Research Article - Journal of Translational Research (2018) Volume 2, Issue 1
Improvement of cancer stem cell expansion with kinetic and static alternating culture system.
Zhang Y1*, Wang Y1, Wang Z1, Farhangfar F2, Zimmerman M1
1Zyxell Inc. Carrollton, TX 75007, USA
2Atrium Health, Charlotte NC, USA
- *Corresponding Author:
- Zhang Y
Zyxell Inc. Carrollton,
Texas,
USA
Phone: (972)975-8600
E-mail: zyx@zyxellinc.com
Accepted date: March 27, 2018
Citation: Zhang Y, Wang Y, Wang Z, et al. Improvement of cancer stem cell expansion with kinetic and static alternating culture system. J Transl Res. 2018;2(1):2-7.
Abstract
Cancer stem cells (CSCs) are rare cell populations in cancers but play an important role in cancer development and could be associated to all malignant processes of cancer. Effective in vitro expansion of CSCs would greatly help the studies on CSCs by providing greater numbers of these cells and a defined native-like niche where their interactions with other cells and molecules can be examined. We have previously shown that kinetic and static alternating cell culture systems (KSACCS) can effectively improve the growth of multiple cell types. In the current study, we demonstrate that KSACCS with the proper program can significantly increase the expansion efficiency of lung cancer stem cells in comparison with static culture and absolute kinetic culture. The KSACCS expanded CSCs maintained the ability to form tumors in immune deficient mice and to gain some features of the corresponding lung cancer cells in immunology and anti-cancer drug sensitivity
Keywords
Cancer stem cell, Cell expansion, Bioreactor, Chemotherapy, Cytotoxic T cell.
Introduction
Cancer stem cells (CSCs) have been identified as rare cell populations in many cancers, including leukemia and solid tumors [1-4]. Accumulating evidence has suggested that CSCs are capable of self-renewal and differentiation into various types of cancer cells [1,2]. It is broadly accepted that CSCs are responsible for cancer initiation, progression, metastasis, recurrence and drug resistance [3,4]. The CSC hypothesis has recently highlighted the potential for discovery and development of CSC-related therapies and the identification of key molecules involved in controlling the unique properties of CSC populations [5-7]. Recent studies have shown that CSCs exhibit different drug sensitivities compared to the bulk population of tumor cells and represent an attractive therapeutic target [8,9]. Studying these cells, however, has been a challenge due to their low abundance in vivo and the phenotypic plasticity they exhibit during expansion. Using current methods, isolated CSCs lose the expression of CSC markers and tumor initiating capacity when cultured in vitro or in vivo in xenograft animal models [10,11]. The proportion of CSCs often trends to an equilibrium level of less than 1% over time, and the cell population derived from CSC cultures typically recapitulates the heterogeneous nature of the original population. Thus, the goal of this study is to meet the critical need of identifying a cell culture system that can specifically grow CSCs for basic and translational research.
Kinetic and static alternating cell culture system (KSACCS) has been successfully applied in the expansion of hematopoietic stem cells (HSCs), mesenchymal stem cells (MSCs), lymphocytes and many cancer cell lines [12-17]. It also exhibited great advantages in anticancer drug screening and evaluation [18]. Since CSCs and hematopoietic stem cells (HSCs) have many common growth features in vitro, among which, the most important is the ability to differentiate into other cell types. This plasticity makes it extremely difficult to maintain in vivo levels of differentiation potential, the critical feature that is the defining characteristic of a stem cell. Interestingly, CSCs of some cancers express CD133 [10-12], a cell maker normally expressed on HSCs. These CSCs lose CD133 as they differentiate into “mature” cancer cells, just as HSCs do. The CSCs isolated from lung cancer in this study are also positive for CD133 and were cultured under multiple conditions, including KSACCS, rotating clutures, and conventional static cultures to determine the optimal conditions for CSC expansion.
