Research Article - Biomedical Research (2017) Volume 28, Issue 18
Hyperthermia as an adjuvant therapy to chemotherapy for the treatment of advanced ovarian cancer complicated by ascites
Lang He1,2,3#, Jun Wang4#, Hong Chen5#, Xun Wu6, Lina Tang6, Xiaoshan Wang7* and Jianguo Lei8*
1Department of Oncology, the Fifth People’s Hospital of Chengdu, North Sichuan Medical College, Chengdu, Sichuan, PR China
2Cancer Center, the Second Clinical Medical College of North Sichuan Medical College, Nanchong, Sichuan, China
3Cancer Center, Suining Central Hospital, Suining, Sichuan, PR China
4Department of Respiration, the Fifth People’s Hospital of Chengdu, North Sichuan Medical College, Chengdu, Sichuan, China
5Tumor Department of TCM, Sichuan Cancer Hospital and Institute, Chengdu, Sichuan, PR China
6Department of Clinical Medicine, North Sichuan Medical College, Nanchong, Sichuan, PR China
7Cancer Center, Academy of Medical Sciences and Sichuan Provincial People’s Hospital, Chengdu, Sichuan, PR China
8Internal Medicine-Cardiovascular Department, the Fifth People’s Hospital of Chengdu, North Sichuan Medical College, Chengdu, Sichuan, PR China
#These authors have contributed equally to this article
- *Corresponding Author:
- Xiaoshan Wang
Cancer Center
Academy of Medical Sciences and Sichuan Provincial People’s Hospital
Chengdu, Sichuan, PR China
Jianguo Lei
Internal Medicine-Cardiovascular Department
The Fifth People’s Hospital of Chengdu
North Sichuan Medical College
Chengdu, Sichuan, PR China
Accepted date: August 29, 2017
Abstract
In this study, the efficacy of hyperthermia as an adjuvant treatment was assessed for patients with advanced epithelial ovarian cancer complicated by ascites. Forty-eight patients with advanced ovarian cancer and ascites were randomly assigned to two groups. Group A was treated with both hyperthermia (BSD-2000 Hyperthermia System) and chemotherapy (the GT regimen). Group B was treated only with the GT regimen. The curative effects, side effects, Karnofsky Performance Score (KPS), and immune indexes were assessed after two cycles of treatment for both groups. The response rate of Group A was significantly higher than that of Group B (50.0% vs. 25.0%, P<0.05). The median progression-free survival time for Group A was 8.2 months, as compared to 4.8 months for Group B (P<0.05). There was no significant difference between the groups in the disease control rate, overall survival, or improvement in the KPS score. Compared with Group B, the number of CD3+, CD4+, and CD8+ cells in Group A increased remarkably, while the CD4+/CD8+ ratio declined after the treatment (P<0.05 for all). These results suggest that hyperthermia is a promising adjuvant therapy for late-stage ovarian cancer. A future large-scale randomized clinical trial is warranted to confirm this conclusion.
Keywords
Hyperthermia, Chemotherapy, Ovarian cancer, Malignant seroperitoneum
Introduction
Ovarian cancer kills over 10,000 women in the US every year, according to the National Institute of Cancer, and is the fifth leading cause of cancer deaths among women [1]. There are several known risk factors for ovarian cancer, such as age, hormone levels, reproductive history, a family history of ovarian cancer and endometriosis [2]. A key reason for the high mortality rate of ovarian cancer is that most cases are already at an advanced stage when diagnosed [3]. Early-stage ovarian cancers are difficult to detect, because the symptoms are subtle and non-specific [4].
Ascites is one of the complications of advanced ovarian cancer; it causes impaired nutrition and chemotherapeutic efficacy [5]. Intraperitoneal injection (IP) chemotherapy was developed for the treatment of advanced ovarian cancer with ascites [6]. Multiple clinical trials have recently reported that IP chemotherapy lengthens the Progression-Free Survival (PFS) and Overall Survival (OS) [7,8].
