figure 1. The data indicates that the 50% DPPH radical scavenging activity was 137.27 μg/ml. The investigated feature reveals effective antioxidant attributes significantly [22] and the amount of half maximal effective concentration (EC50). Previous studies have revealed that free radical-scavenging activity is extremely affected by or correlated with the phenolic components of the plant extract [16].
Thus, the higher free radical scavenging activity of CcSE reported through the present study can be credited to their peculiar composition and the distribution of the essential phenolic compounds [23]. The present results are in conformity with the one previously reported by Einafshar et al. [10] the later reporting that the EC50 of cumin seeds was 0.74 ± 0.10 mg/ml while the EC50 of essential oil of cumin seed was 1.20 ± 0.20. Rebey et al. [18] reported the antiradical activity against the DPPH radical for p-coumaric, trans-2- dihydrocinnamic and rosmarinic acids determined in the seeds employing reversed-phase high-performance liquid chromatography (RP-HPLC). Also, Pandey et al. [24] revealed this activity to the presence of 1-O-β-D-glucopyranoside, β-Dglucopyranoside and cuminoside A and B.
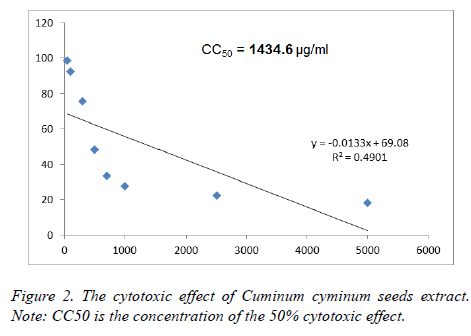
To evaluate cytotoxicity of CcSE, IC50 was determined in HEK293 cultured cell by MTT assay and according to the findings we found that the IC50 value for the CcSE was found to be 1434.6 μg/ml. According to the results of these studies (Figure 2), CcSE has low cytotoxicity and HEK293 cell can tolerate it. The low toxicity of CcSE was reported by Wei et al. [25], they found that cumin essential oil did not display any cytotoxic effect at the concentrations present under their investigation (0.0005-0.01%).
To examine the antiviral property of CcSE against herpes simplex viruses (type 1 and 2), cytopathic inhibitory assay was performed and the data was presented in Table 2. Results of the present investigation, based on the cytopathic effect (CPE) of the virus-infected confluent monolayer of Vero cells, give convincing evidence that CcSE expresses adequate antiviral activity against herpes simplex viruses (Type 1 and 2) at low concentration of 8 μg/ml. In this manner, the cytopathic effect was showed at 4 μg/ml against HSV-1 and at 5 μg/ml against HSV-2.
| CcSE | Cell Cytopathic Effect (CPE) | |
|---|---|---|
| HSV-1 | HSV-2 | |
| 1 µg/ml | + | + |
| 2 µg/ml | + | + |
| 3 µg/ml | + | + |
| 4 µg/ml | - | + |
| 5 µg/ml | - | - |
| 10 µg/ml | - | - |
Table 2. The antiviral activity of Cuminum cyminum seeds extract.
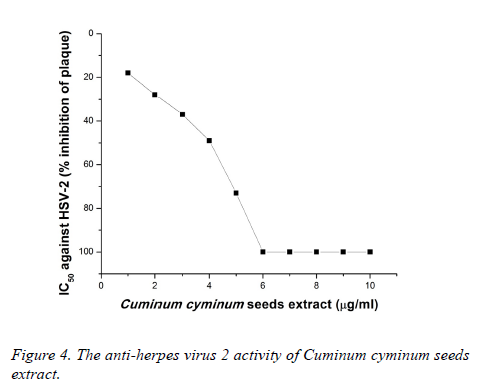
Furthermore, the anti-HSV-2 activity of the CcSE was explored (Figure 4). The HSV-2 was less sensitive to CcSE and according to the current data, CcSE at 4 μg/ml showed 49% inhibition of plaque of HSV-2 and showed 100% inhibition against HSV-2 at 6-10 μg/ml when the extract applied to the Vero cells 2 h before the virus infection. The anti-herpes simplex virus types-1 and 2 could have been due to the binding of phytochemical compounds in cumin with the protein coat of the virus and arrest the absorption of virus into the Vero cells.
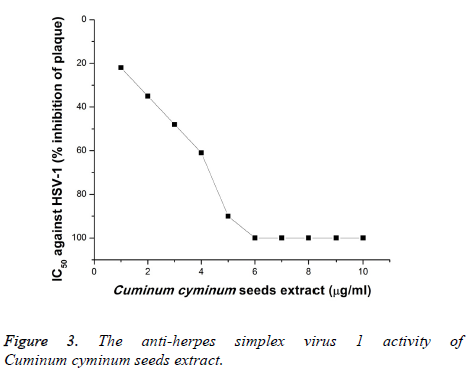
Plaque inhibition assay was performed to determine the IC50. As illustrated in Figure 3, CcSE at 4 μg/ml provided 61% inhibition of plaque of HSV-1 and showed 100% inhibition against HSV-1 at 6-10 μg/ml.
