Review Article - Journal of Molecular Medicine and Therapy (2017) Journal of Molecular Medicine and Therapy (Special Issue 1-2017)
FBN1 and TGFB1: Molecular mechanisms in the pathogenesis of thoracic aortic aneurysms and dissections.
- *Corresponding Author:
- Ramune Sepetiene
Laboratory of Molecular Cardiology Institute of Cardiology Lithuanian University of Health Sciences, Sukileliu 15 Kaunas Lithuania
Tel: +37037302873
E-mail: sepetiene@yahoo.co.uk
Accepted date: December 12, 2016
Citation: Sepetiene R, Simonyte S. FBN1 and TGFß1: Molecular mechanisms in the pathogenesis of thoracic aortic aneurysms and dissections. Mol Med Ther. 2016;1(1):1-7
Abstract
Thoracic aortic aneurysms (TAAD) develop asymptomatically until occurs aortic rupture or dissection often cause of morbidity. A high mortality is determined by TAAD and complications developing. 15,000 people die every year due to the complications of TAAD in USA. It takes 14th place according to the reasons of mortality among 55 years people and older. The main risk factors for TAAD formation still remain under discussion. Hypertension, atherosclerosis, age, gender and eventually genetic predisposition are on the focus for the research. Only in certain cases it is caused by aortitis, atherosclerosis or inherited as a single gene mutation: in the fibrillin genes - Marfan syndrome, by inherited collagen mutations as in Ehler-Danlos syndrome, by mutations of the transforming growth factor-beta gene causing Loeys-Dietz syndrome or by actin gene mutations. Evidence has shown that FBN1 mutations may predispose TAA in the absence of phenotypic characteristics of Marfan syndrome. Recently published data from the genome-wide association study (GWAS) identified novel associations of FBN1 SNPs: namely, rs1036477, rs2118181, rs10519177, rs4774517, rs755251, with sporadic TAAD. These data extend knowledge on the molecular pathways leading to sporadic thoracic disease and connect it with a model of Marfan syndrome. There are anatomical, histological and molecular findings discussed within pathogenesis of TAA development. Fibrillin -1 (FBN1), transforming growth factor (TGF), matrix metalloproteases (MMP) are analyzed in the TAA molecular background and their importance for clinical determination and individualization are more often valuable. How FBN1 or TGFBR mutations lead to the disease are not well understood at a molecular level, the proposed mechanisms trigger the alterations in calcium binding EGF-like domains and increased bioavailability of transforming growth factor. The purpose of this review is to concentrate the available research information with focus on TAA common development trying to find a key for early diagnostic points.
Keywords
Transforming growth factor, Bioavailability, Matrix metalloproteases (MMP), Thoracic aortic aneurysms (TAAs).
Introduction
Thoracic aortic aneurysms (TAAs) present a major group of diseases of cardiovascular pathology affecting 5.9:100,000 worldwide [1]. Aortic aneurysms develop asymptomatically until occurs aortic rupture or dissection often cause of morbidity. The main risk factors for TAA formation still remain the same for all cardiovascular pathology, including hypertension, atherosclerosis, age, gender and eventually genetic predisposition, which is on the focus for ourdays researches [2].
TAA develop and expand as a result of the predominant destruction within remodeling process where the alterations weakening the vascular wall increase the risk of dissection and rupture [3,4]. The extracellular matrix (ECM) responsible for aortas wall resistance to the different blood pressure, vascular smooth muscle cells (VSMC) and endothelial cells involved to the different mechanisms of remodeling play the crucial role in the pathogenesis of TAA [5]. Fibrillin -1 (FBN1), transforming growth factor (TGF), matrix metalloproteases (MMP) are analyzed in the TAA molecular background and their importance for clinical determination and individualization are more often valuable. How FBN1 or TGFBR mutations lead to the disease are not well understood at a molecular level, the proposed mechanisms trigger the alterations in calcium binding EGF-like domains and increased bioavailability of transforming growth factor [5-9].
Extracellular Matrix and Proteases
The main roles of ECM destruction share various proteases: matrix metalloproteases (MMP), chymase, triptase, cathepsins, serine elastase from neutrophils, enzymes from tPA, uPA. This seems to be proved by Dobrin et al. [10,11]. His experiments in vitro showed that elastase and collagenase trigger vessel dilatation and rupture. Following the proteolysis studies a lot of researches’ works were done on abdominal aortas, using reproducible animal models as well [12,13]. The proteolytic activity in tissues remains defendant from a balance between proteases and their inhibitors. Inhibitors of MMPs, tissue plasminogen activator (tPA) are more expressed in atherosclerotic diseases of aorta than in dilatative pathology of vessels, thus ECM destruction by decrease activity of inhibitors in the wall of aortas may lead to their aneurysmatic issue [13]. The role of MMPs pathway has been described using gene and cell transfer to show high expression of TIMP and plasminogen inhibitor (PAI-1) in a remodeling process of aortas tissue [14].