The first-line treatment for advanced ovarian cancer is usually combination chemotherapy including platinum-based drugs. However, platinum or paclitaxel-based chemotherapy regimens are not often recommended for second-line treatment. On the other hand, chemotherapy regimens with altered paclitaxel dosage schedules, such as administration of paclitaxel weekly, have been reported to represent effective second-line therapies, with a 21% response rate [9].
Hyperthermia was developed as an adjuvant therapy for radiotherapy and chemotherapy. In hyperthermia, the temperature of the exposed tissue is increased up to 45°C, which is believed to damage the cancer cells [10]. Various clinical trials have been performed to assess the role of hyperthermia in the treatment of various cancers [11]. The results suggest that, as an adjuvant treatment, hyperthermia improves the efficacy of anticancer drugs [12]. Herein, the efficacy of hyperthermia was assessed for the treatment of advanced ovarian cancer complicated by ascites.
Methods
Patients and samples
The ovarian cancer patient cohort consisted of 48 patients pathologically diagnosed with advanced ovarian epithelioma with severe ascites. Drug resistance was observed in all participants. The patients were diagnosed and treated at the Second Clinical Medical College of North Sichuan Medical College. They were randomly assigned to two groups: Groups A and B. Detailed information about the groups is provided in Table 1. Signed informed consent was obtained from each participant. Traditional Chinese medicine and immunotherapy were not administered to any of the participants during the trial. The study was approved by the Ethics Committee of the Second Clinical Medical College of North Sichuan Medical College.
| Age range | Median age | Stage III C | Stage IV | Total cases | |
|---|---|---|---|---|---|
| Group A | 40-72 | 58 | 13 | 11 | 24 |
| Group B | 45-75 | 63 | 10 | 14 | 24 |
Table 1. Information of the participants.
The patient inclusion criteria were as follows: age between 40 and 75 y, Karnofsky performance score (KPS)>60, and an expected lifespan>3 months. All patients had an International Federation of Gynecologists and Obstetricians (FIGO) cancer stage of IIIC or IV, associated with ascites and drug resistance. Furthermore, all patients had cancer cells detectable in the peritoneal fluids. The white blood cell count, platelet count, creatinine level, prothrombin time and activated partial thromboplastin time were within the normal range. The absolute neutrophil count was ≥ 1.5 × 109/L; hemoglobin was ≥ 100 g/L; the serum bilirubin level ≤ 1.5 times the upper limit of normal; aspartate aminotransferase and alanine aminotransferase levels were ≤ 2.0 times the upper limit of normal and no abnormality was observed on electrocardiogram.
The patient exclusion criteria were mental illness, pregnancy or breastfeeding, other cancers or brain metastasis, severe or uncontrollable disorders or infections, organ failure, and other factors such as the patients refusing to undergo chemotherapy or hyperthermia.
Chemotherapy
Group A was treated with hyperthermia and chemotherapy (the GT regimen). Group B was treated with the GT regimen only. The GT regimen consists of 1000 mg gemcitabine (Jiangsu Hansoh Pharmaceutical Co., Ltd. Lianyungang City, Jiangsu Province, China) and 80 mg paclitaxel (Taiji Group Taiji Pharmaceutical Co., Ltd. Fuling city, Chongqing, China) per square meter of body-surface area on days 1 and 8. Standard premedication was administered to prevent hypersensitivity reactions of paclitaxel. The treatments were administered every 28 d (one cycle), for a total of two cycles. Gemcitabine was infused intravenously (IV) before the IP therapy. For the IP therapy, the peritoneal fluid was located by using ultrasonography. A venous catheter (14 G) was implanted using the Seldinger technique. The infusion of the drugs was performed after the peritoneal fluid had been removed completely or the drainage volume was <100 mL over 24 h. The infused liquid was prepared by paclitaxel (80 mg/m2 in 1500 ml normal saline) and dexamethasone (20 mg in 100 ml normal saline). All patients changed their body positions after the IP injections. Antiemetics, hydration therapy, and diuretics were administered. Tests for blood, liver, and kidney functions were performed weekly. Grade 4 myelosuppression was treated with granulocyte colony-stimulating factor.
