Research Article - Biomedical Research (2017) Volume 28, Issue 3
Hesperetin inhibits the proliferation of cerebrally implanted C6 glioma and involves suppression of HIF-1α/VEGF pathway in rats
Xin-hua Zhang1, Ning-ning Zhang1, Xian-bing Meng1, Yang Zhang2, Yun Qian1 and Yun-jie Xie2*
1Department of Neurosurgery, Affiliated Hospital of Taishan Medical University, China
2Department of Neurosurgery, Jining NO.1 People’s Hospital, China
Accepted date: August 1, 2016
Abstract
Hesperetin is a plant flavonoid known for its anti-oxidant and anti-cancer properties. The effect of hesperetin treatment on cerebrally implanted C6 glioma cells in rats was studied. C6 tumours were implanted in rats (n=20). The animals with C6 gliomas were treated with hesperetin (10 and 20 mg/kg/ day). Tumour weight, survival of animals, regional tumour permeability analysis was performed. Moreover, the concentration of Bax, bcl-2, Caspase-3, Caspase-9, Claudin-1, Cyclin B1, Cyclin D1, PCNA, VEGF, VEGFR2 and ZO-1 was also determined. The proliferation of glioma cells was also estimated by TUNEL and BrdU staining. Prolonged survival was observed in animals with C6 gliomas when treated with hesperetin. The treatment also attenuated cell proliferation, increased apoptosis and caused arrest of cell cycle, as evident from the differential expression of PCNA, Caspase-3, Caspase-9, Bax/bcl-2, Cyclin B1 and Cyclin D1. Hesperetin treatment of C6 gliomas caused a marked decrease in the tumour permeability and oedema and increased expression of tight junction-associated proteins. Hesperetin treatment also down-regulated the HIF-1a/VEGF/VEGFR2 pathway. We could conclude that hesperetin possesses anti-tumour properties in implanted C6 glioma cells in rats. Therefore, hesperetin treatment may be effective against malignant brain gliomas, functioning by attenuating the growth of tumour and suppression of oedema.
Keywords
Glioma, Brain tumour, C6 cells, Hesperetin, Flavonoids.
Introduction
Gliomas are one of the most common types of brain tumours which are known to affect 0.02% of the human population [1,2]. Glioma arises from the glial cells and is characterized by their histological malignancy and proliferation of the malignant cells [3,4]. The standard therapy for brain tumours includes surgery followed chemotherapy. However, the present approach has a median survival rate of only 12 months [5,6]. The implantation of C6 glioma cells in rats has been established as glioma model. Tumours are characterized by cell proliferation. In glioma cell proliferation is coupled by oedema due to increased permeability caused by the disruption of the Blood Brain Barrier (BBB) [7].
Flavonoids are a group of natural substances known for their several anti-inflammatory and anti-oxidative properties [8]. Flavonoids are also well known for their anticancer properties [9]. The anticancer properties of flavonoids have primarily been attributed to their anti-oxidant nature [10]. However, in addition to anti-oxidant properties, several other effects of flavonoids are also known to prevent tumour cell proliferation such as inhibition of angiogenesis [11] and modulation of cell proliferation [12]. Hesperetin is a naturally occurring flavanone found in citrus fruits. Hesperetin is known for its anticancer and antioxidant properties [13,14]. Moreover, hesperetin has been shown to have high permeability for the Blood Brain Barrier (BBB) [15]. In this study we determined the antiproliferative properties of hesperetin against the cerebrally implanted C6 glioma cells in a rat model. We studied the effect of hesperetin treatment on cell proliferation, apoptosis, cell cycle, oedema associated with gliomas. Moreover, the effect of hesperetin treatment on the survival of C6 glioma animals was also determined.
Materials and Methods
Study animals, tumour implantation and experimental design
Adult male Wistar rats (230 to 260 g) were kept in a 12/12 h dark/light circadian cycle under controlled conditions. The animals were fed with the standard laboratory water and diet (ad libitum). The animal experiment was approved by animal ethics committee of the Dalian Medical University. The entire animals up keeping procedures were carried out according to World Medical Association (WMA) animal ethics guidelines (Declaration of Helsinki).