Predisposition of MMP-2 and MMP-9 expression has been reported to be involved to the TAA development caused by ECM destruction [15,16]. Proteases origin, release and role for dilatation the aortic wall is recognized but the regulation process still remains under discussion. Cystic medial necrosis associated with TAA is definitely of non-inflammatory origin. Immunohistochemical staining with monoclonal antibodies against CD3C showed the significantly increased amount of T lymphocytes flattening the media of TAA [17]. Mononuclears as T lymphocytes and macrophages have been observed in various types of TAA within mixed etiologies [18].
This could prove that infiltration of T lymphocytes into TAA occurs through the vasa vasorum due to their excessive amount around the vasa vasorum within revascularization process [19,20]. Tang with colleagues found that Th1-type immune responses with activated CD4 and CD8 producing interferon- γ predominate in TAA suggesting the reason why the vessel wall is expanding [21].
Luminal thrombus occurred due to the atherosclerotic lesion in rare cases of TAA could collaborate to release proteases from polymorph nuclear neutrophils. This was detected by Sangiorgi et al. in type A dissections in humans. It has been found increased MMP-9 levels in plasma as early as 1 h after symptoms onset suggested that degranulation of polymorphonuclears release the proteases [17]. It is possible to speculate that the thrombus modulates the aortic size by trapping neutrophils with proteases release to the aortic wall.
Smooth Muscle Cells
The results of affected SMC exposed to TAA development are multiple and complex. A medial degeneration in TAA is common not in aging process itself but was observed in patients with TAA. Histological investigations indicated that TAA has a greater medial area compare to healthy aorta. Collagen and elastin are significantly reduced in the wall. Paul et al. reported that SMC density is not reduced in TAA [21]. The changes in aortas wall parameters could be spotted due to SMC remodeling during dilatation process and proteolysis excluding the role of apoptosis when contractile type of myocytes turns to synthetic type [22]. The controversial findings were published by He et al. [18]. He used α-actin staining method and noticed the normal aortas had significantly more α-actin staining than pathological TAA. Another team with Zhu et al. investigating MYH11 gene in patients with familial TAA also observed the SMC reduction. Their works presumed the apoptosis might be the main reason of SMC changes in TAA wall despite they did not observe degraded DNA ladder in electrophoresis gel assay [16,21]. The increased knowledge of the genes affecting individually TAA syndrome could explain the differences in SMC density and apoptosis.
Experimental works with abdominal aortas showed that addition of SMC prevents aneurysmal formation, suspending expansion in an already dilated vessel [23,24]. This fact proposes to think the similar mechanism could be discovered for TAA despite the different structure between TAA and AAA (the medial layer is thicker in thoracic aortas). As SMC supports vessel wall hypertrophy and contributes the healing process, when mechanical stimulation of these cells allows producing TGFbeta1, ECM and inhibitors of proteolysis, which all together increase aortas wall size [25]. The other way in contrast the macrophages respond to mechanical damage releasing proteases with effect on ECM and wall destruction during dissection formation. Both mechanisms: destruction and reconstruction or healing depends individually and perform in particular manner. The differences in aortic healing between dissection and aneurysm depend on a level of lesion but differences in patient homeostasis remains unclear [2].
TGF-beta
The role of TGF-beta signaling pathways in ascending TAAs described in Marfan syndrome stimulated a particular interest within non syndromic features of TAA. TGF-beta is a family of soluble proteins, cytokines, including three TGF-beta isoforms that has been involved in various cellular processes: angiogenesis, proliferation, differentiation, apoptosis, wound healing and described in conjunction within modification of the ECM [3,26,27]. A lot of studies for its role in stimulating collagen production and regulation pathways involved in pathogenesis of fibrosis of the liver, heart and the lung [28,29]. The main sources of TGF harvest in the body are bone matrix and the α-granules of platelets. Cells secrete TGF-beta in a biologically inactive, latent form bound to propeptide and called LAP (latency associated peptide). Use of transient acidification release TGFbeta from its non-covalent association with LAP. Other proteins such as proteoglycans, type IV collagen, and fibronectin bind TGF in non-covalent manner. The determination of TGF- beta in blood (free TGF-beta or associated with α2macroglobin-α2M) has been involved in the diagnostics of cancer, immunological disorders, hematology and fibrotic diseases [30,31]. The classical TGF-beta or Smad mediated signaling pathway is important to induce ECM deposition as well as repressing ECM degradation (through TIMP-1, TIMP-3) [32,33].
In the classical TGF-beta signaling pathway all three isoforms dimerize and bind to a heteromeric receptor complex, both of them consisting of two types I and II receptors with serinethreonine kinase activity. Type II receptor activates type I receptor through transphosphorylation way [34,35]. The activated type I receptor phosphorylates a receptor- Smad (molecular signaling intermediates, named for their homologous in Caenorhabditis elegans (sma genes: SMAll, regulators of body size) and Drosophilla (mad genes; mothers against decantaplegic(dpp). The R-Smad interacts with co-Smad (Smad4) and this formed complex working as transcriptional regulator (activates or suppresses) passes the information to the nucleus inducing or repressing genes [36,37].