Hyperthermia
Hyperthermia was performed using the BSD 2000 Hyperthermia System (BSD Medical Corporation, Salt Lake City, Utah, USA). The frequency and output power were 75-120 MHz and 450-550 W, respectively. The hyperthermia treatments were performed in 30 min and 3 d after the IP chemotherapy, for 60 min each. The hyperthermia location was identified by using computed tomography or magnetic resonance imaging. Thermometers were distributed evenly around the tumor. The temperature feedback was collected by a computer so that the tumor temperature was kept at 42.5-43°C. The blood pressure, heart rate, respiratory rate and oxygen saturation were monitored and the rectal temperature was kept at 39-41°C. Patients in Group A received four hyperthermia treatments per cycle.
Assessment criteria
Side effects and quality of life: Toxicity of the treatment was assessed following the National Cancer Institute Common Toxicity Criteria (NCI-CTC, v.4.0). The toxicity was classified into five grades (Grade 0-IV). The performance status was assessed by the KPS. A significant change in the KPS was defined as change>10%. Any change within 10% was considered as a stable KPS. The KPS improvement rate was defined as the percentage of patients with increased and stable KPS.
Efficacy: The response to treatment was assessed by abdominal ultrasound and by evaluating the curative effect of the lesions according to the World Health Organization criteria. The definitions of the responses are summarized in Table 2. The Response Rate (RR) was calculated as the percentage of patients with a Complete Response (CR) or a Partial Response (PR). The Disease Control Rate (DCR) was calculated as the percentage of patients with CR, PR, or Stable Disease (SD).
| Response category | Definition |
|---|---|
| Complete Response (CR) | No ascetic fluid observed for at least four weeks |
| Partial Response (PR) | At least 50% ascetic fluid removed; condition lasts for at least four weeks |
| Stable Disease (SD) | Less than 50% ascetic fluid removed |
| Progressive Disease (PD) | Ascitic fluid volume increases |
Table 2. Definitions of the responses to the treatment.
Progression-free survival and overall survival: The PFS and OS were measured and compared between the groups.
Immune indexes
Peripheral venous blood samples of 2 mL, anti-coagulated with heparin at 1:20 U, were collected under fasting conditions before and after the two cycles of treatment to detect CD3+, CD4+ and CD8+ cells by flow cytometry.
Statistical analysis
Chi-square tests were used in this study. Survival curves were produced by the Kaplan-Meier method and the differences in survival between the groups were compared using the log-rank test. All statistical analyses were performed with SPSS software, version 13.0 (SPSS Institute, Chicago, Illinois, USA).
Results
Side effects and quality of life
The side effects in Group A and B were similar, with all side effects assessed as Grade 0 to Grade III. Blood toxicity, nausea, and vomiting accounted for most side effects. No allergic reactions were observed in either group. There was no significant difference between the two groups in toxicity or side effects (P>0.05). Inhibition of the hemoglobin and platelet syntheses were the most frequently observed blood toxicities. In Group A, we observed 8 and 6 cases of hemoglobin and platelet inhibition, respectively, while the corresponding numbers for Group B were 7 and 4, respectively. These findings are consistent with that of a previous report [13,14]. Fat necrosis, stomach-ache, and constipation were more frequent in Group A than in Group B. These symptoms were relieved after proper treatment. We did not observe any intestinal perforation or obstruction, peritonitis, acute renal failure, or urinary retention in either group. The KPS improvement rates were 66.7% (16/24) and 50.0% (12/24) in Groups A and B, respectively. However, the difference was not significant (Ρ>0.05).