Hesperetin was procured from Sigma Aldrich (USA). Rat C6 glioma cell line was obtained from American Type Culture Collection (CCL-107™). The cells were cultured in Ham’s medium (Sigma Aldrich, USA) supplemented with foetal bovine serum (2.5%, v/v) and antibiotics (gentamycin, 50 μg/ml and fugizone, 2 μg/ml).
Three months old rats were divided into three experimental groups (20 animals each) at random viz. (1) control group, administered with Phosphate Buffered Saline (PBS) (2) Hesperetin 10 mg/kg/day of body weight (3) Hesperetin 20 mg/kg/day of body weight. Hesperetin was administered orally by gavage for 28 days starting from the second day of secondary tumour implantation.
Implantation of tumour cells and establishment of glioma model
Rat C6 glioma cells were obtained from Sigma-Aldrich, USA. The cells were maintained in monolayers in 100 mm dishes at 37˚C under humidified air (5% CO2/95% air). The cells were cultured in HAMs medium supplemented with foetal bovine serum (FBS 10% final concentration), penicillin-G (50 unit/ml) and streptomycin (50 μg/ml). Cells were harvested during the log phase with a solution of 0.05% trypsin and 0.02% EDTA, and resuspended in minimum essential medium supplemented with FBS to a final concentration of 105 cells per 10 μl for the implantation. The animals were anesthetized by administering xylazine (IM, 10 mg/kg) and ketamine (IP, 100 mg/kg). Post anaesthesia, the heads of animals were shaved and sterilized with povidone and 70% ethanol. The animals were secured on a stereoscopic frame. A burr hole was drilled into the right side of the skull with the stereotactic coordinated defined 3.0 mm towards lateral left and 1 mm towards anterior to bregma. The choice of this site was based on previous reports for example Lal et al. and Kobayashi et al. [16,17]. Approximately 1 × 105 cells were implanted using a 10 μl micro syringe. The burr hole was resealed using bone wax and the skin overlying the hole was sutured. The tumours were harvested after they reached a size of 3 × 3 mm. The harvested tumours were fragmented to one tenths of their size and implanted into the brains of animals. The tumour implantation was performed by drilling a small hole near the bregma and lateral to mid line with a 30-guage needle.
Analysis of water content
The animals were euthanized using an overdose of ketamine (200 mg/kg) and xylazine (40 mg/kg). The brain samples were collected and dissected into individual compartments viz. contralateral and ipsilateral hemispheres and tumour. The samples were weighed immediately after the harvest of samples. Vacuum drying of the brain samples was performed for 14 days. The weight of samples was recorded daily during the drying period and percentage water content was estimated.
Analysis of regional tumour permeability
Quantitative assessment of autoradiography was performed for the determination of vascular permeability. For this, the constant ‘Ki’ was estimated according to Groothuis et al. [7]. The traces for permeability viz. (14C) Alpha-Aminoisobuturic Acid (AIB) and (14C) sucrose were administered as bolus (i.v.) with a total dose of 100 mCi. Blood was sampled from arteries at regular intervals to estimate the radioactivity of plasma using a liquid scintillation counter. The radioactivity was estimated using (14C) standards that were quenched appropriately. The animals were killed by decapitation 15 min after administration of radioactivity. The brain tissue samples were isolated from the killed rats and subsequently frozen by immersing in isopentane kept on dry ice. 20 μm sections were sliced from the frozen samples. Subsequently, the thawed samples were mounted on slides. The autoradiographs of the mounted samples were produced by exposing a phosphor screen with samples along with 14C standards. The estimation of radioactivity was performed by analysing the exposed phosphor screens on ImageJ v.55 (NIH) software. The bloodto- brain transfer constant ‘Ki’ was estimated according to [18].