There are many speculations and hypothesis on other way of TGF-beta signaling- alternative or Smad independent within the researchers’ studies. This alternative signaling can proceed with alternative key mediators in the classical way or signals spread directly without type I receptor involvement [37,38]. The lack of Smad and co-Smad activity and abnormal type I receptor; activation of R-Smad by other signaling mediators without direct interaction of TGF-beta receptors may also activate Smad independent signaling, which is not fully understood due its complexity and diversity [39,40].
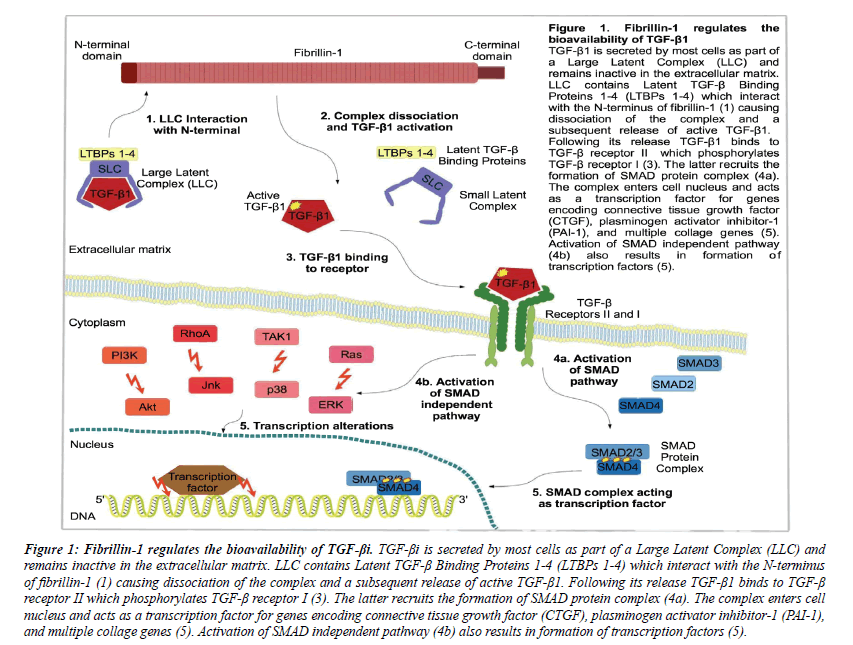
The way of TGF-beta regulation is multiple and depends on different mechanisms: extracellular regulation of ligand availability, regulation at the transcriptional level by activators, repressors and terminators [41]. Also by multiple feedback and cross mechanisms that affect intracellular signal [42]. All these factors and their relationship to modify the response in signaling TGF-beta pathway are in Figure 1.
TGF-beta identified as cell growth regulator is valuable not only for its implication in matrix deposition, but it works in matrix degradation as mediator for structural events of the ECM. It was demonstrated in vitro by treating with TGF-beta and this resulted the production of type I and type II collagen, involved collagen gene expression [43,44]. These studies were important to explain the role of TGF-beta normal fibrogenesis. Later this signaling pathway was implicated for therapeutic target in pathological fibrosis [44,45]. It was observed that the overexpression of TGF-beta1 may stabilize the degeneration within ECM remodeling during aneurysm development. The study with rat abdominal aortic aneurysm when virus mediated overexpression was induced increased endogenous TGFbeta1 level and stabilized dilatation and decreased aortic wall degeneration.
TGF-beta signaling regulates vascular matrix proteins while the abnormalities in its pathway may cause harm to normal vascular structure and function. There are known several vascular disorders in conjunction with alterations within TGFbeta pathway, including aortic aneurysm syndromes, primary pulmonary fibrosis and atherosclerosis [46-48].
TGF-beta and Aneurysm Development
There are two major groups of published investigations: first of them demonstrated different mutations of TGF-beta type I and II receptors within the kinase domain, another group stated that the TAA development is associated with increased proteolysis of ECM due to increased activity of TGF-beta.
Denton et al. with his works in vitro developed transgenic mouse with control of the COL1A2 promoter expressed a fibroblast restricted –kinase deficient TGF-beta RII. They found that (due to overexpression of the mutant receptor) the transgene works with dominant-negative effect on mouse and performs fibroblast specific suppression of TGF-beta signaling developing paradoxical results– the mice appeared with dermal and pulmonary fibrosis. The authors explained that the dominant-negative TGF-beta receptor may increase TGF-beta signaling in functional complexes by interactions of ligands and the presence of the mutant TGF-beta RII may change the orientation of wild type receptors with simplest way for signaling. The increased TGF-beta signaling may be associated with heterozygous TGF-beta receptor mutations which can work accessory [48].