Efficacy
The efficacy of the therapy was assessed by the response of the ascites to the treatment. For Group A, we observed 4, 8, 6 and 6 patients with a CR, PR, SD and progressive disease, respectively. In Group B, the corresponding numbers were 1, 5, 7, and 11 patients, respectively. The response rate of Group A (50.0%, 12/24) was significantly higher than that of Group B (25.0%, 6/24; P<0.05). However, the DCR did not significantly differ between the two groups (75.0% vs. 54.2%, P>0.05). The efficacy assessment data are summarized in Table 3.
| Group | Samples (n) | CR | PR | SD | PD | RR (%) | DCR (%) |
|---|---|---|---|---|---|---|---|
| A | 24 | 4 | 8 | 6 | 6 | 12 (50.0) | 18 (75.0) |
| B | 24 | 1 | 5 | 7 | 11 | 6 (25.0) | 13 (54.2) |
| Total | 48 | 5 | 13 | 13 | 17 | 18 (37.5) | 31 (64.6) |
Table 3. The comparison of effects between the groups.
PFS and OS
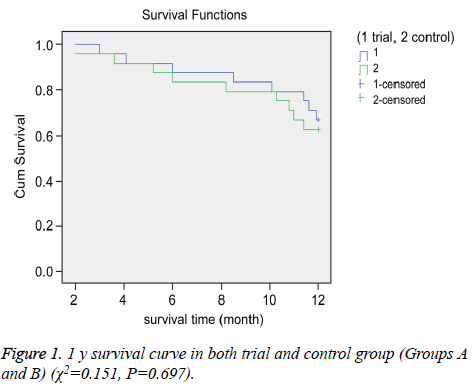
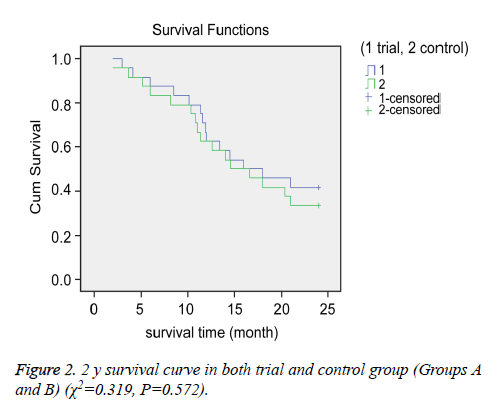
We compared the OS and PFS between the groups. The median OS for Groups A and B were 15.3 and 14.9 months, respectively. The 1 and 2 y survival rates for Groups A and B were 66.7% (16/24) vs. 62.5% (15/24) and 41.7% (10/24) vs. 33.3% (8/24), respectively (Figures 1 and 2).
There were no significant differences in OS between the groups (P>0.05 for all). However, we observed a significant difference in PFS between the two groups. The median PFS for Group A (8.2 months) was longer than that of Group B (4.8 months) (P<0.05).
Immune indexes
After the treatment, the numbers of CD3+, CD4+ and CD8+ cells were significantly increased, while the ratio of CD4+/ CD8+ was decreased, in Group A (P<0.05, Table 4).
| Group | n | Time | CD3+ (%) | CD4+ (%) | CD8+ (%) | CD4+/CD8+ (%) |
|---|---|---|---|---|---|---|
| A | 24 | After test | 54.18 ± 4.01 | 48.42 ± 4.38 | 38.51 ± 3.46 | 1.08 ± 0.71 |
| B | 24 | After test | 43.34 ± 4.06 | 41.39 ± 4.17 | 32.58 ± 3.49 | 1.45 ± 0.54 |
Table 4. The comparison of indexes of immunity between trial and control group after trials (͞x ± s).
