Research Article - Biomedical Research (2017) Volume 28, Issue 21
Expression change and research of the roles of Sirt-2 in liver tissue of sepsis mice
Wen Yang, Yanbing Liang, Hao Tang, Fang Li, Zhibin Chen, Zhenyu Li, Jingguo Wu, Lijin Zeng and Zhongfu Ma*
Department of General Internal Medicine, the First Affiliated Hospital of Sun Yat-Sen University, Guangzhou, Guangdong 510080, PR China
- *Corresponding Author:
- Zhongfu Ma
Department of General Internal Medicine
The First Affiliated Hospital of Sun Yat-Sen University, PR China
Accepted date: October 27, 2017
Abstract
Silent information regulator-2 (SIRT2) is a recently discovered type of dependence on nicotine amines adenine dinucleotide type III histone acetylation enzyme. However, the expression change and research of SIRT2 in macrophage remains unclear. Here in this study, we demonstrate that the regulatory effects of SIRT2 on sepsis and the feasible correlational research. BALB/c mice were used to establish Cecum ligation puncture (CLP)-induced sepsis model. At 3, 6, 12, 24, 48 and 72 h after CLP-induced, the expression of SIRT2 and MMP-9 gene were analyzed using Quantitative real time polymerase chain reaction (Q-PCR), the serum of ALT, inflammatory factor and MCP-1 levels were measured by ELISA kits, pathological changes of liver tissue was observed using HE Dyeing and Immuno-histochemical analysis, and the expressions of NF-κB p65 and p38MAPK protein were surveyed using western blotting. Firstly, the expression of SIRT2 gene and the serum of ALT level in sepsis mice were rapidly increased since 3 h operation and reached peak at 48 h after operation. Next, seep inflammatory cells was surged and the serum of TNF-α, IL-1, IL-6, IL-10 and MCP-1 were observably increased and reached peak at 6 or 12 h after operation in sepsis mice. Lastly, NF-κB p65 and phosphorylation-p38MAPK protein expression, and the MMP-9 gene expression were rapidly increased and reached peak at 12 h or 6 h, respectively, after operation in sepsis mice. Our data suggested that SIRT2 can increase inflammatory of sepsis mice through mediation of NF-κB and p38 MAPK pathway.
Keywords
SIRT2, Sepsis, Inflammatory, p38 MAPK
Introduction
Sepsis is caused by systemic inflammatory response syndrome (SIRS), is the common complications of infection, gen/burns and surgical operations, they are closely related with individual factors including age, genetic, if with chronic disease [1]. Sepsis with organ damage and then develop for severe sepsis, even septic shock, multiple organ dysfunction syndrome [2]. Sepsis is a common disease with high mortality. In the United States, each year at least 750000 people suffer from sepsis, and the patients’ mortality of sepsis was 34.0%, treatment costs was $22100 per person [3]. In China, the incidence rate of sepsis in Intensive Care Unit (ICU) was 8.68%, the mortality was 48.7%, and per people cost of treatment is about 100000 RMB/person [4]. The incidence of sepsis in the world is increasing year by year, and the mortality is still high.
The pathogenesis of sepsis is very complicated; the infection and inflammation reaction in the development of the occurrence of sepsis plays a vital role [5]. After pathogenic microorganism invasion to the body, the pathogen associated molecular patterns through Toll-like receptors and immune cells such as pattern recognition receptor interactions, activate the transmembrane signal transduction pathways, regulate the expression of inflammatory mediators, which produce a large number of inflammatory related molecules, which triggers the inflammatory response [6]. Monocyte/macrophage as important effector cells plays a key role in the body's natural immune system. Monocyte/macrophage on one hand removed by phagocytosis invasion in the body by a variety of pathogenic microorganisms, on the other hand, it is the main source of the body's inflammatory related molecules, by producing a large number of pro-inflammatory/antiinflammatory medium, mediated inflammatory reaction [7].
Silent information regulator-2 (SIRT2) is a recently discovered type of dependence on nicotine amines adenine dinucleotide type III histone acetylation enzyme, it mainly located in cytoplasm, the recently reported main functions involve involved cell cycle, maintain the stability of the genome, and tumorigenesis [8]. SIRT2 through acetylated histone H4K16Ac loci, regulate the cell cycle. H4K16Ac loci acetylation specificity occur between the cell cycle stage of G2 and M, and in this phase, SIRT2 transfer from cytoplasm to nuclei [9]. H4K16 loci of acetylation level reached to the highest in S phase, this state is an important signal in mammalian DNA repair [10]. SIRT2 adjust the acetylation level of H4K16 loci, release DNA damage situation in the cell cycle signal, to participate in regulating the cell cycle [11]. Recent studies reveal that SIRT2 related to the differentiation of fat cells and the neural microglia [12]. SIRT2 in liver cancer research get more and more people's attention in recent years [13]. At present, the study of SIRT2 is concentrated in the tumors, metabolic diseases, etc. And the study of sepsis has not yet clear, this experiment adopts the caecal ligation and puncture, making sepsis model, aimed to explores SIRT2’s influence on sepsis inflammatory reaction and prognosis, provide a new train of thought for the treatment of sepsis. This study is to explore its role in the pathogenesis of sepsis.