Estimation of protein concentration using Western blot densitometry
The homogenate of brain samples containing 25 μg total protein was loaded per well on 12% Sodium Dodecyl Sulphate Polyacrylamide (SDS–PAGE). Thereafter, the proteins were electrophoretically transferred on a Polyvinylidene Difluoride (PVDF) membrane after completion of gel run. Blocking of the membranes was performed for 1.5 hours in Tris-buffered saline which contained 0.2% Tween 20 (TBST) and 2% non-fat dry milk. Binding of primary antibodies onto the membranes was performed by incubating it with primary antibody solution in TBST and 2% non-fat dry milk for 8 hours at 4˚C. Then the membranes were washed thrice with TBST. Finally, the membranes were incubated for 1 hour with Horse Radish Peroxidase (HRP) conjugated secondary antibodies and developed using an ECL detection system. The band density on the membranes was scanned and then analysed using the ImageJ (NIH, USA) software program. Primary antibodies for bax, bcl-2, Caspase-3, Caspase-9, Claudin-1, Cyclin B1, Cyclin D1, Proliferating Cell Nuclear Antigen (PCNA), VEGF, VEGFR2 and ZO-1 were used for analysis. All the primary antibodies were procured from Santa Cruz Laboratory, USA.
Estimation of cell death using TUNEL assay
The tumour samples were fixed with formalin at the end of treatment. Apoptosis was estimated using terminal deoxynucleotidyltransferase- mediated dUTP biotin nick endlabelling (TUNEL) assay. Subsequently, the samples were counterstained with methyl green (0.4%) before observation under an optical microscope.
Identification of proliferating cells
5-Bromodeoxyuridine (BrdU) (40 μg/g of body weight) was injected (i.p). The animals were sacrificed 1 hour after administration of BrdU. The estimation of BrdU was performed using the rat BrdU Cell Proliferation ELISA Kit ELISA Kit (abcam, USA) following the manufacturer’s protocol.
Survival assay of C6 glioma animals
In another set of experiment the survival of C6 glioma rats was studied in the three groups as described above. The survival assay started from the day of tumour implantation. For the recording of data the animals were killed by decapitation when a 25% loss of body weight was observed and they were observed to be feeling difficulty in activities such as feeding, ambulation and grooming.
