Research Article - Journal of Infectious Diseases and Medical Microbiology (2018) Volume 2, Issue 2
Larvicidal and pupicidal activities of Solonum pseudocapsicum fruits compunds against Aedes aegypti, Anopheles stephensi and Culex quinquefasciatus (Diptera: Culicidae).
Jeyasankar A*, Chinnamani TPG & Research Department of Zoology, Government Arts College (Autonomous), Coimbatore, Tamil Nadu, India
- *Corresponding Author:
- Jeyasankar A
PG & Research Department of Zoology
Government Arts College
Coimbatore
Tamil Nadu
India
Tel: 09894214939
E-mail: sankar.alagarmalai@gmail.com
Accepted date: May 8, 2018
Citation: Jeyasankar A, Chinnamani T. Larvicidal and pupicidal activities of Solonum pseudocapsicum fruits compunds against Aedes aegypti, Anopheles stephensi and Culex quinquefasciatus (Diptera: Culicidae). J Infectious Disease Med Microbiol. 2018;2(2):11-16.
Abstract
The aim of this research was to determine the chemical composition and insecticidal activity of ethyl acetate extracts of S. pseudocapsicum fruits parts against the Aedes aegypti, Anopheles stephensi and Culex quinquefasciatus. The maximum larval and pupal mortality was observed in ethyl acetate extracts of S. pseudocapsicum fruits parts respectively. Ethyl acetate extracts of S. pseudocapsicum fruits parts were obtained from hydrodistillation and investigated by GC and GC-MS. A total of 47 components of the ethyl acetate extracts of S. pseudocapsicum fruits parts were identified, respectively. The principal compounds in S. pseudocapsicum fruits parts were n-Propyl acetate, 1,2,3-Propanetriol, monoacetate, Tetradecanoic acid, 1,2,3-Propanetriol, 1-acetate, Triacetin, 9-Hexadecenoic acid, n-Hexadecanoic acid, 9,12-Octadecadienoic acid (Z,Z), methyl ester. The results indicated that this compounds of n-Propyl acetate, 1,2,3-Propanetriol, monoacetate, Tetradecanoic acid, 1,2,3-Propanetriol, 1-acetate, Triacetin, 9-Hexadecenoic acid, n-Hexadecanoic acid, 9,12-Octadecadienoic acid (Z,Z)-, methyl ester show potential in to serve as an alternate botanical mosqticide in the management of Aedes aegypti, Anopheles stephensi and Culex quinquefasciatus.
Keywords
Laricidal activities, Pupicidal activity, Aedes aegypti, Anopheles stephensi, Culex quinquefasciatus, Solonum pseudocapsicum, Ethyl acetate
Introduction
Mosquitoes are the most important single group of insects that known for their public importance, since they act as vector for transmitting many diseases such as dengue fever, yellow fever, chikungunya, malaria, filariasis and encephalitis of different types including, Japanese encephalitis in tropical and subtropical areas [1]. These diseases not only cause high levels of morbidity and mortality, but also inflict great economic loss and social disruption on developing countries such as India, China, etc. India alone contributes around 40% of global filariasis burden and the estimated annual economic loss is about 11,31,90,000 US Dollars [2,3]. Mosquito-borne diseases contribute radically to disease trouble, loss, shortage, and social weakness all greater than the world, mostly in tropical countries. Nevertheless, high cost of synthetic pyrethroids, environment and food safety concerns, unacceptability and toxicity of many organophosphates and organochlorines, and worldwide raise in insecticidal resistance, have argue for stimulated research towards the advance of possible insecticides of botanical origin [4]. In India, about 20,000 medicinal plants have been recorded newly, but advance than 500 established communities use about 800 plant species for remedial different diseases. Plant derived materials are comparatively safer to humans and ecosystem and easily biodegradable [5]. Plant derived natural products have the advantage of being harmless to beneficial non-target organisms and environment when compared to synthetic insecticides [6].
Plants may be an alternative source of insecticidal agents because they constitute a rich source of bioactive chemicals. Much effort has been focused on plant extracts or phytochemicals as potential sources of commercial mosquito-control agents or bioactive chemical compounds [7-9]. The plants already pointed out that the most promising botanical mosquito-control agents are in the family’s Asteraceae, Cladophoraceae, Labiatae, Meliaceae, Oocystaceae, and Rutaceae. Recently, plants in the family Piperaceae have drawn attention because they contain insecticidal principles [10]. In this paper, we report larvicidal activity, pupicidal activity and GC-MS principal compounds analysis from ethyl acetate extracts of S. seudocapsicum fruit against early fourth-instar larvae of Aedes aegypti, Anopheles stephensi and Culex quinquefasciatus.