Materials and Methods
Human CSC isolation and identification
Lung cancer (PLs008, small cell lung cancer [SCLC]) primary tumor tissues were homogenized, isolated and cultured as described previously [12,14,17,18]. Cells were seeded (4 cultures/each) in 10 ml cell culture bags for ZYX bioreactor culture [12-18] at 2 × 104/ml and cultured in SCLC expansion medium (IMDM [ATCC30-2005] supplemented with 10% fetal bovine serum, 100 U/mL penicillin, and 100 μg/mL streptomycin [Gibco-BRL, Germany]). Before and on day 6 post-culture, cells were dissociated with enzyme-free cell dissociation buffer (Thermo-Fisher Scientific, Waltham, MA) and analyzed by flow cytometry for cell counting and cell surface marker examination. CSCs were enriched in the CSC enrichment medium (DMEM medium containing 20 μg/ml EGF, 10 μg/ml bFGF [Gibco-Invitrogen], 50 mg/ml insulin, 100 mg/ml apo-transferrin, 10 mg/ml, putrescine, 0.03mM sodium selenite, 2 mM progesterone, 0.6% glucose, 5mM HEPES, 0.1% sodium bicarbonate, 0.4% BSA, glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin [Gibco-BRL, Germany]) [19]. Different doses (0, 2.5, 5.0 and 10 μmol/L) of Cisplatin [18,20-22] and 5-FU [18,23-26] were used for the evaluation of anticancer drug sensitivity to CSCs. 50% of the medium was changed every three days for total 14 days. When the cells reached 85% confluence, cells were dissociated with enzyme-free cell dissociation buffer and analyzed by flow cytometry for CD133 and CD44 examination. CD133 positive cells were isolated with the positive cell selection program of ZYX Bioreactor as previously described [12-17], and were cultured with SCLC expansion medium and CSC enrichment medium for examining the potential of the CSCs in cancer cell development.
Cell enumeration and flow cytometry
Cells were counted using trypan blue dead cell exclusion and flow cytometry with PI staining. CD8+ CTL and CSC were counted by flow cytometry, with cell markers stained using established protocols [17,27-30]. Specifically, CSCs were stained with FITC-anti-human HLA-A,B,C antibody (Biolegend, Sand Diego, CA, Mouse IgG2a, κ) and PE-antihuman CD133 (Biolegend, Mouse IgG1, κ) and APC-antihuman CD44 (Biolegend, Mouse IgG1, κ). Activated CTLs were stained with PerCP-anti-human CD8 (Biolegend, Mouse IgG1, κ) and PE-anti-human CD137 (Biolegend, Mouse IgG1, κ) [17,27-31].
Immune effector expansion and cytotoxic assays
Commercially available human cancer cells and corresponding autologous mononuclear cells (Buffy from peripheral blood, ZYX Biotech Company, Texas, USA) were used for human CTL evaluation. Mononuclear cells were isolated and seeded in a ZYX Bioreactor (ZYX Btr) (ZYX Biotech Company, Texas, USA) cell culture chamber at 106 /ml and cultured for 6 days in RPMI1640 containing FBS, 8 ng/mL IL-2 and 10 ng/ mL IL-7 (PeproTech, Rocky Hill, NJ, USA), and irradiated cancer cells (seeding density determined by surface area, 80% confluent). Cancer cells were used as stimulators and target cells and mononuclear cells as effectors. Established protocols [12-17,27-31] with slight modification were used in this study. In brief, (1) CSCs were enriched in vitro. (2) Some of these cells were treated with mitomycin C and cultured with the mononuclear cells from the same patient in static culture, continuous suspension bioreactors and ZYX Bioreactors (ZYX Btr) with IL-2 and IL-7 for 6 days as determined by the computer program in the ZYX Btr control system. (3) CD8+ cell isolation was accomplished using the cell sorting program in the ZYX Btr or using a Miltenyi cell separation device for the controls. Additional controls consisted of con A-treated CD8+ cells, un-stimulated CD8+ CTL and/or CD8+ Cells stimulated with different tumor types. (4) in vitro CTL assay were performed as previously described [12-17,27-31] withCD133- tumor cells or CD133+ CSCs as target cells. Based on our preliminary studies, E:T ratios of 4:1, 8:1, and 16:1 were used for the cell lysis tests. LDH Cytotoxicity Detection Kit (Clontech, Cat#630117) was used for the CTL Cytotoxicity analysis by following the Manufacturer’s instructions with a slight modification described previously [12-17,27-31]. In more detail, 1x, 2x, or 4x 105/well activated effector cells were mixed with 25,000/well target cells in triplicate, centrifuged at 150 rpm for 10 minutes in round bottom 96-well plates and incubated for 4 hours. Supernatants were then harvested for LDH detection. CTL activity (% lysis) was calculated using the formula (Test cell mix-Effector controlspontaneous release)/(maximum release-spontaneous release).