Statistical evaluation
The quantitative results were depicted as means ± SD. Oneway Analysis Of Variance (ANOVA) was used for the analysis of quantitative results. The estimation of deviation between control and treatment groups was performed using student’s Ttest. The maximum threshold for statistically significant Pvalues was set at 0.05.
Results
Effect of hesperetin treatment on rat body weight, tumour weight and survival
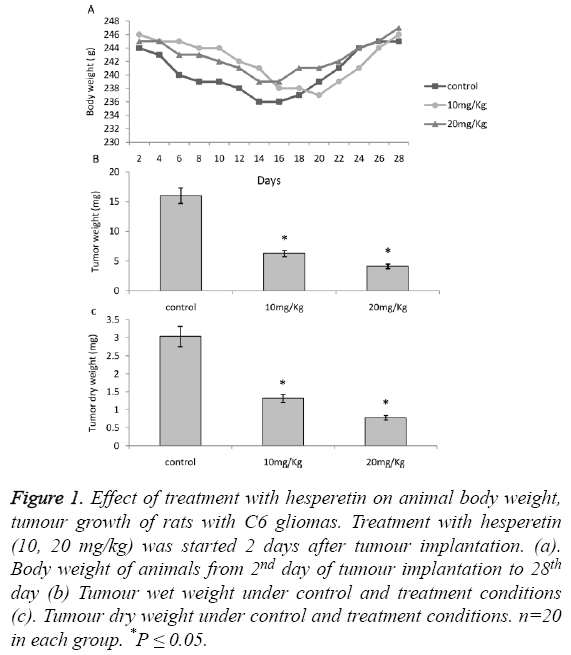
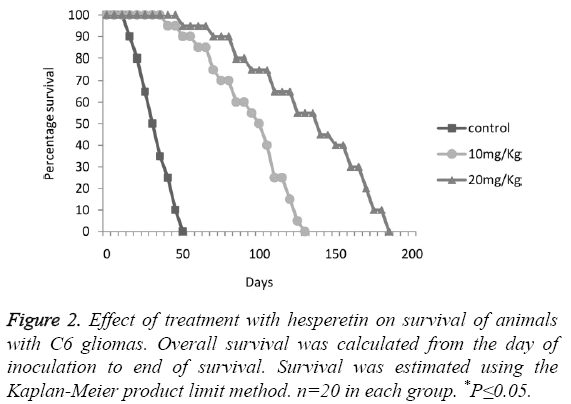
The animals with C6 glioma treated with hesperetin showed no significant difference on body weight as shown in Figure 1A. A significant decrease in tumour weight (wet and dry) was observed 28 days post implantation upon treatment with hesperetin as shown in Figures 1B and 1C. Especially in 20 mg/kg group the decrease was drastic (74.18%). The Kaplan Meier analysis revealed that there was a large and significant variation in the survival curve of control and treatment groups as shown in Figure 2. On a drug dependant manner the treatment groups showed a markedly prolonged lifespan as compared to the control group.
Figure 1: Effect of treatment with hesperetin on animal body weight, tumour growth of rats with C6 gliomas. Treatment with hesperetin (10, 20 mg/kg) was started 2 days after tumour implantation. (a). Body weight of animals from 2nd day of tumour implantation to 28th day (b) Tumour wet weight under control and treatment conditions (c). Tumour dry weight under control and treatment conditions. n=20 in each group. *P ≤ 0.05.
Effect of hesperetin treatment on proliferation of cells, apoptosis and glioma cell cycle
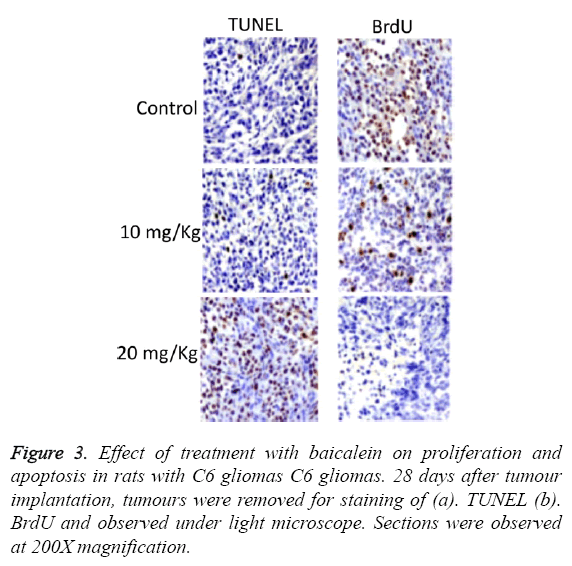
TUNEL and BrdU assays were performed to estimate apoptosis and proliferation of cells respectively as shown in Figure 3. The results of these assays clearly revealed that the treatment with hesperetin caused an increase in TUNEL +ve whereas a large decrease in BrdU +ve cells was observed.