Materials and Methods
Collection of plant materials
The fruits parts of Solonum pseudocapsicum were collected from Pulliansolai, Namakkal District, Tamil Nadu, India. The voucher specimen of S. pseudocapsicum (IPH No. 23) was prepared and deposited in PG and Research Department of Zoology, Government Arts College, Coimbatore, Tamil Nadu, India.
Soxhlet extraction method
The plant materials were thoroughly washed with tap water and shade dried under room temperature (27.0 ± 20ºC and 75 ± 5% RH). After complete drying the plant materials were powdered using electric blender and sieved through a kitchen strainer. 1000 g of plant powder was extracted by soxhlet extraction methods with ethyl acetate solvents and filtered through Whatman’s No. 1 filter paper [11]. The solvent from the crude extract were evaporated to air dried at room temperature. The crude extracts were collected in clean borosil vials and stored in the refrigerator at 40ºC for subsequent bioassay against Aedes aegypti, Anopheles stephensi and Culex quinquefasciatus.
Insect rearing
Eggs of Aedes aegypti, Anopheles stephensi and Culex quinquefasciatus were collected from National Centre for disease control (Communicable Diseases), Government of India, Ministry of health and family welfare, Southern India branch, field station, Mettupalayam, Coimbatore, Tamil Nadu, and India. The collected eggs were maintained in plastic trays containing tap water. Once the larvae are hatched, those were fed with Dog biscuit and Baker’s yeast in the ratio of 3:1 every day. The pupae formed were transferred in a cup containing tap water and placed in the oviposition cage (44 × 44 × 43). Emerged adults were continuously fed with 10% sucrose solution. Adults were given blood meal from a Broiler chicken (Gallus gallus domesticus) from fifth day on wards. Small plastic bowls of tap water lined with filter paper were placed inside the cage for oviposition. The whole setup was maintained at 28 ± 2ºC and 70-80% relative humidity under the 14:10 light and dark cycles [12].
Larvicidal bioassay
The larvicidal activity of plant crude extract assessed by using the standard method as prescribed by WHO (2005) [13]. From the stock solution, four different concentrations viz., 125, 250, 500 and 1000ppm for crude extracts was prepared and tested against the freshly moulted (0-6 hours) 4th instar larvae of A. aegypti, A. stephensi and C. quinquefasciatus. Polysorbate 20 (polyoxyethylene sorbitan monolaurate, Tween 20) used as emulsifier and distilled water treated as control. Twenty five larvae of each mosquito species were introduced in 500 ml plastic cups containing 250 ml of aqueous medium (249 ml of dechlorinated water + 1 ml of emulsifier) and the required amount of plant extracts were added. The larval mortality were observed and recorded after 12 and 24 hours of post treatment. For each experiment, five replicates were maintained at a time. The percentage of larval mortality was calculated by using Abbott's formula [14].
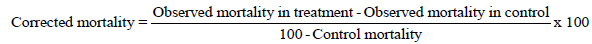
Pupicidal bioassay
The pupicidal activity of plant crude extract of using the standard method as prescribed by WHO (2005) [13]. Similar test concentrations as stated in the previous experiments were prepared and tested against the pupae of A. aegypti, A. stephensi and C. quinquefasciatus Tween 20 (emulsifier) in water were treated as control. The pupae of these mosquito species (10 pupae) were introduced in 250 ml plastic cups containing 100 ml of aqueous medium (100 ml of dechlorinated water + 2 drops of tween 20) and the required amount of plant extract were added. The pupal mortality were observed and recorded after 24 hours of post treatment. For each experiment, five replicates were maintained at a time. The percentage of mortality was calculated by using Abbott's formula [14].
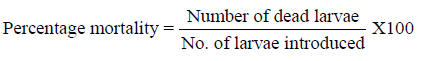
Gas chromatography analysis
Alanalysis was carried out on a varian-gas chromatography euipped with flame ionization detector and a BPI (100% demethyl polysiloxane) capillary column. Helium at a rate of flow 1 ml/min; Split ratio: 1:10 was employed as a carrier gas. The oven temperature was programmed from 40ºC (10 minutes) at 7ºC/min to 200ºC (5 minutes) at 8ºC/min to 250ºC (3 minutes) and injector temperature was 280ºC. The sample (0.2 μL) was injected with 1:20 split ratio.