Mice and grouping
Animals were maintained at Zyxell Inc in accordance with an IRB approved protocol and according to the principles expressed in the National Institutes of Health, USPHS, and Guide for the Care and Use of Laboratory Animals. Female 12-16 week-old NOD/scid mice (Charles River Laboratories, Wilmington, MA) were housed in a specific pathogen-free environment in which cages covered with barrier filters were housed in laminar flow hoods. Twenty four hours before cancer cell inoculation, mice received 3.5 Gy gamma-irradiation as previously described [12- 17,31]. Each irradiated NOD/scid mouse (3 per cohort) received 1 × 106 lung cancer cells or 1 × 106 CD133+ CSCs subcutaneously in the dorsal lateral thorax.
Statistics analysis
ANOVA and Student t-test were used for comparison of means, including those for cell number and their percentages. SAS and SAS Statview (SAS Institute, Cary, NC, USA) were used to perform the statistical analysis. A p-value <0.05 was considered significant.
Results
Isolation and identification of lung cancer CSCs
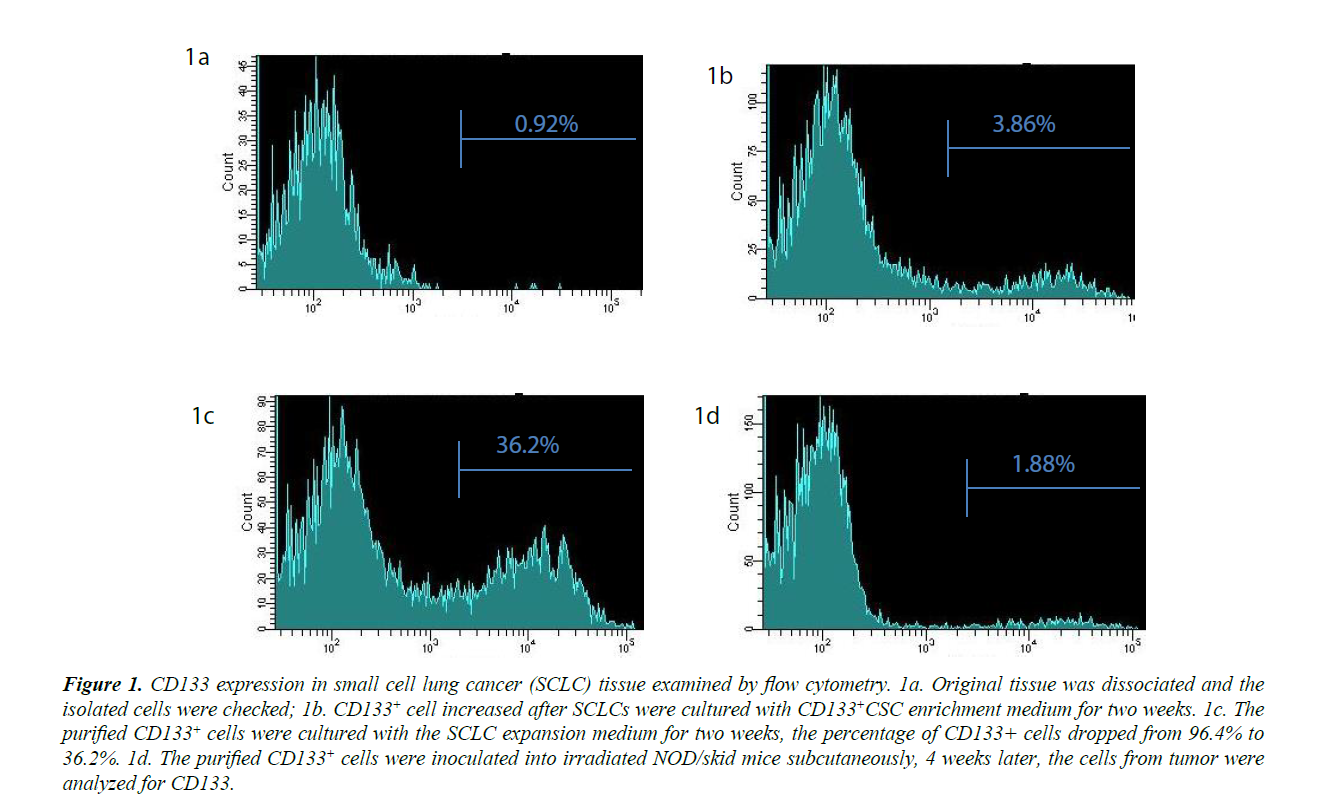
PLs008 small cell lung cancer cells do not express CD133. The percentage of CD133+ cells was below 1%, the threshold level for negative controls (Figures 1A). After enrichment CD133+ cells reached 3.86% (Figure 1B). The enriched CD133 cells were then purified to 96.2% by positive selection. When these isolated CD133+ cells were further cultured in SCLC expansion medium, they gradually lost the CD133 marker and only 36.2% were positive for CD 133 on day 28 (Figure 1B and 1C). NOD/ skid mice inoculated with these CD133+ cells developed a tumor up to (9 × 11 × 15 mm3) 8 weeks later. These tumors contained CD133+ cell numbers (Figure 1D) that were similar to those of the original SCLC tissue (1.67%) when examined by flow cytometry. These data demonstrated that the CD133+ cells isolated from PLs008 cancer cells could differentiate into cancer cells in vitro and in vivo and lose their CD 133 marker. The CD44 levels for these cells were also examined by flow cytometry before and after CSC enrichment. However, the CD44 levels for these cells was similar to that of the negative controls, suggesting CD44 is not co-expressed with CD133 as a CSC marker of PLs008 lung cancer.
