- Biomedical Research (2015) Volume 26, Issue 2
HPLC Determination of Glycyrrhizic and Glycyrrhetinic acids in Weiyanning granule.
Shiqiong Zhou1#, Xiangyou Zhang2#, Xufei Duan3*, Hong Mei11Division of GI, The Children Hospital of Wuhan, Wuhan 430016, China
2Pharmacy Department, The Traditional Chinese Medicine Hospital of Jiangxia, Wuhan 430016, China
3Department of General Surgery, The Children Hospital of Wuhan, Wuhan 430016, China.
#Authors contributed equally to this work.
- *Corresponding Author:
- Xufei Duan
Department of General Surgery
The Children Hospital of Wuhan
100Xianggang Road
Wuhan 430016, China
Accepted date: December 21 2014
Abstract
To establish a method for determination of glycyrrhizic and glycyrrhetinic acids in Weiyanning Granule. The contents of glycyrrhizic and glycyrrhetinic acids are determined by HPLC under conditions as follows: Diamonsil C18 (4.6 mm × 250 mm, 5 μm) column, mobile phase of acetonitrile- 0.05 mol/L ammonium acetate solution (A: 0.05 mol/L ammonium acetate solution; B: acetonitrile, gradient elution: 0~30 min: 32% B; 30~60 min: 75% B); flow rate of 1.0 ml/min; and detection wavelength of 250 nm. The determined linear range of glycyrrhizic acid is 0.320~1.920 μg, and average recovery is 98.6%, with RSD = 1.98%; linear range of glycyrrhetinic acid is 0.039~0.234 μg, and average recovery is 97.3%, with RSD = 3.20%. The method established in this paper is simple, accurate and reproducible, which provides a new way for the quality control of Weiyanning Granule.
Keywordsf
Weiyanning Granule; glycyrrhizic acid; glycyrrhetinic acid; HPLC
Introduction
Weiyanning Granule is composed of ten Chinese medicinal materials including Lignum Santali albi, Fructus Crataegi, Fructus Mume and Radix Glycyrrhizae. It has the middle warming, spleen refreshing, stomach harmonizing, adverse qi descending, dampness eliminating and indigestion curing functions, which is used clinically for the treatment of atrophic gastritis, superficial gastritis, dyspepsia due to overeating and other digestive diseases [1-2].
Radix Glycyrrhizae is the major component of Weiyanning Granule, it contains large amounts of flavonoids and phenolic acids [3-5], of which glycyrrhizic and glycyrrhetinic acids are major active constituents [6-7]. Pilot study has found that Weiyanning Granule contains detectable glycyrrhizic and glycyrrhetinic acids. The drug is not listed in the 2010 Edition of "Chinese Pharmacopoeia"; to effectively control the quality of the preparation, and provide more comprehensive and simpler approach for the research of extraction process and pharmacology of effective parts of Radix Glycyrrhizae, the determination of glycyrrhizic and glycyrrhetinic acids in Weiyanning Granule is hereby reported as follows.
Instruments, Reagents and Drug
Agilent 1200 HPLC; ultrasonic cleaner, purchased from Ningbo Ultrasonic Instrument Co., Ltd.; 0.01 mg accuracy electronic analytical balance, purchased from Tianjin Guangfa Precision Instrument Factory. Acetonitrile was purchased from Sigma (HPLC grade), water was redistilled water, and other reagents were all of analytical grade. Glycyrrhizic acid reference substance was purchased from the National Institute for the Control of Pharmaceutical and Biological Products (batch No.: 965- 200125), glycyrrhetinic acid reference substance was purchased from the National Institute for the Control of Pharmaceutical and Biological Products (batch No.: 111517-200632), Weiyanning Granule was Purchased from Yikang Pharmaceutical Co., Ltd. (batch No.: 20140314, 20140315, 20140316).