Discussion
Ovarian cancer has one of the highest mortality rates among all cancers. Most ovarian cancers are in the advanced stages when diagnosed. Ascites is a frequent complication in patients with advanced ovarian cancer. The treatment options for ovarian cancer with ascites are limited. The standard treatment for advanced ovarian cancer consists of complete cytoreductive surgery and IV combination chemotherapy with a platinum compound and a taxane; the addition of hyperthermia to the standard therapy is intended to prolong survival by reducing peritoneal recurrences [14]. Hyperthermia increases the local temperature of the tumor and is a promising adjuvant treatment for ovarian cancer with ascites. However, the efficacy of hyperthermia for cancer treatment is controversial. We therefore assessed the performance of hyperthermia for advanced ovarian cancer by comparing the efficacy of a chemotherapy-hyperthermia combination with that of chemotherapy only.
Two prior phase III clinical studies have demonstrated that the PFS and OS of ovarian cancer patients were prolonged with the use of first-line postoperative IP chemotherapy. Although the current first-line treatment of advanced ovarian carcinoma is platinum-based chemotherapy, the clinical outcome remains poor, owing to drug resistance. For second-line and recurrence treatment, platinum-based and paclitaxel-based chemotherapy regimens are not recommended, and enrolment in a clinical trial or observational study should be considered for these patients [15]. However, some researchers have pointed out that patients could show remission again by adjusting the dosing regimen of paclitaxel and the efficacy of weekly administration of paclitaxel has been reported to be 21% [16,17]. Based on these previous studies, we altered the chemotherapy regimen and changed the administration method of paclitaxel to the GT regimen (gemcitabine 1000 mg/m2 IV on days 1 and 8; paclitaxel 80 mg/m2 IP on days 1 and 8 per cycle, with each cycle comprising 28 d), which has been previously combined with hyperthermia in order to explore the treatment efficacy for ovarian cancer with malignant peritoneal effusion [18]. In the present study, we adjusted the paclitaxel administration from IV on day 1 and IP on day 8 [19] to IP on days 1 and 8. In addition, the BSD 2000 Hyperthermia System was employed for hyperthermia delivery in Group A. Our revised treatment resulted in reduced hematological toxicity of the chemotherapy and extended the time window for restoring marrow function and regaining physical strength. Given this situation, it is clear that Group A experienced more benefits than Group B.
Hyperthermia is a novel treatment for cancer. It is controlled by a computer, which can adjust the amplitude and phase of each channel, forming a thermal field suitable for the specific tumor shape as a means to reduce the damage to the surrounding normal tissue. Hyperthermia not only has a direct killing effect on tumor cells, but also enhances the sensitivity of radiotherapy and chemotherapy, induces apoptosis of tumor cells, and inhibits tumor angiogenesis. Specifically, it expands the blood vessels inside the tumor, increases the concentration of the internalized drug in the tumor tissue and catalyzes the interaction between the drug and the cancer cell DNA, and improves the curative effect of the chemotherapy [20]. If the response of the ascites is considered the criterion for efficacy, the response rate of the group with hyperthermia treatment (Group A) was 2-fold higher than that of the control group without hyperthermia therapy (Group B) in this study. Although the DCR and quality of life showed no significant differences between the two groups, the DCR and KPS scores in Group A tended to be higher than those in Group B. If the sample size was larger, the difference might have reached statistical significance. Thus, although few patients achieved CR, with most patients experiencing progressive disease after being temporarily stable or improved, the addition of hyperthermia still appears to represent an effective treatment, with a benefit for half of the patients who were drug resistant or had recurrent disease.
Hyperthermia may increase the local effect of the treatment on malignant peritoneal diseases and reduce systemic cytotoxicity [21]. It has been noted that hyperthermia does not significantly enhance the OS rate, and, accordingly, our data indicated no survival rate difference between the group treated with hyperthermia and the control group at 1 and 2 y. However, we did observe an increased PFS in the group treated with hyperthermia. This suggests that hyperthermia may help control the progression of cancer. Although the survival time was not prolonged, the disease control by hyperthermia is still clinically meaningful. In addition, this study found that the numbers of CD3+, CD4+, and CD8+ cells were significantly increased, while the ratio of CD4+/CD8+ cells was clearly decreased in Group A compared to that in Group B after the treatment, indicating that the immune functions of the patients treated with hyperthermia were strengthened. Because of the significant improvement in serous effusion in Group A, the possibility of an immune reaction mediated by hyperthermia should be taken into consideration, as hyperthermia has an effect on neutralizing the immune suppression caused by chemotherapy. Specifically, in the necrocytosis process, heat shock protein produced by hyperthermia is released into the blood and mediates the maturation of dendritic cells to produce specific immune functions; activates natural killer, CD4+ and CD8+ cells to promote the release of cytokines such as interleukin-12 and activates the immune system to eliminate tumor cells in vivo [22].