Gas chromatography and mass spectroscopy analysis
Gas chromatograpgy-mass spectrometer analsyis was performed on an Elmer Clarus 500 Software GC equipped with capillary coloumn Elite-5MS (5% Phenyl 95 dimethylpolysiloxane). The oven temperature was programmed from 200ºC to 150ºC at the rate of 4oC minutes 1 and held at this temperature for 5 minutes. The inlet and interface temperature were 250ºC to 280 ºC. The carrier gas was helium at s flow rate of 1.0 mL minute 1 (constant flow). The sample (1.0 μL) was injected with split 20:1. Electron impact mass spectrometry was carried out at 70 eV. Ions source and quadrupole temperature were maintained at 230ºC to 150ºC. The Mass range was 40 amu to 600 amu and compounds were identified using the library-NIST 2005.
Statistical analysis
Data analysis was carried out using Microsoft Excel 2007. One -Way ANOVA was performed for all the expe-rimental data from that Least Significant Difference was calculated and the significant differences were marked with different alphabet. LC50, LC90 was carried out using SPSS 16.00.
Results and Discussion
Larvicidal activity of ethyl acetate extracts of Solonum pseudocapsicum against Aedes aegypti, Anopheles stephensi and Culex quinquefasciatus
The previous experiment of ethyl acetate extracts of S. pseudocapsicum was tested for their larvicidal activity against Aedes aegypti, Anopheles stephensi and Culex quinquefasciatus on perusal of the revealed that larvicidal activity against Aedes aegypti showed the LC50(LCL-UCL), LC90(LCL-UCL) X2 12 hours value of 630.44 (537.88-747.73), 1535.38(1293.48- 1950.47) 2.152 and 24 hours value of 304.15(132.97-476.05), 824.26(606.02-1473.28) 9.600 respectively. Accordingly, Culex quinquefasciatus showed the LC50 (LCL-UCL), LC90 (LCL-UCL) X2 12 hours value of 509.68(290.55- 892.19), 1400.72(973.98-318.56) 7.826 and 24 hours value of 279.50(169.00-391.76), 730.68(569.75-1094.05) 5.930 respectively. Likewise, for Anopheles stephensi showed the LC50 (LCL-UCL), LC90 (LCL-UCL) X2 12 hours value of 582.18(370.16-1030.83), 1438.17(1004.21-3224.36) 8.326 24 hours value of 335.99(193.15-488.53), 881.27(671.37-1403.83) 7.234 respectively (Tables 1 and 2).
Table 1. Larvicidal activity of ethyl acetate extracts of Solonum pseudocapsicum against 4th instar larvae of Aedes aegypti, Anopheles stephensi and Culex quinquefasciatus was calculated by spss 16.00 in 12 hrs.
| Mosquitoes | Concentration (ppm) |
12 hrs | 95% Confidence Limits (ppm) | ||
|---|---|---|---|---|---|
| Larval Mortality (%) |
LC50 (LCL-UCL) |
LC90 (LCL-UCL) |
χ2 | ||
| Aedes | 125 | 19.66 ± 3.23a | 630.44 (537.88-747.73) |
1535.38 (1293.48-1950.47) |
2.152 |
| 250 | 31.43 ± 2.54b | ||||
| 500 | 47.22 ± 1.45c | ||||
| 1000 | 67.80 ± 2.65d | ||||
| Culex | 125 | 21.22 ± 2.18a | 509.68 (290.55-892.19) |
1400.72 (973.98-318.56) |
7.826 |
| 250 | 41.20 ± 4.33b | ||||
| 500 | 57.55 ± 3.44c | ||||
| 1000 | 71.44 ± 1.23d | ||||
| Anopheles | 125 | 20.50 ± 1.85a | 582.18 (370.16-1030.83) |
1438.17 (1004.21-3224.36) |
8.326 |
| 250 | 39.20 ± 1.22b | ||||
| 500 | 53.60 ± 2.26c | ||||
| 1000 | 69.33 ± 2.35d | ||||
*Values are mean ± S.D of fivereplication; Number of larvae =10; LC50=Lethal concentration 50 and LC90=Lethal concentration 95; Values with different alphabet in column are statistically significant (p<0.05 level; DMRT)
Table 2. Larvicidal activity of ethyl acetate extracts of Solonum pseudocapsicum against 4th instar larvae of Aedes aegypti, Anopheles stephensi and Culex quinquefasciatus was calculated by spss 16.00 in 24 hrs.