TGF-beta signaling increase by kinase deficient receptors alters receptor stability. This process is regulated by receptor interactions with accessory proteins that interacts TGF-beta signaling. Guglielmo et al. stated that TGFBR2 mutations in TAA syndromes may facilitate interactions with SARA (Smad anchor for receptor activation) or decrease relation with Smad7, subsequently resulting prolongation of TGF-beta signal transmission [49].
There is the possibility that TGF-beta receptor complexes, incorporating kinase deficient receptors with lack of direct signal, able to form signaling platforms with interactions among signaling components. The stimulation of human mesangial cells with TGF-beta resulted collagen gene COL1A2 expression, which was dependent on kinase (P13K) activity. With use of P13K inhibitors and mutants of Smad3 was concluded that the binding of TGF-beta allows Smad3 mediated collagen gene expression with no dependency on phosphorylation by TGFbeta RI. Thus, TGF-beta RII mutations, which are able to bind TGF-beta, could induce alternatively signaling without receptor kinase activity [50,51].
As mentioned previously increased TGF-beta activity is related with TAA formation inducing matrix deposition, regulating its degradation through MMP activity. TGF-beta induced ECM degradation may take place with or without Smad mediated pathways [52,53]. TGF-beta role within the aneurysm syndromes has been described in Marfan (MFS) context, where the dilatated aorta is very common among other genetically determined features [54].
Despite the hypothesis and all proposals explaining the cellular response to TGF-beta the mechanisms “how it works” are still unclear. There are different cells in aortas tissue and as known during the studies, the different type of cells reacts differentially. Thus, the complex of various mechanisms and reactions should take place in aortas aneurysms development. (See the Chapters “Extracellular matrix and proteases” and “Smooth muscle cells”).
Fibrillin-1 and Aneurysms
The FBN1 gene is very large, app 200 kb in size and its coding sequence divided into 65 exons, located on chromosome 15q- 21.1 [55,56]. It encodes fibrillin- 1 protein, which is large enough – 350 kDa. Fibrillin-1 protein consists of epidermal growth factor domains and a small number of TGF-beta1 domains [57-70].
Genetic predisposition in the etiology of thoracic aortic aneurysm and dissection has a very high value. Thoracic aortic disease is inherited in an autosomal dominant manner with or without syndromic features. There are known syndromes as Marfan (MFS), Loeys-Dietz, Weill-Marchesani which are associated with various mutations in FBN1 gene. The absence of phenotypic characteristics may be not enough evaluated with the potential risk to develop aneurysm and/or dissection bearing in mind that FBN1 mutations may have asymptomatic features and genetic determination can predispose the risk [5].
LeMaire et al. performed a multistage genome-wide association study on a spectrum of sporadic thoracic aneurysm and dissection (STAAD) to identify the single nucleotide polymorphisms (SNPs) associated with FBN1. This study was performed on 327 628 SNPs for 765 patients with sporadic thoracic aneurysm and/or dissection and compared with healthy by cardiovascular point more than 4000 individuals. None of 765 patients have had a history of Marfan syndrome or other familial inheritance. Five SNPs among all of them were identified with genome-wide significance (p<5 × 10-8). All five SNPs (rs1051977, rs4774517, rs755251, rs1036477, rs2118181) fall into an area of linkage disequilibrium app 305kb in size, encompassing the entire FBN1 gene. An important finding was a very strong association of rs10519177 with dissections of aortic aneurysms (OR=1.8, with p=1.2 × 10-8) [70]. Others two studies with FBN1 SNPs and TAAD association were performed by Iakubova with colleagues at Yale University [71] and by Lesauskaite leading her team at Lithuanian University of Health Sciences [72]. Quite valuable findings were established within the latter study: patients with ascending aortic aneurysm had higher minor allele frequency in FBN1 SNPs rs755251s, rs10519177, and rs4774517, as compared to the reference group (p=0.003). Minor alleles of all FBN1 SNPs studied were more frequent (p ≤ 0.0005) among patients with Stanford A dissection as compared to the reference group subjects [72]. The studies demonstrate the association of several polymorphisms, encompassing the FBN1 gene with sporadic TAAD in the absence of syndromes or etiology of familial history of TAAD. The future experiments and growing knowledge of FBN1 mutations may be valuable to diagnose and determine the risk of TAAD.
FBN1 and TGF-beta
Fibrillin-1 works as a major structural component of ECM microfibrils and also regulates TGF-beta1 activity [57] (See Figure 1).