Figure 1: CD133 expression in small cell lung cancer (SCLC) tissue examined by flow cytometry. 1a. Original tissue was dissociated and the isolated cells were checked; 1b. CD 133+ cell increased after SCLCs were cultured with CD 133+CSC enrichment medium for two weeks. 1c. The purified CD 133+ cells were cultured with the SCLC expansion medium for two weeks, the percentage of CD 133+ cells dropped from 96.4% to 36.2%. 1d. The purified CD 133+ cells were inoculated into irradiated NOD/skid mice subcutaneously, 4 weeks later, the cells from tumor were analyzed for CD133.
in vitro expansion of CSCs with Kinetic-Static cell culture system
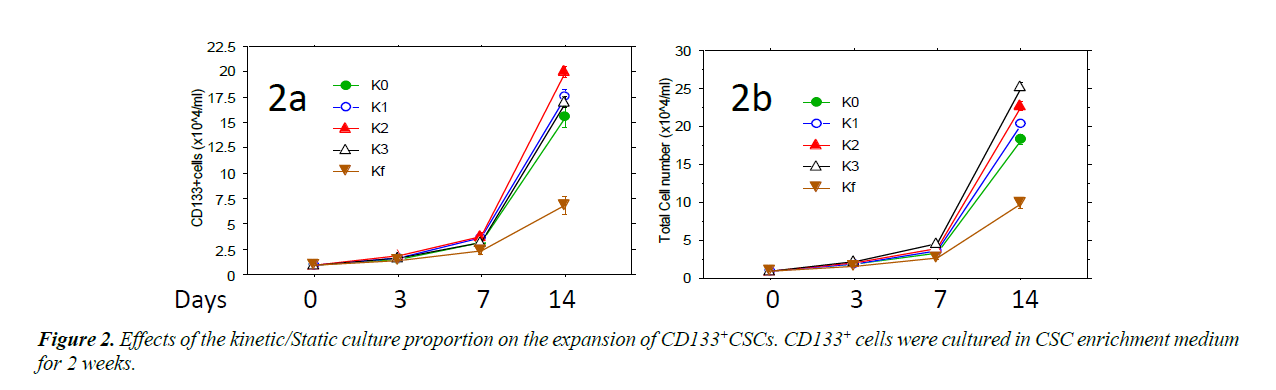
Isolated CD133+ cells were expanded in ZYX bioreactor with different expansion programs. The CD133+CSCs were cultured in CSC enrichment medium using different programs (K0, K1, K2, K3 and Kf) of ZYX Bioreactors to provide different ratios between static and kinetic states [18]. In these programs, Kf was a purely kinetic culture setting in which the bioreactor was constantly rotated and K0 provided a static control in which cultures were not rotated during the culture. For the programs K1, K2 and K3, cultures were vertically rotated once, twice, or three times every 24 hours, respectively. Rotation times started with 5 minutes each and increased gradually and automatically as the cell expansion fold increased until they reaches Kf level. Cells with each program were tested four times and the results show that K2 has the highest CD 133+ cells and K3 has the highest total cell yield.
Different programs of ZYX bioreactor was used for the cell expansion. The proportion of kinetic culture was gradually increased from K0 to Kf, in which K0 was complete static culture and Kf was complete kinetic culture. CD133+ cells proliferated fast with program K2 (Figure 2A) while total cell number increased fastest with program K3 (Figure 2B). On day 14, the CD133+ cell number with K2 was significantly higher than (P<0.01) that with K0 and Kf, the total cell number with K3 was also higher than under purely static or kinetic conditions (P<0.01).