| Mosquitoes | Concentration (ppm) |
24hrs | 95% Confidence Limits (ppm) | ||
|---|---|---|---|---|---|
| Larval Mortality (%) | LC50 (LCL-UCL) |
LC90 (LCL-UCL) |
χ2 | ||
| Aedes | 125 | 26.54 ± 2.70a | 304.15 (132.97-476.05) |
824.26 (606.02-1473.28) |
9.600 |
| 250 | 53.60 ± 3.23b | ||||
| 500 | 73.34 ± 1.66c | ||||
| 1000 | 92.80 ± 2.22d | ||||
| Culex | 125 | 31.30 ± 3.18a | 279.50 (169.00-391.76) |
730.68 (569.75-1094.05) |
5.930 |
| 250 | 53.10 ± 1.33b | ||||
| 500 | 76.33 ± 3.44c | ||||
| 1000 | 96.20 ± 2.11d | ||||
| Anopheles | 125 | 29.55 ± 3.55a | 335.99 (193.15-488.53) |
881.27 (671.37-1403.83) |
7.234 |
| 250 | 60.70 ± 1.22b | ||||
| 500 | 76.50 ± 3.43c | ||||
| 1000 | 91.22 ± 1.22d | ||||
*Values are mean ± S.D of fivereplication; Number of larvae =10; LC50=Lethal concentration 50 and LC90=Lethal concentration 95; Values with different alphabet in column are statistically significant (p<0.05 level; DMRT)
Larvicidal activity of ethyl acetate, butanol, and petroleum ether extracts of five species of Euphorbiaceae plants, Jatropha curcas, Pedilanthus tithymaloides, Phyllanthus amarus, Euphorbia hirta, and Euphorbia tirucalli, were tested against the early fourth instar larvae of Aedes aegypti. The larval mortality was observed after 24 hours of exposure. All extracts showed low larvicidal effects. The highest larval mortality was found in petroleum ether extract. The LC50 value of petroleum ether extracts of J. curcas, P. tithymaloides, P. amarus, E. hirta, and E. tirucalli were 8.79, 55.26, 90.92, 272.36, and 4.25 ppm, respectively, against A. aegypti [15]. Efficiency of leaf chloroform extract of Nyctanthes arbortristis have been reported with LC50 value of 526.3, 780.6 ppm (24 hours) and 303.2, 518.2 (48 hours) for A. aegypti and A. stephensi [16]. Previous studies showed that ethanol extracts from fruit endocarps of Melia azedarach and Azadirachta indica, two members of the family Meliaceae, were found to have lethal effects on A. aegypti larvae, with LC50 values ranging from 0.017 g to 0.034 g% [17]. Moreover, ethanolic extracts derived from three species of the Piperaceae (pepper) family, Piper longum, P. ribesoides and P. sarmentosum had toxic effect on A. aegypti 4th instar larvae. Their LC50 values ranged from 2.23 ppm to 8.13 ppm.
Pupicidal activity of ethyl acetate extracts of Solonum pseudocapsicum fruits against Aedes aegypti, Anopheles stephensi and Culex quinquefasciatus
The previous experiment of ethyl acetate extracts of S. pseudocapsicum fruits was tested for their pupicidal activity against Aedes aegypti, Anopheles stephensi and Culex quinquefasciatus on perusal of Table 3 revealed that pupicidal activity against Aedes aegypti showed the LC50(LCL-UCL), LC90(LCL-UCL) X2 24 hours 456.39(391.36-517.60), 1040.20(922.23-1211.13) 1.336 respectively. Accordingly, Culex quinquefasciatus showed the LC50 (LCL-UCL), LC90 (LCL-UCL) X2 24 hours value of 351.47(281.27-416.33), 972.75, (853.68-1150.70) 1.808 respectively. Likewise, for Anopheles stephensi showed the LC50 (LCL-UCL), LC90 (LCLUCL) X2 24 hours value of 414.83 (350.46-478.82), 1015.23 (896.98-1188.33) 2.693 respectively (Table 3). Jeyasankar et al. have observed that the ethyl acetate extract of Phyllanthus emblica Linn exhibited more than 90% larval and pupal mortality at 250 ppm on C. quinquefaciatus [18]. The toxicity to the third instar larvae of A. aegypti, C. quinquefaciatus and A. stephensi by the ethyl acetate leaf extract of Andrographis paniculata showed the LC50 value of 20.85 and LC95 444.41 ppm respectively [19,20]. Jeyasankar and Ramar have reported that the petroleum ether extract of Andrographis paniculata exhibited more than 85% Pupal mortality and 100% Ovicidal activity at 250 ppm on A. aegypti, C. quinquefaciatus and A. stephensi [20].