The treatment of herpes simplex virus (HSV-1 and HSV-2) infections with the available synthetic therapies as Acyclovir often leads to the problems such as viral resistance to the drug [26] and virus latency duration [27]. However, studies based on alternative medicines showed that some of the natural products including medicinal plants have a therapeutic application and can be potent candidate as an antiviral agent. So studying medicinal plants and evaluating the efficacy and efficiency may give way for treatment of, apart from others, HSV-1 and HSV-2 infections [28]. For instance, spiroketal-enol ether derivative (E)-2-(2,4-hexadiynyliden)-1,6- dioxaspiro[4.5]dec-3-ene extracted from the rhyzomes of tansy plant (Tanacetum vulgare) has been found to check a key step in controlling the viral replication through the expression of viral genes [6]. Also, the hydrolyzable tannins such as chebulagic acid and punicalagin extracted from Terminalia chebula Retz. (Combretaceae) act as cell surface glycosaminoglycan (GAG) competitors can prevent HSV-1 entry and cell-to-cell spread [29].
In the current experiment, we reported the anti-HSV-1 and anti-HSV-2 activities of C. cyminum seeds extract and the plant has exhibited antimicrobial activity as indicated by other studies. Iacobellis et al. [14] reported that p-mentha-1,4-dien-7- al, cuminaldehyde, ɣ-terpinene, and β-pinene isolated from cumin seeds oil have a potential use for the control of bacterial diseases. Ani et al. [30] demonstrated the antimicrobial activity by the extract of this species on the impact of food-borne pathogenic and spoilage bacteria and suggested the antimicrobial activity of cumin seeds due to the presence of polyphenols and flavonols. However, the exact mechanism of action against HSV-1 and HSV-2 activities has not been reported yet. It might be due to the interaction of some components of CcSE including polyphenolics or flavonoids with Vero cell membrane and/or herpes simplex viruses’ envelope.
In conclusion, C. cyminum seeds extract demonstrated antiretroviral activity with low cytotoxicity and it could be used as a promising candidate for new and potent antiviral herbal preparation with fewer side effects.
Acknowledgements
The authors extend their sincere appreciation to the Deanship of scientific Research at King Saud University-Saudi Arabia for funding this work through Research Group number (RG-1436-242).
References
- Koelle DM, Corey L. Recent progress in herpes simplex virus immunobiology and vaccine research. Clin Microbiol Rev 2003; 16: 96-113.
- Khan MT, Ather A, Thompson KD, Gambari R. Extracts and molecules from medicinal plants against herpes simplex viruses. Antiviral Res 2005; 67: 107-119.
- Wagner EK. Basic virology. 3rd ed. Oxford: Blackwell Pub, 2008
- Berger JR, Houff S. NEurological complications of herpes simplex virus type 2 infection. Archives of Neurology. 2008; 65: 596-600.
- Schillinger JA, Xu F, Sternberg MR, Armstrong GL, Lee FK, Nahmias AJ. National seroprevalence and trends in herpes simplex virus type 1 in the United States, 1976-1994. Sex Transm Dis. 2004; 31: 753-60.
- Alvarez AL, Habtemariam S, Abdel Moneim AE, Melon S, Dalton KP, Parra F. A spiroketal-enol ether derivative from Tanacetum vulgare selectively inhibits HSV-1 and HSV-2 glycoprotein accumulation in Vero cells. Antiviral Res. 2015; 119: 8-18.
- Johri RK. Cuminum cyminum and Carum carvi: An update. Pharmacogn Rev. 2011; 5: 63-72.
- Thippeswamy NB, Naidu KA. Antioxidant potency of cumin varieties—cumin, black cumin and bitter cumin—on antioxidant systems. European Food Research and Technology. 2005; 220: 472-476.
- Allahghadri T, Rasooli I, Owlia P, Nadooshan MJ, Ghazanfari T, Taghizadeh M. Antimicrobial property, antioxidant capacity, and cytotoxicity of essential oil from cumin produced in Iran. J Food Sci. 2010; 75: H54-61.
- Einafshar S, Poorazrang H, Farhoosh R, Seiedi SM. Antioxidant activity of the essential oil and methanolic extract of cumin seed (Cuminum cyminum). European Journal of Lipid Science and Technology. 2012; 114: 168-74.
- Kedia A, Prakash B, Mishra PK, Dwivedy AK, Dubey NK. Biological activities of Cuminum cyminum seed oil and its major components against Callosobruchus chinensis and Sitophilus oryzae. J. Asia-Pac. Entomol. 2015; 18: 383-388.
