Letter to Editor - Journal of Cholesterol and Heart Disease (2017) Volume 1, Issue 1
Familial Hypercholesterolemia and PCSK9 Inhibitors: A Cautious Comment
Ossama K. Abou Hassan, Georges Nemer*
Department of Biochemistry and Molecular Genetics, American University of Beirut, Beirut, Lebanon
- Corresponding Author:
- Georges Nemer
Department of Biochemistry and Molecular Genetics American University of Beirut
Beirut, Lebanon
Tel: 9611350000 Ext 4876
E-mail: gn08@aub.edu.lb
Accepted Date: February 06, 2017
Citation: Hassan OKA, Nemer G. Familial Hypercholesterolemia and PCSK9 Inhibitors: A Cautious Comment. J Cholest Heart Dis. 2017;1(1):1-2
Abstract
We have read with much interest the recent expert consensus by Lloyd-Jones et al. [1] on the role of non-statin therapies for LDLF- cholesterol lowering. They have reinforced and expanded the recommendations for the use of non-statin therapies for patients with high LDL from their earlier published guidelines in 2013. In particular, the role of genetically determined disease is plainly stated and emphasized in the subgroup of patients with high risk for cardiovascular disease. Those patients have primary severe elevations of LDL-C = 190 mg/dL, and are more likely to have genetic heterozygous (HeFH) or homozygous (HoFH) familial hypercholesterolemia. In the opinion of the Expert Consensus Writing Committee, in such patients who fail statin mono-therapy (<50% reduction in LDL-C), it is reasonable to take advantage of the greater LDL-C lowering effect of PCSK9 inhibitors as a first step treatment. Inadequate response to such therapies supports the use of mipomersan, lomitapide or LDL apheresis. This allows the use of the PCSK9 inhibitor (evolocumab) before committing to LDL apheresis in phenotypic HoFH patients unless a “receptor negative” mutation has been documented.
The notion of “receptor negative” practically means a nonfunctioning LDL receptor - patients with less than 2% of LDL uptake – as classified by Raal et al. [2]. Homozygous receptor negative familial hypercholesterolemia patients do not respond to PCSK9 treatment – the LDL cholesterol in those patients even show slight increase compared to baseline levels. Homozygous non-negative (with 2-25% LDL uptake) mutations are amenable to evolucomab therapy [2]. Heterozygous or compound heterozygous familial hypercholesterolemia patients have a significant mean reduction in LDL levels [3,4]. The mean reduction in LDL cholesterol reached 40.8% in those with one or two defective alleles, and no response in double receptor negative alleles. The opinion of the expert consensus written committee on receptor negative homozygous familial hypercholesterolemia is derived from this finding and is based on one Lebanese patient with the p.C681X “Lebanese Allele” homozygous mutation.
In Lebanon, most of the familial hypercholesterolemia patients have a founder LDLR mutation with high demographic burden for the disease. We have previously identified in one cohort 18 different families with the homozygous mutations c.2043 C>A (p.C681X) in LDLR accounting for almost 50% of all families with clinically identified FH (Table 1) [5].
| Any LDLR mutation | No LDLR mutation | ||||
|---|---|---|---|---|---|
| Heterozygous any Allele | Homozygous Leb Allele | Homozygous other Allele/s | |||
| Number of patients | 29 | 18 | 6 | 11 | |
| Average Years | 26.3 | 24.1 | 23.3 | 26.1 | |
| Average Lipid Levels (mg/dl) | TC | 567 | 600 | 655 | 500 |
| LDL | 467 | 493 | 562 | 380 | |
Table 1: Clinical indicators by type of mutation. (TC: total cholesterol, LDL: low density lipoprotein). Adapted from Fahed et al.
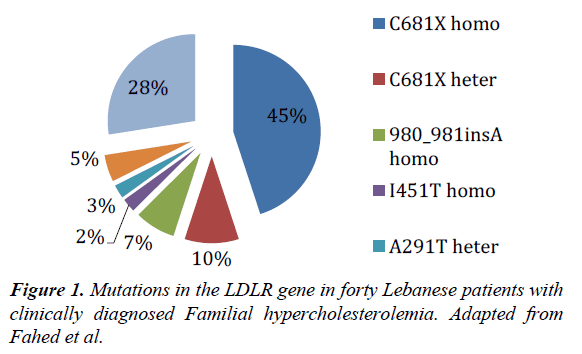
These patients presented with an average LDL-C level of 493 mg/dl (ideal below 100 mg/dl) and typical signs and symptoms of familial hypercholesterolemia. They were managed with apheresis after their failure to respond adequately to statin therapy. Our finding on the genetic basis of the disease in Lebanon puts a red flag on the consensus’s opinion to provide non-statin therapy before going into apheresis. We expect failure of therapy to occur in 45% of the clinically identified FH patients in Lebanon, who will be harbor an underlying homozygous “negative receptor” mutation (Figure 1).
This effect might not be seen in other countries as population studies in South Africa, Netherlands and Italy failed to show such a drastic disease-linked mutation [6-8]. We recommend population based understanding of the disease prior to drug use. In populations where there is high risk of therapy failure, and where a recurrent founder mutation is of the negative receptor type, genetic screening on individual basis before therapy trial should thus be indicated.
Φ Low Density Lipoprotein
Ψ Proprotein Convertase Subtilisin/Kexin type 9
References
- Lloyd-Jones DM, Morris PB, Ballantyne CM, et al. ACC Expert Consensus Decision Pathway on the Role of Non-Statin Therapies for LDL-Cholesterol Lowering in the Management of Atherosclerotic Cardiovascular Disease Risk: A Report of the American College of Cardiology Task Force on Clinical Expert Cons. J Am CollCardiol.
- Raal FJ, Honarpour N, Blom DJ, et al. Inhibition of PCSK9 with evolocumab in homozygous familial hypercholesterolaemia (TESLA Part B): a randomised, double-blind, placebo-controlled trial. Lancet (London, England). 2015;385(9965):341-50.
- Raal FJ, Stein EA, Dufour R, et al. PCSK9 inhibition with evolocumab (AMG 145) in heterozygous familial hypercholesterolaemia (RUTHERFORD-2): a randomised, double-blind, placebo-controlled trial. Lancet (London, England). 2015;385(9965):331-40.
- McNutt MC, Ahmad Z. So Far, PCSK9 Inhibitors Work for All Heterozygous FH Patients. CircCardiovasc Genet. 2015;8(6):749-51.
- Fahed AC, Safa RM, Haddad FF, et al. Homozygous familial hypercholesterolemia in Lebanon: a genotype/phenotype correlation. Mol Genet Metab. 2011;102(2):181-8.
- Sjouke B, Kusters DM, KindtI, et al. Homozygous autosomal dominant hypercholesterolaemia in the Netherlands: prevalence, genotype-phenotype relationship, and clinical outcome. Eur Heart J. 2015;36(9):560–5.
- Raal FJ, Pilcher GJ, Panz VR, et al. Reduction in mortality in subjects with homozygous familial hypercholesterolemia associated with advances in lipid-lowering therapy. Circulation. 2011;124(20):2202-7.
- Bertolini S, Pisciotta L, Rabacchi C, et al. Spectrum of mutations and phenotypic expression in patients with autosomal dominant hypercholesterolemia identified in Italy. Atherosclerosis. 2013;227(2):342-8.