Resistance of CSCs to cytotoxic T lymphocytes
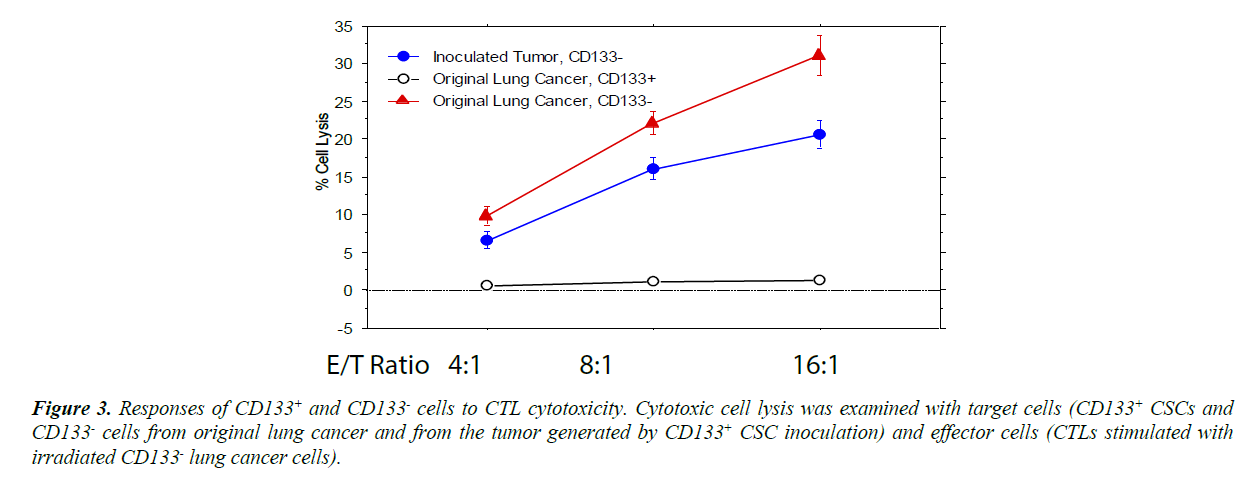
When the CD133+ and CD 133- cells from cultures expanded under program K2 were used as target cells in CTL assays [12- 17,27-31], CD133+ cells did not response to the cytotoxicity of CTLs while CD133- were significantly lysed by CTLs (Figure 3). Since the cytotoxicity of CTLs to the target cells also relies on the MHC class I expression level of the cells, MHC class I expression of these cells was also examined with flow cytometry. However, the MHC class I expression level of CD133+ cells was slightly higher than CD 133- cells (the difference is not significant [P>0.2]), suggesting the diminished cytotoxic responses to CTL in CD133+ cells were not caused by the decreased MHC class I expression. It is possible that CD133+ cells display a lower level of the cancer cell specific antigen(s).
Figure 3: Responses of CD 133+ and CD 133- cells to CTL cytotoxicity. Cytotoxic cell lysis was examined with target cells (CD 133+ CSCs and CD 133- cells from original lung cancer and from the tumor generated by CD 133+ CSC inoculation) and effector cells (CTLs stimulated with irradiated CD 133- lung cancer cells).
The CD133- cells cultured and purified from the original lung cancer were lysed more effectively by the CTL cytotoxicity (P<0.01), but CD133+ CSCs did not show any response. When the CSCs differentiated into CD133- cancer cells, the cells partially recovered their responses to CTL cytotoxicity.