Figure 1: Fibrillin-1 regulates the bioavailability of TGF-βi. TGF-βi is secreted by most cells as part of a Large Latent Complex (LLC) and remains inactive in the extracellular matrix. LLC contains Latent TGF-β Binding Proteins 1-4 (LTBPs 1-4) which interact with the N-terminus of fibrillin-1 (1) causing dissociation of the complex and a subsequent release of active TGF-β1. Following its release TGF-β1 binds to TGF-β receptor II which phosphorylates TGF-β receptor I (3). The latter recruits the formation of SMAD protein complex (4a). The complex enters cell nucleus and acts as a transcription factor for genes encoding connective tissue growth factor (CTGF), plasminogen activator inhibitor-1 (PAI-1), and multiple collage genes (5). Activation of SMAD independent pathway (4b) also results in formation of transcription factors (5).
In the wild type population fibrillin-1 acts regulating the activation of TGF-beta, while abnormal fibrillin-1 with deficient functionality causes excessive amounts of active TGF-beta to be released from the matrix. It was demonstrated in mice with deficiency of fibrillin-1 and that aortic aneurysm in these mice can be prevented by administration of TGF-beta neutralizing antibodies [62,63].
The angiotensin pathway provides another way to target TGFbeta. Angiotensin II is a vasoconstrictor that signals through angiotensin II type I receptor and has been known in animal experiments by Everett et al. in 1994 [64]. Also angiotensin II activates trombospondin -1, which is an activator of TGFbeta signaling. The medicaments, which can block angiotensin receptor (Losartan- AT1 receptor antagonist) usually use for hypertension treatment can decrease TGF-beta signaling and its activity in plasma.
Brooke et al. reported that losartan was effective in within TGFbeta control in Marfan syndrome patients. This study highlighted the importance of cytokine activation through ECM and may be valuable in other conditions where TGF-beta is increased (organ fibrosis, cancer progression and others) [65]. The angiotensin receptor blocator Losartan is effective to reduce the thoracic aneurysm formation in Marfan syndrome [64].
The same study of Brooke et al. [65] demonstrated a significant decrease in the aortic root enlargement in population of aortic disease with several etiologies with administration of ARB. The very interesting finding was performed by Chung and coworkers with their experiments on mice [66]. They showed that long term doxycycline administration together with atenolol (beta blocker) may prevent thoracic aortic aneurysm formation in Marfan syndrome through the inhibition of matrix metalloproteinase-2 and -9 by multiple actions. As previous researches have been focused on doxycycline effect within abdominal aneurysma and was showed the suppression of aneurysmatic expansion due to the proteases inhibition [66-68].
There are controversial findings with TGF-beta1 concentration measurement levels, within different populations published since 2013. The scientist group from Japan stated that circulating TGF-beta1 is not a diagnostic and therapeutic marker for MFS patients because they didn’t find any statistical difference between MFS patients and controls by measuring TGB-beta concentration in plasma [69]. Another study provided by The Netherlands scientists stated an opposite opinion that elevated TGF-beta1 level in MFS patients is correlated with larger aortic root diameters and may predict cardiovascular events serving as prognostic biomarker [70]. A researchers’ team from Lithuania [73] measured the TGF beta1 concentration for sporadic TAAD and found to be a valuable marker, especially for dissections. They found the association of the TGF beta1 concentration with different genotypes of FBN1 SNPs [74]. The main reason why such different results among studies could explore might be a genetic difference, the presence of a mutation in TGF-beta receptor genes rather than in the FBN1 gene. That is to say, further and wider analysis is needed by mentioned TGF-beta and FBN1 interaction mechanism to assess.
Conclusions
Over 600 FBN1 mutations have been discovered in connection with Marfan syndrome and eventually this list continues to grow. The genetic findings may be the basic diagnostic background to determine the risk of non syndromic TAAD. However, there are many questions requiring explanation or discovery in pathogenesis of TAAD development. First, the interactions of FBN1 and TGF-beta whether the fibrillin-LTBP junction is needed to protect the LLC from proteolytic activation or whether FBN1 functions more directly in controlling assembly or stability of latent TGF-beta complexes. Second, the balance between the need for TGF-beta in normal development and suppression of its activity in the treatment of disease must be evaluated in future works. Third, aortic diseases are heterogeneous along the whole vessel, different determinants and contribution of SMC, inflammation and thrombus formation, a failure of reconstruction and all these matters according to the different embryonic origin of abdominal and thoracic parts. Thus, future works to be done on namely different cellular and molecular mechanisms, diameter and length increase, rupture and dissection. The clinical application of the genetic studies on FBN1 polymorphisms must have a wide approach in practice and complexity of multiple studies.
References
- Anderson CA. Ascending aortic aneurysms. Cardiac surgery in adult. New York: McGraw-Hill; 2003; 1123-1148.
- Allaire E, Schneider F, Saucy F, Dai J, Cochennec F. New insight in aetiopathogenesis of aortic diseases. Eur J Vasc Endovasc Surg 2009; 37: 531-537.
- Jones JA, Francis GS, John SI. Transforming growth factor-β signaling in thoracic aortic aneurysm development: a paradox in pathogenesis. Journal of vascular research 2008; 46: 119-137.