- Zhaveh S, Mohsenifar A, Beiki M, Khalili ST, Abdollahi A,Rahmani-Cherait T, Tabatabaei M. Encapsulation of Cuminum cyminum essential oils in chitosan-caffeic acid nanogel with enhanced antimicrobial activity against Aspergillus flavus. Ind. Crop. Prod. 2015; 69: 251-256.
- Siow HL, Gan CY. Extraction, identification, and structure-activity relationship of antioxidative andα-amylase inhibitory peptides from cumin seeds (Cuminum cyminum). J Funct foods. 2016; 22: 1-12.
- Iacobellis NS, Lo Cantore P, Capasso F, Senatore F. Antibacterial activity of Cuminum cyminum L. and Carum carvi L. essential oils. J Agric Food Chem. 2005; 53: 57-61.
- Abdel Moneim AE, El-Khadragy MF. The potential effects of pomegranate (Punica granatum) juice on carbon tetrachloride-induced nephrotoxicity in rats. J Physiol Biochem. 2013; 69: 359-370.
- Abdel Moneim AE. The Neuroprotective Effects of Purslane (Portulaca oleracea) on Rotenone-Induced Biochemical Changes and Apoptosis in Brain of Rat. CNS Neurol Disord Drug Targets. 2013.
- Rasooly R, Bradley H, Xiaohua H, Mendel F. Plant compounds enhance the assay sensitivity for detection of active Bacillus cereus toxin. Toxins. 2015; 835-845.
- Rebey IB, Zakhama N, Karoui IJ, Marzouk B. Polyphenol composition and antioxidant activity of cumin (Cuminum cyminum L.) seed extract under drought. J Food Sci. 2012; 77: C734-739.
- Settharaksa S, Jongjareonrak A, Hmadhlu P, Chansuwan W, Siripongvutikorn S. Flavonoid, phenolic contents and antioxidant properties of Thai hot curry paste extract and its ingredients as affected of pH, solvent types and high temperature. Int Food Res J. 2012; 19: 1581-1587.
- Molyneux P. The use of the stable free radical diphenylpicryl-hydrazyl (DPPH) for estimating antioxidant activity. Songklanakarin J Sci Technol. 2004; 26: 211-219.
- Kataky A, Handiquel PJ. Bioassay of micropropagated Andrographis paniculata: An overview. Asian J Sci Techn. 2011; 4: 17-23.
- Iqbal S, Younas U, Sirajuddin, Chan KW, Sarfraz RA, Uddin K. Proximate Composition and Antioxidant Potential of Leaves from Three Varieties of Mulberry (Morus sp.): A Comparative Study. Int J Mol Sci. 2012; 13:6651-6664.
- Bettaieb I, Bourgou S, Wannes WA, Hamrouni I, Limam F, Marzouk B. Essential oils, phenolics, and antioxidant activities of different parts of cumin (Cuminum cyminum L.). J Agric Food Chem. 2010; 58: 10410-10418.
- Pandey S, Patel MK, Mishra A, Jha B. Physio-Biochemical Composition and Untargeted Metabolomics of Cumin (Cuminum cyminum L.) Make It Promising Functional Food and Help in Mitigating Salinity Stress. PLoS One. 2015; 10: e0144469.
- Wei J, Zhang X, Bi Y, Miao R, Zhang Z, Su H. Anti-Inflammatory Effects of Cumin Essential Oil by Blocking JNK, ERK, and NF-kappaB Signaling Pathways in LPS-Stimulated RAW 264.7 Cells. Evid Based Complement Alternat Med. 2015; 2015: 474509.
- Frobert E, Ooka T, Cortay JC, Lina B, Thouvenot D, Morfin F. Herpes simplex virus thymidine kinase mutations associated with resistance to acyclovir: a site-directed mutagenesis study. Antimicrob Agents Chemother. 2005; 49: 1055-1059.
- Farahani M. Anti-Herpes Simplex Virus Effect of Camellia sinesis, Echiumamoenum and Nerium oleander. J Appl Environ Microbiol . 2014; 2: 102-105.
- Lin LT, Hsu WC, Lin CC. Antiviral natural products and herbal medicines. J Tradit Complement Med. 2014; 4: 24-35.
- Lin LT, Chen TY, Chung CY, Noyce RS, Grindley TB, McCormick C. Hydrolyzable tannins (chebulagic acid and punicalagin) target viral glycoprotein-glycosaminoglycan interactions to inhibit herpes simplex virus 1 entry and cell-to-cell spread. J Virol. 2011; 85: 4386-4398.
- Ani V, Varadaraj MC, Naidu KA. Antioxidant and antibacterial activities of polyphenolic compounds from bitter cumin (Cuminum nigrum L.). European Food Research and Technology. 2006; 224: 109-115.