Difference in anticancer drug sensitivity between CD133+ CSCs and CD 133- cancer cells
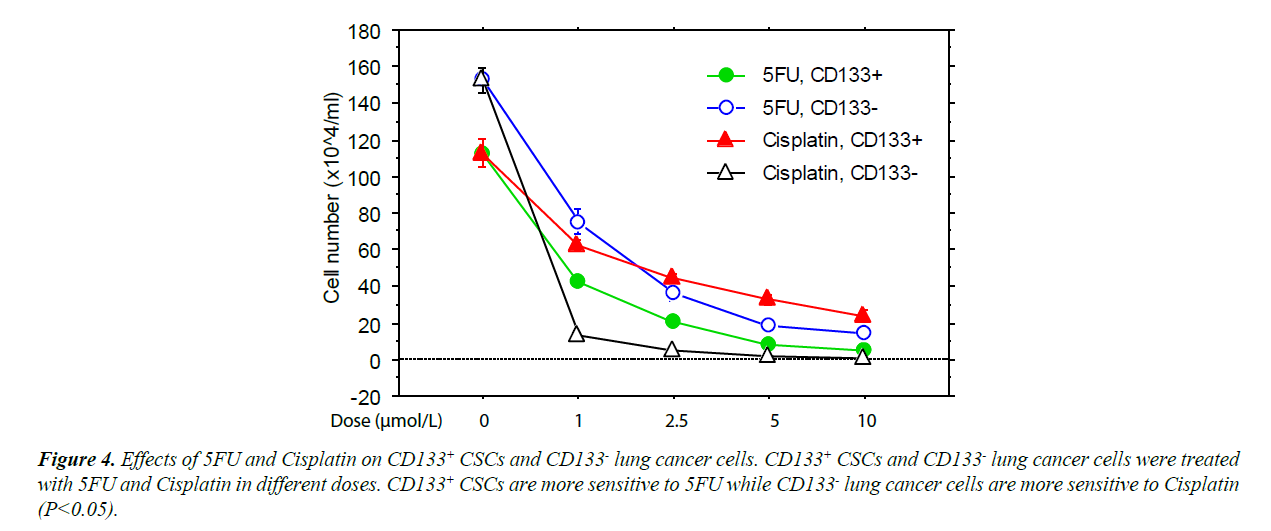
The CD133+ and CD 133- cells expanded with program K2 were further cultured with Cisplatin or 5-FU [18]. These cultures showed that the CD133+ cells were significantly less sensitive to Cisplatin but slightly more sensitive to 5-FU than CD133- cancer cells (Figure 4). This finding suggests that the differentiation state of CSCs affects their resistance to chemotherapeutic agents.
Discussion
CSCs can be found within tumors or hematological cancers and possess characteristics similar to normal stem cells including the ability to give rise to all cell types found in the particular cancer type. CSCs of solid tumors are tumorigenic in contrast to other non-tumorigenic cancer cells. CSCs persist in tumors as a distinct population and cause relapse and metastasis by giving rise to new tumors. CSCs usually express different cell makers from those cancer cells which they potentially develop to. Among their surface markers, CD133 and CD44 are the most frequently reported positive on CSCs [32,33], with CD133 being reported as the key marker for CSCs in lung cancer [19]. In the current study, CD133+ cells were successfully isolated from small cell lung cancer tissue and used to establish solid tumors in immune deficient mice. Most of the cells in the newly developed tumors no longer expressed the CD133 marker. These data are consistent with the findings of other studies of CSCs from SCLC [19].
The efficacy of cancer treatments is usually measured by the ablation fraction of tumor mass. For this reason, CSCs may not necessarily be selected as targets for anti-cancer drugs, since they are a small component of tumors. It is very possible that the cancer cells and their CSCs have different sensitivities to the selected chemotherapies. In our current study, we showed that the CSCs isolated from PLs008 SCLC exhibited significantly higher sensitivity to 5-FU and lower sensitivity to Cisplatin compared to PLs008 SCLC. Since 5-FU is often used to treat leukemia [18,23] and hematopoietic stem cells are normally positive for CD133, it is suggested that 5-FU, as well as other anti-leukemia drugs, could be a potential options for the relatively more effective elimination of CD133+ CSCs.