- Albornoz G. Familial thoracic aortic aneurysms and dissections-incidence, modes of inheritance, and phenotypic patterns. The Annals of thoracic surgery 2006; 82: 1400-1405.
- Milewicz DM. Genetic basis of thoracic aortic aneurysms and dissections: focus on smooth muscle cell contractile dysfunction. Annu. Rev. Genomics Hum. Genet 2008; 9: 283-302.
- Milewicz DM, Michael K, Fisher N, Coselli JS, Markello T, Biddinger A. Fibrillin-1 (FBN1) mutations in patients with thoracic aortic aneurysms. Circulation 1996; 94: 2708-2711.
- LeMaire SA. Genome-wide association study identifies a susceptibility locus for thoracic aortic aneurysms and aortic dissections spanning FBN1 at 15q21.1. Nature genetics 2011; 43: 996-1000.
- Guo, DC. Familial thoracic aortic aneurysms and dissections identification of a novel locus for stable aneurysms with a low risk for progression to aortic dissection. Circulation: Cardiovascular Genetics 2011; 4: 36-42.
- Chung AWY. Loss of elastic fiber integrity and reduction of vascular smooth muscle contraction resulting from the up regulated activities of matrix metalloproteinase-2 and-9 in the thoracic aortic aneurysm in Marfan syndrome. Circulation research 2007; 101: 512-522.
- National Centre of Health Statistics in USA, 2012.
- Dobrin PB, William HB, William GC. Elastolytic and collagenolytic studies of arteries: implications for the mechanical properties of aneurysms. Archives of Surgery 1984; 119: 405.
- Anidjar S. Elastase-induced experimental aneurysms in rats. Circulation 1990; 82: 973-981. Defawe, Olivier D, TIMP-2 and PAI-1 mRNA levels are lowering in aneurysmal as compared to athero-occlusive abdominal aortas. Cardiovascular research 2003; 60: 205-213.
- Natzi S, Raymond L, Defawe OD. Abdominal aortic aneurysm. The Lancet 2005; 365: 1577-1589.
- Defawe OD. TIMP-2 and PAI-1 mRNA levels are lowering in aneurysmal as compared to athero-occlusive abdominal aortas. Cardiovascular research 2003; 60: 205-213.
- Carmeliet P. Urokinase-generated plasmin activates matrix metalloproteinases during aneurysm formation. Nature genetics 1997; 17: 439-444.
- Lesauskaite V. Expression of matrix metalloproteinases, their tissue inhibitors, and osteopontin in the wall of thoracic and abdominal aortas with dilatative pathology. Human pathology 2006; 37: 1076-1084.
- Sangiorgi G. Plasma levels of metalloproteinases-9 and-2 in the acute and subacute phases of type A and type B aortic dissection. Journal of cardiovascular medicine 2006; 7: 307-315.
- He R. Characterization of the inflammatory and apoptotic cells in the aortas of patients with ascending thoracic aortic aneurysms and dissections. The Journal of thoracic and cardiovascular surgery 2006; 131: 671-678.
- Kirsch EW. Heterogeneity in the remodeling of aneurysms of the ascending aorta with tricuspid aortic valves. The Journal of Thoracic and Cardiovascular Surgery 2006; 132: 1010-1016.
- Tang PC. Transmural inflammation by interferon-γ-producing T cells correlates with outward vascular remodeling and intimal expansion of ascending thoracic aortic aneurysms. The FASEB journal 2005; 19: 1528-1530.
- Tang PC. Hyperplastic cellular remodeling of the media in ascending thoracic aortic aneurysms. Circulation 2005; 112: 1098-1105.
- Lesauskaite V. Smooth muscle cells of the media in the dilatative pathology of ascending thoracic aorta: morphology, immunoreactivity for osteopontin, matrix metalloproteinase, and their inhibitors. Human pathology 2001; 32: 1003-1011.
- Zhu L. Mutations in myosin heavy chain 11 cause a syndrome associating thoracic aortic aneurysm/aortic dissection and patent ductus arteriosus. Nature genetics 2006; 38: 343-349.
- Allaire E. Paracrine effect of vascular smooth muscle cells in the prevention of aortic aneurysm formation. Journal of vascular surgery 2002; 36: 1018-1026.
- Allaire E. Vascular smooth muscle cell endovascular therapy stabilizes already developed aneurysms in a model of aortic injury elicited by inflammation and proteolysis. Annals of surgery 2004; 239: 417.
- O’Callaghan CJ, Bryan W. Mechanical Strain–Induced Extracellular Matrix Production by Human Vascular Smooth Muscle Cells Role of TGF-β1. Hypertension 2000; 36: 319-324.
- Bertolino P. Transforming growth factor-β signal transduction in angiogenesis and vascular disorders. CHEST Journal 2005; 128: 585S-590S.
- Feng XH, Derynck R. Specificity and versatility in TGF-β signaling through Smads. Annu. Rev. Cell Dev. Biol. 2005; 21: 659-693.