Table 3. Pupicidal activity of ethyl acetate extracts of Solonum pseudocapsicum against 4th instar larvae of Aedes aegypti, Anopheles stephensi and Culex quinquefasciatus was calculated by spss 16.00 in 24 hrs.
| Mosquitoes | Concentration (ppm) |
24hrs | 95% Confidence Limits (ppm) | ||
|---|---|---|---|---|---|
| Pupal Mortality (%) | LC50 (LCL-UCL) |
LC90 (LCL-UCL) |
χ2 | ||
| Aedes | 125 | 21.10 ± 1.50a | 456.39 (391.36-517.60) |
1040.20 (922.23-1211.13) |
1.336 |
| 250 | 33.20 ± 3.33b | ||||
| 500 | 58.30 ± 2.26c | ||||
| 1000 | 86.80 ± 3.20d | ||||
| Culex | 125 | 28.33 ± 2.58a | 351.47 (281.27-416.33) |
972.75 (853.68-1150.70) |
1.808 |
| 250 | 43.30 ± 1.23b | ||||
| 500 | 66.23 ± 3.40c | ||||
| 1000 | 89.30 ± 1.10d | ||||
| Anopheles | 125 | 23.50 ± 2.50a | 414.83 (350.46-478.82) |
1015.23 (896.98-1188.33) |
2.693 |
| 250 | 36.10 ± 3.20b | ||||
| 500 | 63.50 ± 3.43c | ||||
| 1000 | 87.20 ± 3.20d | ||||
*Values are mean ± S.D of fivereplication; Number of larvae =10; LC50=Lethal concentration 50 and LC90=Lethal concentration 95; Values with different alphabet in column are statistically significant (p<0.05 level; DMRT)
Perusal of data revealed that ethyl acetate extracts of S. pseudocapsicum showed significant larvaicidal and pupicidal activities against tested mosquito species. Further, these extract were subjected to hydrodistillation process and investigated by GC and GC-MS analysis. A total of 47 compounds were obtained from ethyl acetate extracts of S. pseudocapsicum. The major principal compounds was found in GC-MS analysis such as n-Propyl acetate, 1,2,3-Propanetriol, monoacetate, 1,2,3-Propanetriol, 1-acetate, Triacetin, Tetradecanoic acid, 9-Hexadecenoic acid, n-Hexadecanoic acid, 9,12-Octadecadienoic acid (Z,Z)-, methyl ester which may responsible for mosquitocidal properties (Table 4). Recently, Jeyasankar and Chinnamani reported that compounds n-Propyl acetate, 1,2,3-Propanetriol, monoacetate, Tetradecanoic acid, 1,2,3-Propanetriol, 1-acetate, Triacetin, 9-Hexadecenoic acid, n-Hexadecanoic acid, 9,12-Octadecadienoic acid (Z,Z)-methyl ester were isolated from S. pseudocapsicum found insect growth inhibitory activity larvae of Spodoptera litura and Helicoverpa armigera [21].
Table 4. List of compounds identified from GC-MS analysis of S. pseudocapsicum fruits.