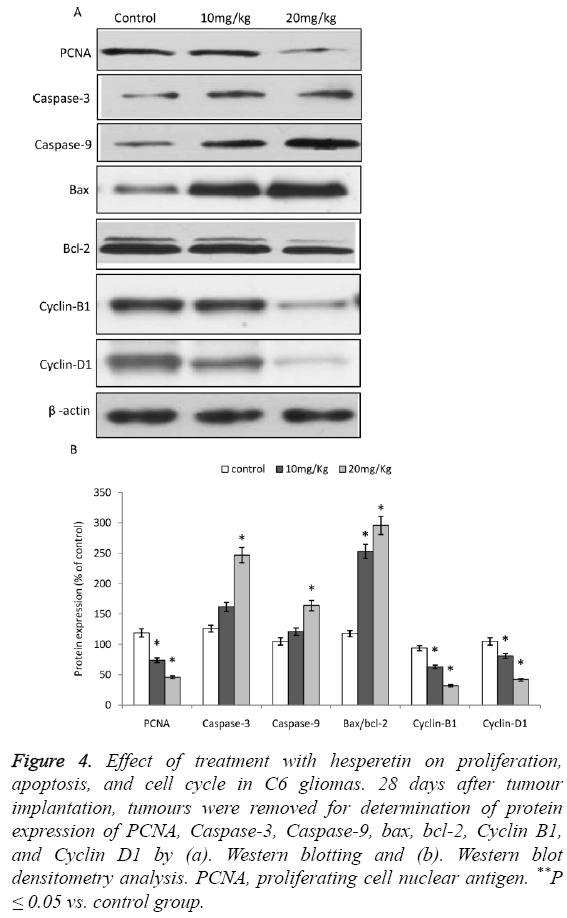
As evident from the Western blot analysis, the expression of PCNA, a marker for tumour proliferation [19] was significantly decreased upon treatment with hesperetin as shown in Figures 4A and 4B. Whereas, the expressions of Caspase-3 and 9 were significantly up-regulated. However, the expression of Caspase-3 and 9 were not significantly larger than control in the 10 mg/kg group. Moreover, the Bax/bcl-2 ratio was increased upon treatment of C6 glioma with hesperetin. The cell cycle proteins Cyclin B1 and D1 showed a reduced expression.
Figure 4: Effect of treatment with hesperetin on proliferation, apoptosis, and cell cycle in C6 gliomas. 28 days after tumour implantation, tumours were removed for determination of protein expression of PCNA, Caspase-3, Caspase-9, bax, bcl-2, Cyclin B1, and Cyclin D1 by (a). Western blotting and (b). Western blot densitometry analysis. PCNA, proliferating cell nuclear antigen. **P ≤ 0.05 vs. control group.
Effect of hesperetin treatment on tumour oedema and permeability
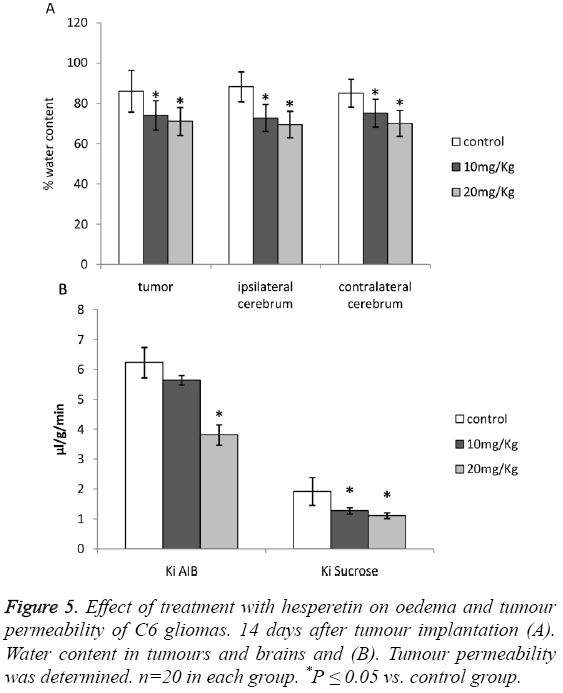
A significant reduction in the water content of tumours and that of ipsilateral and contralateral cerebrums was observed as shown in Figures 5A and 5B. The results pointed out a significant attenuation of oedema caused due to C6 glioma. Although Ki of AIB was higher than control in the 10 mg/kg group, the difference was not significant.