- Bataller R, David AB. Liver fibrosis. Journal of Clinical Investigation 2005; 115: 209-218.
- Lijnen PJ, Petrov VV, Fagard RH. Induction of Cardiac Fibrosis by Transforming Growth Factor-β. Molecular genetics and metabolism 2000; 71: 418-435.
- Bartram U, Christian PS. The role of transforming growth factor β in lung development and disease. CHEST Journal 2004; 125: 754-765.
- Kalluri R, Yuchi H. Targeting TGF-β and the extracellular matrix in Marfan's syndrome. Developmental cell 2008; 15: 1-2.
- Kwak HJ. Transforming growth factor-β1 induces tissue inhibitor of metalloproteinase-1 expression via activation of extracellular signal-regulated kinase and Sp1 in human fibro sarcoma cells. Molecular cancer research 2006; 4: 209-220.
- García-Alvarez J. Tissue inhibitor of metalloproteinase-3 is up-regulated by transforming growth factor-β 1 in vitro and expressed in fibroblastic foci in vivo in idiopathic pulmonary fibrosis. Experimental lung research 2006; 32: 201-214.
- Guo DC, Pannu H, Fadulu VT, Papke CL, Yu RK, Avidan N, Bourgeois S, Estrera AL, Safi HJ, Sparks E, Amor D, Ades L, McConnell V, Willoughby CE, Abuelo D, Willing M, Lewis RA, Kim DH, Scherer S, Tung PP, Ahn C, Buja LM, Rama CS, Shete SS, Milewicz DM. Mutations in smooth muscle α-actin (ACTA2) lead to thoracic aortic aneurysms and dissections. Nature genetics 2007; 39: 1488-1493.
- Wrana JL. Mechanism of activation of the TGF-b receptor. Nature 1994; 370: 341-346.
- Feng XH, Derynck R. Specificity and versatility in TGF-β signaling through Smads. Annu. Rev. Cell Dev. Biol. 2005; 21: 659-693.
- Leivonen SK. Smad3 Mediates Transforming Growth Factor-β-induced Collagenase-3 (Matrix Metalloproteinase-13) Expression in Human Gingival Fibroblasts EVIDENCE FOR CROSS-TALK BETWEEN Smad3 AND p38 SIGNALING PATHWAYS. Journal of Biological Chemistry 2002; 277: 46338-46346.
- Yu Li, Mindy CH, Ying EZ. TGF-β receptor-activated p38 MAP kinase mediates Smad-independent TGF-β responses. The EMBO journal 2002; 21: 3749-3759.
- Dumont N, Andrei VB, Carlos LA. Autocrine transforming growth factor-β signaling mediates Smad-independent motility in human cancer cells. Journal of Biological Chemistry 278.5 (2003): 3275-3285.
- Yakymovych I. Regulation of Smad signaling by protein kinase C. The FASEB Journal 2001; 15: 553-555.
- Runyan CE, William SH, Anne-Christine P. The phosphatidylinositol 3-kinase/Akt pathway enhances Smad3-stimulated mesangial cell collagen I expression in response to transforming growth factor-β1. Journal of Biological Chemistry 2004; 279: 2632-2639.
- Xu L. Regulation of Smad activities. Biochimica Biophysica Acta (BBA)-Gene Structure and Expression 2006; 1759: 503-513.
- Wicks SJ. Reversible ubiquitination regulates the Smad/TGF-beta signalling pathway. Biochemical Society Transactions 2006; 34: 761.
- Ghosh AK. Factors involved in the regulation of type I collagen gene expression: implication in fibrosis. Experimental Biology and Medicine 2002; 227: 301-314.
- Verrecchia F, Alain M. Transforming growth factor-beta and fibrosis. World Journal of Gastroenterology 2007; 13: 3056-3062.
- Ihn H. Pathogenesis of fibrosis: role of TGF-[beta] and CTGF. Current opinion in rheumatology 2002; 14: 681-685.
- Dai J. Overexpression of transforming growth factor-β1 stabilizes already-formed aortic aneurysms a first approach to induction of functional healing by endovascular gene therapy. Circulation 2005; 112: 1008-1015.
- Newman JH. Genetic basis of pulmonary arterial hypertension Current understanding and future directions. Journal of the American College of Cardiology s1 2004; 43: S33-S39.
- Loeys B, Chen J, Neptune ER, Judge DP, Podowski M, Holm T, Meyers J, Leitch CC, Katsanis N, Sharifi N, Xu FL, Myers LA, Spevak PJ, Cameron DE, De Backer J, Hellemans J, Chen Y, Davis YC, Webb CL, Kress W, Coucke P, Rifkin DB, De PAM, Dietz HC. A syndrome of altered cardiovascular, craniofacial, neurocognitive and skeletal development caused by mutations in TGFBR1 or TGFBR2. Nature genetics 2005; 37: 275-281.