| S.No. | Name of the Compounds | Molecular Formula | Molecular Weight | Retention Time | Peak Area | % Peak area |
|---|---|---|---|---|---|---|
| Propanoic acid, ethyl ester | C5H10O2 | 102 | 4.80 | 6531158 | 0.1515 | |
| n-Propyl acetate | C5H10O2 | 102 | 4.90 | 63493828 | 1.473 | |
| 3,5-Dimethyl-5-hexen-3-ol | C8H16O | 128 | 7.57 | 1052588 | 0.0244 | |
| p-Xylene | C8H10 | 106 | 13.84 | 577839 | 0.0134 | |
| Pyrimidine, 4-hydroxy- | C4H4N2O | 96 | 15.74 | 362589 | 0.0084 | |
| Hexanoic acid | C6H12O2 | 116 | 19.62 | 1096527 | 0.0254 | |
| Phenol | C6H6O | 94 | 20.07 | 605486 | 0.0140 | |
| Glycerin | C3H8O3 | 92 | 20.59 | 16735126 | 0.3883 | |
| 1-Hepten-3-ol, 3-methyl | C8H16O | 128 | 20.98 | 4304152 | 0.0999 | |
| 1,1-Ethanediol, diacetate | C6H10O4 | 146 | 22.03 | 3017018 | 0.0700 | |
| 1,2,3-Propanetriol, monoacetate | C5H10O4 | 134 | 22.59 | 68387504 | 1.586 | |
| Benzenepentanoic acid, 4-methyl-ë-oxo- | C12H14O3 | 206 | 23.95 | 3922464 | 0.0910 | |
| Benzenecarboxylic acid | C7H6O2 | 122 | 24.60 | 6406623 | 0.1486 | |
| Dianhydromannitol | C6H10O4 | 146 | 24.88 | 2705639 | 0.0628 | |
| 1,2,3-Propanetriol, 1-acetate | C5H10O4 | 134 | 25.26 | 172108240 | 3.993 | |
| 5-Acetoxymethyl-2-furaldehyde | C8H8O4 | 168 | 26.49 | 4461149 | 0.1035 | |
| 2-Methoxy-4-vinylphenol | C9H10O2 | 150 | 26.72 | 7258137 | 0.1684 | |
| Triacetin | C9H14O6 | 218 | 26.82 | 31844884 | 0.7388 | |
| 3-Acetonylcyclopentanone | C8H12O2 | 140 | 27.05 | 1169476 | 0.0271 | |
| Benzene, 2-(1,3-butadienyl)-1,3,5-trimethyl- | C13H16 | 172 | 27.31 | 399419 | 0.0093 | |
| Naphthalene, 1,6-dimethyl- | C12H12 | 156 | 28.52 | 1734789 | 0.0402 | |
| 5,9-Undecadien-2-one, 6,10-dimethyl-, (E)- | C13H22O | 194 | 28.86 | 798020 | 0.0185 | |
| 1,4-Diacetyl-3-acetoxymethyl-2,5-methylene-l-rhamnitol | C14H22O8 | 318 | 29.61 | 6723022 | 0.1560 | |
| 3(2H)-Furanone, dihydro-2-methyl- | C5H8O2 | 100 | 30.44 | 1252576 | 0.0291 | |
| 1,3,4-Oxadiazole, 2-(acetyloxy)-2,5-dihydro-2,5,5-trimethyl- | C7H12N2O3 | 172 | 30.62 | 3624327 | 0.0841 | |
| Dodecanoic acid | C12H24O2 | 200 | 31.28 | 6665647 | 0.1546 | |
| 4-(2,6,6-Trimethylcyclohexa-1,3-dienyl)but-3-en-2-one | C13H18O | 190 | 31.54 | 465640 | 0.0108 | |
| 1,3-Propanediol, 2,2-dimethyl-, diacetate | C9H16O4 | 188 | 31.64 | 537289 | 0.0125 | |
| 3-Hydroxy-4-methoxybenzoic acid | C8H8O4 | 168 | 32.37 | 6419206 | 0.1489 | |
| 2-Heptadecenal | C17H32O | 252 | 33.46 | 5684738 | 0.1319 | |
| Tetradecanoic acid | C14H28O2 | 228 | 34.81 | 112279992 | 2.604 | |
| Benzoic acid, 4-amino-, ethyl ester | C9H11NO2 | 165 | 35.84 | 1206668 | 0.0280 | |
| 2-Pentadecanone, 6,10,14-trimethyl- | C18H36O | 268 | 35.99 | 2903305 | 0.0674 | |
| Pentadecanoic acid | C15H30O2 | 242 | 37.05 | 5755714 | 0.1335 | |
| 2-Nonadecanone | C19H38O | 282 | 37.63 | 721578 | 0.0167 | |
| (E,E)-7,11,15-Trimethyl-3-methylene-hexadeca-1,6,10,14-tetraene | C20H32 | 272 | 37.86 | 3882320 | 0.0901 | |
| 5,9,13-Pentadecatrien-2-one, 6,10,14-trimethyl-, (E,E)- | C18H30O | 262 | 38.00 | 1139650 | 0.0264 | |
| Hexadecanoic acid, methyl ester | C17H34O2 | 270 | 38.22 | 7578437 | 0.1758 | |
| 9-Hexadecenoic acid | C16H30O2 | 254 | 39.42 | 78250360 | 1.8154 | |
| n-Hexadecanoic acid | C16H32O2 | 256 | 40.38 | 2138582784 | 49.615 | |
| Estra-1,3,5(10)-trien-17á-ol | C18H24O | 256 | 40.86 | 17330716 | 0.4021 | |
| 9,12-Octadecadienoic acid (Z,Z)-, methyl ester | C19H34O2 | 294 | 42.08 | 46558292 | 1.080 | |
| 15-Octadecenoic acid, methyl ester | C19H36O2 | 296 | 42.18 | 20666826 | 0.4795 | |