CD133 is very significant in studying CSC-associated cancer relapse and metastasis [1-4]. However, the extremely small proportion of CSCs in total cancer cells often makes such studies very difficult. Therefore, a highly efficient method of expanding CSCs has great value. Several current bioreactor systems have been utilized as candidate systems for CSC and/ or HSC expansion. However, these systems all have specific disadvantages [13]. The culture systems most commonly used for this purpose are prepared with gel or gel-like cell support materials such as hydrogel to maintain cells in a 3-dimensional (3D) growth environment. These materials are non-physiologic and often artificial. Moreover, gel-based 3D culture systems do not provide appropriate conditions for suspension cell (i.e. HSC and leukemia cells) growth since the latter typically remain in motion in most physiologic situations. Rotation–based 3D culture systems do not need gel-like materials to support cells. Their slow vertical rotation maintains suspension cells or bead-attached cells in 3D growth states. However, in kinetic culture systems, the cells accumulate at the curved bottom when rotation ceases. Such an accumulation prevents normal cell growth. Moreover, rotation systems maintain continuous shear-stress forces, resulting in significant CSC and HSC damage and non-specific differentiation. Other kinetic cell culture bioreactors can exert even higher levels of shear-stress on cells in suspension [13]. The ZYX Btr permits cell culture to alternate between static and kinetic states, maintaining even cell distribution at the bottom or the surface of agitators in the change from kinetic to static state so that cells receive maximum metabolic support and bear minimal shear-stress forces, minimizing non-specific differentiation. KSACCS in ZYX Btr has been successfully applied to the expansion of hematopoietic stem cells, lymphocytes and for anti-cancer drug screening and evaluation [12-17]. In this study, KSACCS also exhibited its potential for the efficient expansion of CSC. We showed that that excessive kinetic culture would be harmful for the growth of CSCs as it was shown to be for a number of other cell types [18]. After in vitro expansion, CSCs maintained their capacity for differentiation and tumorigencity. Also consistent with our previous studies [12-18], the kinetic portion of the culture could be adjusted using the ZYX Btr to improve the expansion of CSCswhile avoiding potentially damaging excessive agitation.
We demonstrated that SCLC or resulting KSACCS-cultureexpanded cancer cells could be lysed by the specific CTLs induced by these cancer cells, though we have not yet identified the specific antigen(s). In contrast, the CSCs do not respond to the cytotoxicity of the PLs008 SCLC-specific CTLs, while the cancer cells from newly formed tumors recovered the response to CTL cytotoxicity. Collectively these findings show that the cancer cell surface antigen expression levels changed over the course of the differentiation of CSCs into SCLC.
In summary, KSACCS promoted the expansion efficiency of CSCs and the expanded CSCs still maintained the capacity for differentiation and tumorigenicity. To further confirm the advantages of KSACCS in CSC expansion, the expansion of the CSCs from different cancers needs to be evaluated. CSCs might not be sensitive to the specific CTLs and chemotherapy which are effective for the corresponding cancer cells, but they could be sensitive to other anticancer drugs. It could be anticipated in future studies that a proper combination of different anticancer drugs would significantly reduce the cancer recurrence by killing both cancer cells and CSCs.
References
- Beck B, Blanpain C. Unraveling cancer stem cell potential. Nature Reviews Cancer. 2013;13(10):727.
- Shackleton M, Quintana E, Fearon ER, et al. Heterogeneity in cancer: cancer stem cells versus clonal evolution. Cell. 2009;138(5):822-9.
- Reya T, Morrison SJ, Clarke MF, et al. Stem cells, cancer, and cancer stem cells. Nature. 2001;414(6859):105.
- Dalerba P, Cho RW, Clarke MF. Cancer stem cells: models and concepts. Annu Rev Med 2007;58:267-84.
- Al-Hajj M, Wicha MS, Benito-Hernandez A, et al. Prospective identification of tumorigenic breast cancer cells. Proceedings of the National Academy of Sciences. 2003;100(7):3983-8.
- O’Brien CA, Pollett A, Gallinger S, et al. A human colon cancer cell capable of initiating tumor growth in immune-deficient mice. Nature. 2007;445(7123):106.
- Zhang S, Balch C, Chan MW, et al. Identification and characterization of ovarian cancer-initiating cells from primary human tumors. Cancer Research. 2008;68(11):4311-20.
- Li C, Heidt DG, Dalerba P, et al. Identification of pancreatic cancer stem cells. Cancer Research. 2007;67(3):1030-7.
- Maitland NJ, Collins AT. Prostate cancer stem cells: a new target for therapy. Journal of Clinical Oncology. 2008;26(17):2862-70.
- Kim YS, Kaidina AM, Chiang JH, et al. Molecular markers of cancer stem cells verified in vivo. Biomeditsinskaia khimiia. 2016;62(3):228-38.
- Alvero AB, Chen R, Fu HH, et al. Molecular phenotyping of human ovarian cancer stem cells unravels the mechanisms for repair and chemoresistance. Cell Cycle. 2009;8(1):158-66.
- Zhang Y, Wang Y, Decker WK, et al. Adaptive Modulation of MHC Class I Expression and Immune Evasion to Cytotoxic Immunocytes in Cancer Cells.
- Zhang Y, Wang X, Pong M, et al. Application of Bioreactor in Stem Cell Culture. Journal of Biomedical Science and Engineering. 2017;10(11):485.