Methods and Results
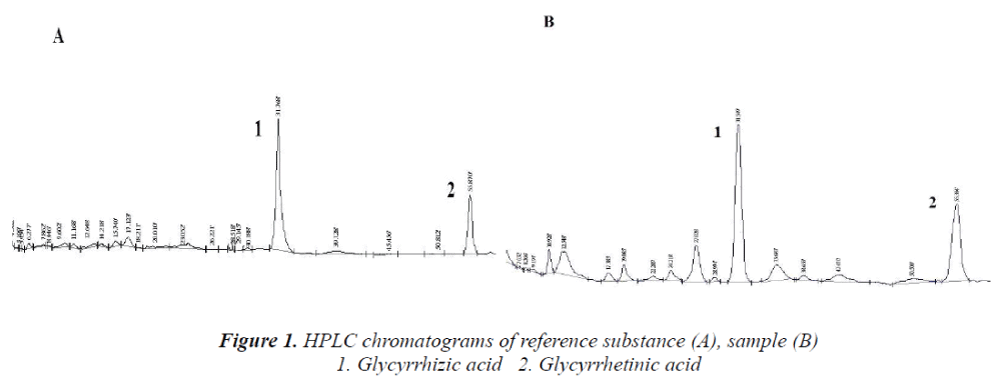
Chromatographic conditions Column: Diamonsil C18 column; mobile phase: A: 0.05 mol/L ammonium acetate solution, B: acetonitrile; gradient elution: 0~30 min: 32% B; 30~60 min: 75% B. Detection wavelength: 250 nm. Flow rate: 1.0 mL/min; column temperature: 30°C, injection volume: 20 μL. HPLC chromatograms are shown in Figure 1.
Preparation of reference solutions
10 mg of glycyrrhizic acid and 2 mg of glycyrrhetinic acid reference substances were precisely weighed, and dissolved to 5 mL with methanol as the reference stock solutions, which were then diluted to obtain mixed reference solutions with glycyrrhizic acid concentration of 0.0320 mg/mL, and glycyrrhetinic acid concentration of 0.0039mg/mL.
Preparation of test solution
0.2 g of Weiyanning Granule was precisely weighed, added with mobile phase (A:B of 1:1), and ultrasonicated at 80°C for 30 min, then diluted to 50 mL, filtered, and the resulting filtrate was used as the test solution.
Investigation of linear relations
2 mL of mixed reference solutions under item "2.2" were accurately weighed, and diluted to 25 mL with methanol, 1, 2, 3, 4, 5 and 6 mL of the resulting solutions were then taken, and diluted to 10 mL with methanol to obtain a total of six concentrations of reference solutions. 20 μL of the solutions were precisely drawn, respectively, and determined under conditions prescribed in item "2.1", linear regression was carried out with injection volume X as the abscissa and peak area Y as the ordinate, and regression equation, correlation coefficients and linear range were obtained as follows: Y=1231.8X+0.9872, r=0.9998, 0.320~1.920 μg (glycyrrhizic acid); Y=1437X-4.296, r=0.9997, 0.039~0.234 μg (glycyrrhetinic acid).
Accuracy test
20 μL of reference solutions were precisely drawn, injected continuously six times, and their peak areas were measured, glycyrrhizic acid RSD = 0.23%, glycyrrhetinic acid RSD = 1.29%.
Reproducibility test
6 aliquots of test solutions were prepared as per the method under item "2.3", 20 μL of each solution was precisely drawn and determined under conditions prescribed in item "2.1", glycyrrhizic acid RSD = 1.98%, glycyrrhetinic acid RSD = 3.20%.
Stability test
Aliquots of 20 μL of the same test solutions were precisely drawn, and determined at 0, 4, 8, 12 and 24 h, respectively, glycyrrhizic acid RSD = 1.37%, glycyrrhetinic acid RSD = 2.13%, which indicated that the test solution was stable within 24 h.
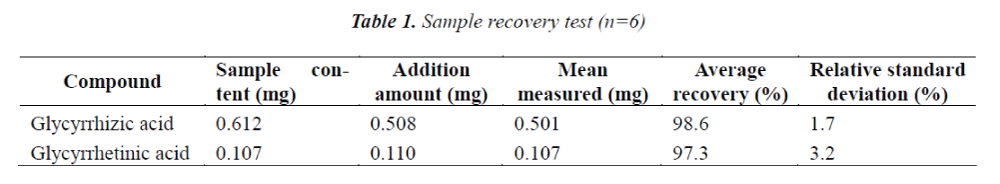
Sample recovery test
6 aliquots of 0.1 g of Weiyanning Granule of known contents were accurately weighed, and added separately with certain amount of reference stock solution, next, test solution was prepared according to the method under item "2.3", and determined under conditions prescribed in item "2.1", the results are shown in Table 1.
Determination of sample content
Weiyanning Granule with three different batch numbers were taken, and prepared into test solutions as per the method under item "2.3", followed by content determination. The results are shown in Table 2.
Discussion
The method established in this paper allows simultaneous determination of glycyrrhizic and glycyrrhetinic acid contents, which can effectively control the quality of Weiyanning Granule, and provides a new way for the quantitative determination of compound preparations containing Radix Glycyrrhizae.