| 9,12-Octadecadienoic acid (Z,Z)- | C18H32O2 | 280 | 43.74 | 209456432 | 4.859 | |
| 9,17-Octadecadienal, (Z)- | C18H32O | 264 | 44.09 | 141477280 | 3.282 | |
| 9,17-Octadecadienal, (Z)- | C18H32O | 264 | 44.20 | 689662912 | 16.000 | |
| 9,17-Octadecadienal, (Z)- | C18H32O | 264 | 44.33 | 402402624 | 9.335 |
The present study agreed with earlier works, the compounds isolated from ethanol extracts of Leucas aspera relies such as tetracosahexane, 2,6,10,15,19,23-hexamethyl, oxiraneundecanoic acid, 3-pentyl methylester, tetradecane 2,6,10- trimethyl, catechin, 1-hexadeconol, 2-methyl, 3,7,11,15 tetramethyl-2-hexadec-1-ol, 9,12-octadecadienoic acid- methyl ester, eicosanoic acid and methylester showed excellent mosquitocidal activity against Aedes aegypti, Anopheles stephensi and Culex quinquefasciatus was reported [22]. The results suggest a likely use of the extracts of these two plant species for the control of A. aegypti. The presence of phytocompounds in H. indicum plant such as Benzene acetaldehyde, 5H-1- Pyrindine, Benzene acetic acid, Dodecanoic acid, Benzene acetic acid, 2,5-Dihydroxy-, 3,7,11,15-Tetramethyl-2 Hexadecen- 1-ol, 9,12-Octadecadienoic acid, Ethyl Ester, 1-(+)-Ascorbic acid 2,6 Dihexadeconate, 4-((1E)-3-Hydroxy-1-propenyl)-2- Methoxy Phenol, 2-Furancarboxaldehyde, 5-(Hydroxymethyl)- were effective in mosquito control [23]. Larvicidal activity of Tetradecanoic acid, 9-Hexadecenoic acid and n-Hexadecanoic acid tested against A. aegypti showed significant larval mortality [24].
A study on the potential of phenolic extract of leaves and flowers of (pyrethrum) Chrysanthemum cinerariaefolium have been investigated and evaluated using GC-MS analysis. The analysis showed also that 3,7,11,15-Tetramethyl-2-hexadecen-1-ol, 3-Buten-2-one, and 4-(2-hydroxy-2,6,6-trimethylcyclohexyl) was found in both leaves and flowers extract. Then bioassay of these two phenolic extract were tested against all larval instar of C. quinquefaciatus and showed that the first instar larvae was more sensitive than other preceding instars [25].
Conclusion
The bioprospect of utilizing plant product for testing its efficacy in controlling medically important mosquitoes as larvicides is a recent phenomenon for facilitating the development of a more potent and environmental friendly. Identification of the bioactive principles compounds in S. pseudocapsicum fruits parts were n-Propyl acetate, 1,2,3-Propanetriol, monoacetate, Tetradecanoic acid, 1,2,3-Propanetriol, 1-acetate, Triacetin, 9-Hexadecenoic acid, n-Hexadecanoic acid, 9,12-Octadecadienoic acid (Z,Z)-, methyl ester and their mode of action and field trials are necessary to recommend an effective formulation as an mosquito vector control programs.
References
- Das NG, Goswami D, Rabha B. Preliminary evaluation of mosquito larvicidal efficacy of plant extracts. J Vect Borne Dis. 2007;44:145-48.
- Rahuman AA, Bagavan A, Kamaraj C, et al. Efficacy of larvicidal botanical extracts against Culex quinquefasciatus Say (Diptera: Culicidae). Parasitol Res. 2009;104:1365-72.
- Kamaraj C, Rahuman AA, Bagavan A, et al. Larvicidal and repellent activity of medicinal plant extracts from Eastern Ghats of South India against malaria and filariasis vectors. Asian Pac J Trop Med. 2011;4:698-705.
- Moore SJ. Plant-based insect repellents: a review of their efficacy, development and testing. Malaria Journal. 2011;10:S11.
- Kalu IJ, Ofoegbu U, Eroegbusi J, et al. Larvicidal activities of ethanol extract of Allium sativum (garlic bulb) against the filarial vector, Culex quinquefasciatus. J Med Plant Res. 2010;4:496-98.