- Lutgens E. Transforming growth factor-β mediates balance between inflammation and fibrosis during plaque progression. Arteriosclerosis, thrombosis, and vascular biology 2002; 22: 975-982.
- Wong SH. Endoglin expression on human microvascular endothelial cells. European Journal of Biochemistry 2000; 267: 5550-5560.
- Di G, Gianni M. Distinct endocytic pathways regulate TGF-β receptor signalling and turnover. Nature cell biology 2003; 5: 410-421.
- Runyan CE, William HS, Anne-Christine P. The phosphatidylinositol 3-kinase/Akt pathway enhances Smad3-stimulated mesangial cell collagen I expression in response to transforming growth factor-β1. Journal of Biological Chemistry 2004; 279: 2632-2639.
- Lee KS, Hong SH, Bae SC. Both the Smad and p38 MAPK pathways play a crucial role in Runx2 expression following induction by transforming growth factor-beta and bone morphogenetic protein. Oncogene 2002; 21: 7156-7163.
- Feng XH, Derynck R. Specificity and versatility in TGF-β signaling through Smads. Annu. Rev. Cell Dev. Biol. 2005; 21: 659-693.
- Dietz HC. Recent progress towards a molecular understanding of Marfan syndrome. American Journal of Medical Genetics Part C: Seminars in Medical Genetics. 2005; 139: 4-9.
- Biery NJ. Revised Genomic Organization of< i> FBN1</i> and Significance for Regulated Gene Expression. Genomics 1999; 56: 70-77.
- Pepin MS, Ulrike S, Andrea SF, Byers PH. Clinical and genetic features of Ehlers–Danlos syndrome type IV, the vascular type. New England Journal of Medicine 2000; 342: 673-680.
- Pereira L. Genomic organization of the sequence coding for fibrillin, the defective gene product in Marfan syndrome. Human molecular genetics 1993; 2: 961-968.
- Mc Gettrick AJ. Molecular effects of calcium binding mutations in Marfan syndrome depend on domain context. Human molecular genetics 2000; 9: 1987-1994.
- Ramirez F, Harry CD. Fibrillin‐rich micro fibrils: Structural determinants of morphogenetic and homeostatic events. Journal of cellular physiology 2007; 217: 326-330.
- Holm TM. Noncanonical TGFβ signaling contributes to aortic aneurysm progression in Marfan syndrome mice. Science 2011; 332: 358-361.
- Everett AD. Angiotensin receptor regulates cardiac hypertrophy and transforming growth factor-beta 1 expression. Hypertension 1994; 23: 587-592.
- Brooke BS. Angiotensin II blockade and aortic-root dilation in Marfan's syndrome. New England Journal of Medicine 2008; 358: 2787-2795.
- Chung AWY. Long-term doxycycline is more effective than atenolol to prevent thoracic aortic aneurysm in marfan syndrome through the inhibition of matrix metalloproteinase-2 and-9. Circulation research 2008; 102: e73-e85.
- Bartoli MA. Localized administration of doxycycline suppresses aortic dilatation in an experimental mouse model of abdominal aortic aneurysm. Annals of vascular surgery 2006; 20: 228-236.
- Curci JA. Pharmacologic suppression of experimental abdominal aortic aneurysms: a comparison of doxycycline and four chemically modified tetracyclines. Journal of vascular surgery 1998; 28: 1082-1093.
- Ogawa N, Imai Y, Nishimura H, Kato M, Takeda N, Nawata K, Hirata Y. Circulating Transforming Growth Factor β-1 Level in Japanese Patients With Marfan Syndrome. International heart journal, 2012; 54: 23-26.
- Franken R, den HAW, de WV, Engele L, Radonic T, Lutter R, Mulder BJ. Circulating transforming growth factor-β as a prognostic biomarker in Marfan syndrome. International journal of cardiology 2013; 168: 2441-2446.
- Iakoubova OA, Tong CH, Rowland CM, Luke MM, Garcia VE, Catanese JJ. Genetic variants in FBN1 and risk for thoracic aortic aneurysm and dissection. PLoS One 2014; 0091437.
- Lesauskaite V, Sepetiene R, Jariene G, Patamsyte V, Zukovas G. FBN1 polymorphisms in patients with the dilatative pathology of the ascending thoracic aorta. Eur J Cardiothorac Surg. 2015; 47: e124-130.
- Sepetiene R, Patamsyte V, Zukovas G, Jariene G, Stanioniene Z, Benetis R, Lesauskaite V. Blood Plasma TGF-β1 Concentration in Sporadic Dilatative Pathology of Ascending Aorta: More Questions than Answers. PloS one, 2015; 10: e0129353.
- Sepetiene R, Patamsyte V, Zukovas G, Jariene G, Stanioniene Z, Benetis R, Lesauskaite V. Association between Fibrillin1 Polymorphisms (rs2118181, rs10519177) and Transforming Growth Factor β1 Concentration in Human Plasma. Molecular Medicine 2015; 21: 735.