Effect of hesperetin treatment on tight junction (TJ)- proteins and HIF-1a/VEGF/VEGFR2 pathway
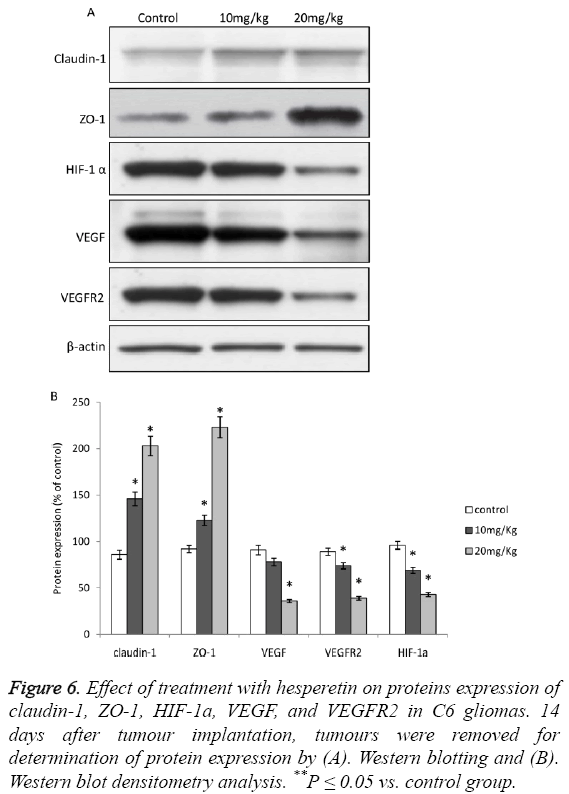
Upon treatment of animals induced with C6 glioma with hesperetin caused significant increase in the expression of TJassociated proteins viz. Claudin-1 and ZO-1 as shown in Figures 5A and 5B. The effect of hesperetin on C6 glioma was also evident on the HIF-1a/VEGF/VEGFR2 proteins where the expression of these proteins was significantly decreased as shown in Figures 6A and 6B. However the expression of VEGF in 10 mg/kg group did not show significant difference from the control group. Overall, these results showed that the HIF-1a/VEGF pathway was down-regulated upon treatment with hesperetin.
Figure 6: Effect of treatment with hesperetin on proteins expression of claudin-1, ZO-1, HIF-1a, VEGF, and VEGFR2 in C6 gliomas. 14 days after tumour implantation, tumours were removed for determination of protein expression by (A). Western blotting and (B). Western blot densitometry analysis. **P ≤ 0.05 vs. control group.
Discussion
In this study we evaluated the effect of flavonoid hesperetin on orthotopic C6 gliomas in rats. The weight of tumour was significantly decreased whereas the survival of animals was significantly increased upon treatment with hesperetin.
With hesperetin treatment, PCNA was found to be downregulated. PCNA is a well-known marker for detection of cell proliferation [19]. Down-regulation of PCNA indicated a depression in tumour growth. The arrest of tumour growth is also indicated by up-regulation of apoptotic pathways. Apoptosis proteins, Caspase-3 and -9 have been observed to get up-regulated in glioma [20]. In this study the up-regulation of caspase-3 and 9 clearly indicated elevated apoptosis in tumor cells. Moreover, the Bax/bcl-2 ratio was also increased. An elevated Bax/bcl-2 ratio is also an indicator of apoptosis [20]. The cell cycle proteins, Cyclin B1 and D1 showed marked down-regulation upon treatment with hesperetin in C6 glioma. These proteins are commonly observed to be upregulated in tumour cells [21,22] and their down-regulation indicates arrest of the cell cycle.
A significant attenuation of oedema was observed upon treatment with hesperetin. Cerebral oedema is frequently observed in glioma patients. Moreover, oedema is primary cause of clinical deficits in the patients of glioma [23,24]. Therefore, the attenuation of oedema with hesperetin may help in the animal survival rate. In humans, oedema of tumour is primarily caused by the disruption of the BBB [25] which is detected by the presence of plasma proteins such as HIF-1a, VEGF and VEGFR2 in the parenchyma of tumour. An upregulated HIF-1α/VEGF pathway indicates induction of oedema in tumours [26]. In this study, the HIF-1α/VEGFR2 pathway was fund to be significantly down-regulated in C6 gliomas when treated with hesperetin thus indicating suppression of oedema. Moreover, the expression of TJassociated proteins was found to be up-regulated in the hesperetin treatment groups. In humans, down-regulation of the TJ-associated proteins is known to be associated with gliomas [27,28]. TJ-associated proteins have been shown to regulate the VEGF pathway [29]. These proteins also mediate the process of increase in permeability by VEGF in gliomas [30].