- Pitasawat B, Champakaew D, Choochote W, et al. Aromatic plant-derived essential oil: An alternative larvicide for mosquito control. Fitoterapia. 2007;78:205-10.
- Kim SI, Shi OK, Song C, et al. Insecticidal activities of aromatic plant extracts against four agricultural insects. Agric Chem Biotechnol 2001;44:23-26.
- Arnason JT, Philogene BJR, Morand P. Insecticides of plant origin. ACS Symp Ser. 1989;387.
- Sukumar K, Perich MJ, Boobar LR. Botanical derivatives in mosquito control: a review. J Am Mosq Control Assoc. 1991;7:210-37.
- MacKinnon S, Chauret D, Wang M, et al. Botanicals from the Piperaceae and Meliaceae of the American neotropics: Phytochemistry. ACS Symp Ser. 1997;658:49-57.
- Pandey A, Tripathi S. Concept of standardization, extraction and pre phytochemical screening strategies for herbal drug. J Pharmacogn Phytochem. 2014;2:115-19.
- Kamaraj C, Bagavan A, Rahuman AA, et al. Larvicidal potential of medicinal plant extracts against Anopheles subpictus Grassi and Culex tritaeniorhynchus Giles (Diptera: Culicidae). Parasitol Res. 2009;104:1163-71.
- World Health Organization. Guidelines for laboratory and field testing of mosquito larvicides. WHO, Geneva. 2005.
- Abbott WS. A method of computing the effectiveness of an insecticide. J Econ Entomol. 1925;18:265-67.
- Rodrigues ENL. Fauna araneológica (Arachnida, Araneae) arborícola de duas áreas em uma mata de restinga no sul do Brasil. Acta Biologica Leopoldensia. 2005;27:73-92.
- Mathew N, Anitha MG, Bala TS, et al. Larvicidal activity of Saraca indica, Nyctanthes arbor-tristis, and Clitoria ternatea extracts against three mosquito vector species. Parasitol Res. 2009;104:1017-25.
- Wandscheer CB, Duque JE, da Silva MAN, et al. Larvicidal action of ethanolic extracts from fruit endocarps of Melia azedarach and Azadirachta indica against the dengue mosquito Aedes aegypti. Toxicon. 2004;44:829-35.
- Jeyasankar A, Premalatha S, Elumalai K. Larvicidal activity of Phyllanthus Emblica Linn. (Euphorbiaceae) leaf extracts against important human vector mosquitoes (Diptera: Culicidae). Asian Pac J Trop Dis. 2012;2:S399-S403.
- Jeyasankar A, Ramar G. Larvicidal properties of Breyenia vitis-idea (Burm.F) Fischer (Euphorbiaceae) against important vector mosquitoes (Diptera: Culicidae). J Vector Borne Dis. 2014;51:239-41.
- Jeyasankar A, Ramar G. Larvicidal activity of Andrographis paniculata (Acanthaceae) against important human vector mosquitoes (Diptera: Culicidae). Int J Adv Res Biol Sci. 2015;2:156-60.
- Jeyasankar A, Chinnamani T. Chemical composition and growth inhibitory activities of Solonum pseudocapsicum against Spodoptera litura and Helicoverpa armigera (Lepidoptera: Noctuidae). Int J Entomol Res. 2017;2:60-68.
- Elumalai D, Hemalatha P, Kaleena PK. Larvicidal activity and GC-MS analysis of Leucas aspera against Aedes aegypti Anopheles stephensi and Culex quinquefasciatus. Journal of the Saudi Society of Agricultural Sciences. 2015;16:306-13.
- Macedo ME, Consoli RA, Grandi TS, et al. Screening of Asteraceae (Compositae) plant extracts for larvicidal activity against Aedes fluviatilis (Diptera: Culicidae). Mem Inst Oswaldo Cruz. 1997;92:565-70.
- Sivakumar R, Jebanesan A, Govindarajan M. Larvicidal and repellent activity of tetradecanoic acid against Aedes aegypti (Linn.) and Culex quinquefasciatus (Say.) (Diptera:Culicidae). Asian Pac J Trop Med. 2011;4:706-10.
- Kareem RO, Annon MR. GC-MS analysis of bioactive compounds in phenolic extracts of leaves and flowers of Chrysanthemum cinerariaefolium and their efficacy against larvae of Culexquin quefaciatus Say (Diptera: Culicidae). J Chem Pharm Res. 2016;8:782-87.