To ensure the sensitivity of the determination method, the detection wavelength was set to 250 nm, the maximum absorption wavelength of glycyrrhizic and glycyrrhetinic acids. As to the selection of mobile phase, the present study has tried acetonitrile-water, acetonitrile- water (1% acetic acid), acetonitrile-ammonium acetate and acetonitrile-methanol systems as well as elution with different gradients, acetonitrile-ammonium acetate system not only allowed better isolation between glycyrrhizic and glycyrrhetinic acids, but also rather good isolation of other ingredients in the formulation, so after comprehensive analysis, elution with acetonitrile-ammonium acetate gradient was adopted. For the selection of column temperature and flow rate, flow rates of 0.8, 1.0 and 1.2 mL/min, and column temperatures of 30°C and 20°C were investigated, which found that chromatographic peaks were better separated at 30°C, so column temperature was set as 30°C. Further investigation of flow rate found that comparatively favorable effect was achieved at a flow rate of 1.0 mL/min.
During the course of this experiment, the extraction solvents of 30%, 50% and 80% methanol, as well as mobile phases of acetonitrile-0.03 mol/L ammonium acetate (1:4; 1:2; 1:1) have been investigated, which revealed that the extraction yield was higher for mobile phase (1:1).
Gastritis is a common gastrointestinal disease [9-10], according to the reported literature, glycyrrhizic acid has a gastritis curing effect [8,11], so we have chosen glycyrrhizic acid and a similar compound, glycyrrhetinic acid, in Weiyanning Granule as the index components for determination, which are able to fully reflect the accuracy and reasonableness of the topic in terms of both activity basis and analysis and determination conditions. Weiyanning Granule is favored by the majority of patients, with huge market sales, so this study not only has laid the foundation for the research of quality control of pharmaceutical preparations, but has also provided the necessary guarantee for health and safety of society.
References
- Wang ZQ, Zhang XR, Zhang XS. 312 Cases of Weiyanning Granule Treating Chronic Atrophic Gastritis. Research of traditional chinese medicine 2001; 5: 12-13.
- Chai XJ, Shi L, Zhu WP, Huang Q. Major Pharmacodynamics Research on Weiyanning Granule. Journal of Chengdu University of TCM 2003; 2: 44-47.
- Kitagawa I, Chen WZ, Hori K, Harada E, Yasuda N, Yoshikawa M, Ren J. ChemInform Abstract: Chemical Studies of Chinese Licorice-Roots. Part 1. Elucidation of Five New Flavonoid Constituents from the Roots of Glycyrrhiza glabra L. Collected in Xinjiang. ChemInform 1995; 3: 126-127.
- Kitagawa I, Chen WZ, Hori K, Harada E, Yasuda N, Yoshikawa M, Ren J. Chemical studies of Chinese licorice-roots. I. Elucidation of five new flavonoid constituents from the roots of Glycyrrhiza glabra L. collected in Xinjiang. Chem Pharm Bull (Tokyo) 1994; 5: 1056-1118.
- Kitagawa i, Chen WZ, Hori K, Harada E, Yasuda N, Yoshikawa M, Ren JL. Chemical studies of chinese licorice-roots .1. Elucidation of 5 new flavonoid constituents from the roots of glycyrrhiza-glabra l collected in xinjiang. Chemical & pharmaceutical bulletin 1994; 5: 1056-1062.
- Gupta S, Sharma R, Pandotra P, Jaglan S, Gupta AP. Chromolithic method development, validation and system suitability analysis of ultra-sound assisted extraction of glycyrrhizic acid and glycyrrhetinic acid from Glycyrrhiza glabra. Nat Prod Commun 2012; 8: 991-994.
- Kruhenbühl S, Hasler F, Krapf R. Analysis and pharmacokinetics of glycyrrhizic acid and glycyrrhetinic acid in humans and experimental animals. Steroids 1994; 2: 121-121.
- Braun L. Liquorice Glycyrrhiza glabra. Journal of complementary medicine 2007; 4: 59-61.
- Sergejs I, Inta LK, Dainius J, Georgijs M, Viesturs P, Konrads F, Ilze K, Aigars V, Ivars T, Marcis L. Gastritis staging: interobserver agreement by applying OLGA and OLGIM systems. Virchows Archiv 2014; 4: 403-407.
- Du YQ, Bai Y, Xie P, Fang JY, Wang XZ, Hou XH, Tian D, Wang CD, Liu YD, Sha WH, Wang BM, Li YQ, Zhang GL, Li Y, Shi RH, Xu JM, Li YM, Huang MH, Han SX, Liu J. Chronic gastritis in China: a national multi-center survey. BMC Gastroenterology 2014; 1: 1-21.
- Valli G, Giardina EGV. Benefits, adverse effects and drug interactionsof herbal therapies with cardiovascular effects. Journal of the American College of Cardiology 2002; 7: 1083-1095.