Conclusion
The plant flavonoid hesperetin could function as a therapeutic agent for glioma. This treatment can not only caused decreased the cell proliferation and oedema associated with gliomas but also increased the apoptosis in the tumour cells. Hesperetin also appeared to down-regulate the HIF-1α/VEGFR2 pathway. However, further studies are needed to identify weather attenuation of oedema was caused due to down-regulation of HIF-1α/VEGFR2 pathway and up-regulation of TJ-associated proteins.
Acknowledgements
YX conceptualized the study, YX, XZ, NZ, XM, YZ and YQ carried out the experiments, YX wrote the paper.
Conflict of interest
The authors declare no conflict of interest associated with this work.
References
- Furnari FB, Fenton T, Bachoo RM, Mukasa A, Stommel JM, Stegh A, Hahn WC, Ligon KL, Louis DN, Brennan C. Malignant astrocytic glioma-genetics, biology, and paths to treatment. Genes develop 2007; 21: 2683-2710.
- Fisher PG, Buffler PA. Malignant gliomas in 2005-where to GO from here? Jama 2005; 293: 615-617.
- Fu YJ, Du J, Yang RJ, Yin LT, Liang AH. Potential adenovirus-mediated gene therapy of glioma cancer. Biotechnol Lett 2010; 32: 11-18.
- Ohgaki H, Kleihues P. Epidemiology and etiology of gliomas. Acta Neuropathol 2005; 109: 93-108.
- Ohgaki H, Dessen P, Jourde B, Horstmann S, Nishikawa T. Genetic pathways to glioblastoma-a population-based study. Cancer Res 2004; 64: 6892-6899.
- Stupp R, Mason WP, Van Den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 2005; 352: 987-996.
- Groothuis DR, Fischer JM, Pasternak JF, Blasberg RG, Vick NA, Bigner DD. Regional measurements of blood-to-tissue transport in experimental RG-2 rat gliomas. Canc res 1983; 43: 3368-3373.
- Nijveldt RJ, van Nood E, van Hoorn DE, Boelens PG, van Norren K. Flavonoids: a review of probable mechanisms of action and potential applications. Am J Clin Nutr 2001; 74: 418-425.
- Galati G, OBrien PJ. Potential toxicity of flavonoids and other dietary phenolics: significance for their chemopreventive and anticancer properties. Fr Radic Biol Med 2004; 37: 287-303.
- Yvonne O, Driss F, Dang PMC, Elbim C, Gougerot-Pocidalo MA, Pasquier C, Benna J. Antioxidant effect of hydroxytyrosol, a polyphenol from olive oil: scavenging of hydrogen peroxide but not superoxide anion produced by human neutrophils. Biochem pharmacol 2004; 68: 2003-2008.
- Afaq F, Saleem M, Krueger CG, Reed JD, Mukhtar H. Anthocyanin-and hydrolyzable tannin-rich pomegranate fruit extract modulates MAPK and NF-B pathways and inhibits skin tumorigenesis in CD-1 mice. Int J Canc 2005; 113: 423-433.
- Yang CS, Landau JM, Huang MT, Newmark HL. Inhibition of carcinogenesis by dietary polyphenolic compounds. Ann rev nutr 2001; 21: 381-406.
- Alshatwi AA, Ramesh E, Periasamy V, Subash-Babu P. The apoptotic effect of hesperetin on human cervical cancer cells is mediated through cell cycle arrest, death receptor, and mitochondrial pathways. Fund clin pharmacol 2013; 27: 581-592.