In summary, based on our clinical data, we conclude that hyperthermia may play a meaningful role in the treatment of advanced ovarian cancer with ascites. However, quality of life and OS time, the most important clinical criteria for cancer treatments, were not significantly improved by the addition of hyperthermia. Our results are consistent with the results of the study by Sugimachi. Based on our findings, several points should be clarified and noted. First, hyperthermia is a promising technique for cancer treatment. We should not deny or underestimate its importance. Our data show that the PFS can be improved by hyperthermia; optimization of the chemotherapy regimen might improve the efficacy of hyperthermia. Second, our study is very preliminary, with a limited sample size and follow-up time. Although the statistical analysis indicated that the OS was not significantly lengthened, this should not be taken to mean that hyperthermia does not play a role in cancer treatment. Considering that, in our study, various factors could have affected the long-term curative effect, such as the age, stage, medical history, and other conditions of the patients, a randomized trial with more participants is necessary to reach a more reliable conclusion.
Conclusion
A preliminary trial was performed to test the value of hyperthermia in the treatment of advanced ovarian cancer with ascites. The results indicated that hyperthermia could improve the tumor response rate and prolongs PFS. However, hyperthermia did not result in significant improvements in the quality of life (assessed by the KPS), DCR, or OS. Based on our data, we propose that a randomized trial with a larger number of participants should be designed to verify our current conclusion.
Acknowledgment
This work was supported by Doctoral start-up fund of North Sichuan Medical College (NO. CBY15-QD09) and Technology support program of Science and Technology Department of Sichuan Province (No. 2014SZ0020-7).
Conflict of Interest
All authors have no conflict of interest regarding this paper.
References
- Howlader N, Noone AM, Krapcho M, Garshell J, Neyman N, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z. SEER Cancer statistics review 1975-2010. National Cancer Institute, Bethesda, 2013.
- Berchuck A, Schildkraut JM, Marks JR, Futreal PA. Managing hereditary ovarian cancer risk. Cancer 1999; 86: 2517-2524.
- Fleming GF, Ronnett BM, Seidman J. Epithelial ovarian cancer. Principles and practice of gynecologic oncology (5th ed). Williams and Wilkins, Lippincott, Philadelphia 2009; 763-836.
- Goff BA. Ovarian cancer: screening and early detection. Obstet Gynecol Clin North Am 2012; 39: 183-194.
- White MA, Agle SC, Padia RK, Zervos EE. Denver peritoneovenous shunts for the management of malignant ascites: a review of the literature in the post LeVeen Era. Am Surg 2011; 77: 1070-1075.
- Sfakianos GP, Havrilesky LJ. A review of cost-effectiveness studies in ovarian cancer. Cancer Control 2011; 18: 59-64.
- Helm CW. Current status and future directions of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in the treatment of ovarian cancer. Surg Oncol Clin N Am 2012; 21: 645-663.
- Tsubamoto H, Itani Y, Ito K, Kanazawa R, Toyoda S, Takeuchi S. Phase II study of interval debulking surgery followed by intra-peritoneal chemotherapy for advanced ovarian cancer: a Kansai Clinical Oncology Group study (KCOG9812). Gynecol Oncol 2013; 128: 22-27.