- Zhang Y, Wang Y, Decker WK, et al. Adaptive Modulation of MHC Class I Expression and Immune Evasion to Cytotoxic Immunocytes in Cancer Cells. Journal of Translation Research 2017;1(1):12-19.
- Zhang Y, Wang Y, Zhang M, et al. Restoration of Retarded Influenza Virus-specific Immunoglobulin Class Switch in Aged Mice. Journal of Clinical & Cellular Immunology. 2016;7(2).
- Zhang Y, Farhangfar F, Giblin L, et al. Assessment of Differentiation States of Hematopoietic Stem Cells Following in vitro Culture Using Side and Forward Scatter of Flow Cytometry. World Journal of Research and Review 2017;5(3):75-83.
- Zhang Y, Wang Y, Wang Z, et al. Cancer Specific CTL Expansion with ZYX Bioreactor. Journal of Clinical & Cellular Immunology. 2016;7:398.
- Zhang Y, Wang Y, Wang Z, et al. in vitro Evaluation of Anticancer Drugs with Kinetic and Static Alternating Cell Culture System. Journal of Cancer Therapy. 2017;8(09):845.
- Eramo A, Lotti F, Sette G, et al. Identification and expansion of the tumorigenic lung cancer stem cell population. Cell Death and Differentiation. 2008;15(3):504.
- Yamazoe H, Hagihara Y, Kobayashi H. Multicomponent Co-culture System of Cancer Cells and Two Types of Stromal Cells for in vitro Evaluation of Anticancer Drugs. Tissue Engineering Part C: Methods. 2015;22(1):20-9.
- Barr MP, Gray SG, Hoffmann AC, et al. Generation and characterization of cisplatin-resistant non-small cell lung cancer cell lines displaying a stem-like signature. PloS one. 2013;8(1):e54193.
- Shen DW, Akiyama SI, Schoenlein P, et al. Characterization of high-level cisplatin-resistant cell lines established from a human hepatoma cell line and human KB adenocarcinoma cells: cross-resistance and protein changes. British Journal of Cancer. 1995;71(4):676.
- Yang CZ, Luan FJ, Xiong DS, et al. Multidrug resistance in leukemic cell line K562/A02 induced by doxorubicin. Zhongguo yao li xue bao Acta pharmacologica Sinica. 1995;16(4):333-7.
- Chu E, Zinn S, Boarman D, et al. Interaction of γ interferon and 5-fluorouracil in the H630 human colon carcinoma cell line. Cancer Research. 1990;50(18):5834-40.
- Johnston PG, Drake JC, Trepel J, et al. Immunological quantitation of thymidylate synthase using the monoclonal antibody TS 106 in 5-fluorouracil-sensitive and-resistant human cancer cell lines. Cancer Research. 1992;52(16):4306-12.
- De Angelis PM, Svendsrud DH, Kravik KL, et al. Cellular response to 5-fluorouracil (5-FU) in 5-FU-resistant colon cancer cell lines during treatment and recovery. Molecular Cancer. 2006;5(1):20.
- Mbawuike IN, Zhang Y, Couch RB. Control of mucosal virus infection by influenza nucleoprotein-specific CD8+ cytotoxic T lymphocytes. Respiratory Research. 2007;8(1):44.
- Zhang Y, Qiu J, Zhou Y, et al. Plasmid-based vaccination with candidate anthrax vaccine antigens induces durable type 1 and type 2 T-helper immune responses. Vaccine. 2008;26(5):614-22.
- Zhang Y, Wang Y, Gilmore X, et al. Apoptosis and reduced influenza A virus specific CD8+ T cells in aging mice. Cell Death and Differentiation. 2002;9(6):651.
- Zheng B, Zhang Y, Mbawuike I, et al. Rectification of age-associated deficiency in cytotoxic T cell response to influenza A virus by immunization with immune complexes. J Immunol. 2007;179(9):6153-9.
- Zhang Y, Wang Y, Decker W, et al. Inhibition of Cancer Cell Immune Evasion by Combined Application of Cytotoxic T-Lymphocytes and Natural Killer Cells. Journal of Translation Research 2017;1(1):12-19.
- Sahlberg SH, Spiegelberg D, Glimelius B, et al. Evaluation of cancer stem cell markers CD133, CD44, CD24: association with AKT isoforms and radiation resistance in colon cancer cells. PloS one. 2014;9(4):e94621.
- Kim WT, Ryu CJ. Cancer stem cell surface markers on normal stem cells. BMB reports. 2017;50(6):285.