- Cho J. Antioxidant and neuroprotective effects of hesperidin and its aglycone hesperetin. Arch Pharm Res 2006; 29: 699-706.
- Youdim KA, Dobbie MS, Kuhnle G, Proteggente AR, Abbott NJ, Rice-Evans C. Interaction between flavonoids and the blood-brain barrier: in vitro studies. J neurochem 2003; 85: 180-192.
- Lal S, Lacroix M, Tofilon P, Fuller GN, Sawaya R, Lang FF. An implantable guide-screw system for brain tumor studies in small animals. J neurosurg 2000; 92: 326-333.
- Kobayashi N, Allen N, Clendenon NR, Ko LW. An improved rat brain-tumor model. J Neurosurg 1980; 53: 808-815.
- Nomura T, Inamura T, Black KL. Intracarotid infusion of bradykinin selectively increases blood-tumor permeability in 9L and C6 brain tumors. Brain res 1994; 659: 62-66.
- Louis DN, Edgerton S, Thor AD, Hedley-Whyte ET. Proliferating cell nuclear antigen and Ki-67 immunohistochemistry in brain tumors: a comparative study. Acta Neuropathol 1991; 81: 675-679.
- Chen TC, Lai KC, Yang JS, Liao CL, Hsia TC, Chen GW, Lin JJ, Lin HJ, Chiu TH, Tang YJ. Involvement of reactive oxygen species and Caspase-dependent pathway in berberine-induced cell cycle arrest and apoptosis in C6 rat glioma cells. Int J oncol 2009; 34: 1681-1690.
- Velpula KK, Dasari VR, Tsung AJ, Gondi CS, Klopfenstein JD. Regulation of glioblastoma progression by cord blood stem cells is mediated by downregulation of cyclin D1. PLoS One 2011; 6: e18017.
- Janss AJ, Maity A, Tang CB, Muschel RJ, McKenna WG, Sutton L, Phillips PC. Decreased cyclin B1 expression contributes to G2 delay in human brain tumor cells after treatment with camptothecin. Neuro oncol 2001; 3: 11-21.
- Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, Ludwin SK, Allgeier A, Fisher B, Belanger K. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study-5-year analysis of the EORTC-NCIC trial. lancet oncol 2009; 10: 459-466.
- Papadopoulos MC, Saadoun S, Davies DC, Bell BA. Emerging molecular mechanisms of brain tumour oedema. Br J Neurosurg 2001; 15: 101-108.
- Groothuis DR, Lapin GD, Vriesendorp FJ, Mikhael MA, Patlak CS. A method to quantitatively measure transcapillary transport of iodinated compounds in canine brain tumors with computed tomography. J Cereb Blood Fl Metabol 1991; 11: 939-948.
- Gerstner ER, Duda DG, di Tomaso E, Ryg PA, Loeffler JS, Sorensen AG, Ivy P, Jain RK, Batchelor TT. VEGF inhibitors in the treatment of cerebral edema in patients with brain cancer. Nature Rev Clin Oncol 2009; 6: 229-236.
- Lee J, Baird A, Eliceiri BP. In vivo measurement of glioma-induced vascular permeability. Methods Mol Biol 2011; 763: 417-422.
- Rascher G, Fischmann A, Kroger S, Duffner F, Grote EH, Wolburg H. Extracellular matrix and the blood-brain barrier in glioblastoma multiforme-spatial segregation of tenascin and agrin. Acta neuropatholog 2002; 104: 85-91.
- Wang W, Dentler WL, Borchardt RT. VEGF increases BMEC monolayer permeability by affecting occludin expression and tight junction assembly. American Journal of Physiology-H Circul Physiol 2001; 280: 434-440.
- Herr D, Sallmann A, Bekes I, Konrad R, Holzheu I, Kreienberg R, Wulff C. VEGF induces ascites in ovarian cancer patients via increasing peritoneal permeability by downregulation of Claudin 5. Gynecol oncol 2012; 127: 210-216.