- Markman M, Blessing J, Rubin SC, Connor J, Hanjani P, Waggoner S. Phase II trial of weekly paclitaxel (80 mg/m2) in platinum and paclitaxel-resistant ovarian and primary peritoneal cancers: a Gynecologic Oncology Group study. Gynecol Oncol 2006; 101: 436-440.
- Hildebrandt B, Wust P, Ahlers O, Dieing A, Sreenivasa G, Kerner T, Felix R, Riess H. The cellular and molecular basis of hyperthermia. Crit Rev Oncol Hematol 2002; 43: 33-56.
- Wust P, Hildebrandt B, Sreenivasa G, Rau B, Gellermann J, Riess H, Felix R, Schlag PM. Hyperthermia in combined treatment of cancer. Lancet Oncol 2002; 3: 487-497.
- Frampton JE. Catumaxomab: in malignant ascites. Drugs 2012; 72: 1399-1410.
- Katsumata N, Yasuda M, Takahashi F, Isonishi S, Jobo T, Aoki D, Tsuda H, Sugiyama T, Kodama S, Kimura E, Ochiai K, Noda K. Dose-dense paclitaxel once a week in combination with carboplatin every 3 weeks for advanced ovarian cancer: a phase 3, open-label, randomised controlled trial. Lancet 2009; 374: 1331-1338.
- Mulier S, Claes JP, Dierieck V, Amiel JO, Pahaut JP, Marcelis L, Bastin F, Vanderbeeken D, Finet C, Cran S, Velu T. Survival benefit of adding Hyperthermic Intraperitoneal Chemotherapy (HIPEC) at the different time-points of treatment of ovarian cancer: review of evidence. Curr Pharm Des 2012; 18: 3793-3803.
- Markman M, Rothman R, Hakes T, Reichman B, Hoskins W, Rubin S, Jones W, Almadrones L, Lewis JL Jr. Second-line platinum therapy in patients with ovarian cancer previously treated with cisplatin. J Clin Oncol 1991; 9: 389-393.
- Ritter M, Teudt IU, Meyer JE, Schröder U, Kovács G, Wollenberg B. Second-line treatment of recurrent HNSCC: tumor debulking in combination with high-dose-rate brachytherapy and a simultaneous cetuximab-paclitaxel protocol. Radiat Oncol 2016; 11: 6.
- Sharma R, Graham J, Mitchell H, Brooks A, Blagden S, Gabra H. Extended weekly dose-dense paclitaxel/carboplatin is feasible and active in heavily pre-treated platinum-resistant recurrent ovarian cancer. Br J Cancer 2009; 100: 707-712.
- Landrum LM, Java J, Mathews CA, Lanneau GS, Copeland LJ, Armstrong DK, Walker JL. Prognostic factors for stage III epithelial ovarian cancer treated with intraperitoneal chemotherapy: a Gynecologic Oncology Group study. Gynecol Oncol 2013; 130: 12-18.
- Armstrong DK, Bundy B, Wenzel L, Huang HQ, Baergen R, Lele S, Copeland LJ, Walker JL, Burger RA. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med 2006; 354: 34-43.
- Stein U, Jurchott K, Walther W, Bergmann S, Schlag PM, Royer HD. Hyperthermia-induced nuclear translocation of transcription factor YB-1 leads to enhanced expression of multidrug resistance-related ABC transpotters. J Biol Chem 2003; 276: 28562-28569.
- Krastev N, Djurkov V, Vladimirov B, Penkov V, Koleva M, Belev N, Grudeva-Popova J, Petrov P, Kiprin G, Asenov K, Rusev P. Intraperitoneal perfusion chemotherapy with hyperthermia in some malignant ascites. Khirurgiia (Sofiia) 2013; 4: 11-18.
- Wang SZ, Wang L, Gao XD, Cheng Z, Bi HG, Wang DZ. Effect of hyperthermic chemotherapy on expression of heat shock protein 70 in maxillofacial squamous cell carcinoma. West Chin J Stomatol 2005; 23: 277-279.