Materials and Methods
Grouping
Mixing Sumianxin, Ketamine and saline by volume ratio of 2:1.5:3.5, 4°C keep aside using 0.05 to 0.06 ml/one for BALB/c mice anesthesia (anesthesia by intraperitoneal injection).
Sepsis modelling
Cecum ligation puncture (CLP) method was used to construct sepsis model (main indicators of model construct success are the modeling survival rate of mice for 20-30%).Using 75% alcohol dip in surgical instruments. Maxing Sumianxin, Ketamine and saline by volume ratio of 2:1.5:3.5, 4°C saved for later use. The anaesthetic abdominal cavity injection by 50-60 μl/mice, about 2 to 4 min later, the mice lose consciousness. After completely anesthesia, mice will be fixed on the operating table, with 75% alcohol disinfection abdomen. Exposed mice abdominal cavity, under xiphoid one finger long refers to the midline of abdomen from top to bottom cut about 2 cm long incision, and cut open abdominal peritoneum along the Hunter's line, reveal the abdominal cavity. Pulling the cecum from the lower left corner of the abdominal cavity, about ligation on the cecum two-thirds distance to caecum, ligation about 2/3 width (not ligation too tight to prevent intestinal necrosis and influence the results). Using 8 needles on the outer edge of the ligation puncture intestinal wall for 2 times, pay attention to avoid loss of capillaries on the intestinal wall, to prevent bleeding from the walls of intestines, squeeze right amount of contents carefully. Using line suture needle to step by step stitch peritoneum and muscle layer (with the "8" suture). Control group (Sham) treatment in mice, just pulling out the cecum and then put the cecum back into the abdominal cavity, do not need the cecum ligation perforation. In this study, animal processing method is according to the ethics standard.
Tissue paraffin embedding
Fresh organization fixed in 4% paraformaldehyde for more than 24 h. Remove tissue from a fixed liquid, repair the purpose tissue to flat with scalpel in the fuming cupboard, put the repaired organization and the corresponding labels in a dehydrated box; Dehydration, put the dehydration box in the hanging basket in the dryer, in turn, gradient alcohol dehydration.75% ethyl alcohol 4 h-85% ethyl alcohol 2 h-90% ethyl alcohol 2 h-95% ethyl alcohol 1 h-absolute ethyl alcohol I 30 min-absolute ethyl alcohol II 30 min-Alcohol benzene 5-10 min-dimethylbenzene I 5-10 min-dimethylbenzene II 5-10 min-wax I 1 h-wax II 1h-wax III 1 h; Embedding: The tissue will be immersed within the organization on the embedding machine wax embedding. Melting wax into the embedding box first, stay before wax solidification and remove tissue from the dehydration box in accordance with the standard embedding the into the embedding box with the requirements and the paste the corresponding label. Cooling in -20°C refrigerator, after wax solidification to remove wax block from the embedding box and repairing the wax block; Slice: Dressed wax block in paraffin wax for microtome section 4 μm thick. Slicing machine will float in 40°C warm water and organization flattening by stand machine, use glass slide to get the organize salvage, and placed into 60°C oven to bake.
HE Dyeing experiment steps
Putting slice in turn into dimethylbenzene I 20 mindimethylbenzene II 20 min-absolute ethyl alcohol I 10 minabsolute ethyl alcohol II 10 min-95% ethyl alcohol 5 min-90% ethyl alcohol 5 min-80% ethyl alcohol 5 min-70% ethyl alcohol 5 min-distilled water to wash; Sliced into the Harris logwood dye 3-8 min, water washing, 1% hydrochloric acid alcohol differentiation into a few seconds, tap water rinse, 0.6% ammonia return to blue, water flushing; Sliced into the red dye solution dyed 1-3 min; Slice was in turn into the 95% alcohol I 5 min-95% alcohol II 5 min-absolute ethyl alcohol I 5 min-the anhydrous ethanol II 5 min-dimethylbenzene I 5 mindimethylbenzene II dehydration transparent in 5 min, the slice out of dimethylbenzene for a bit dry, neutral gum sealing piece.
Immuno-histochemical analysis method
Image pro-plus 6.0 software analysis method of immunehistochemical image: Within each group of each section of at least three times the 200 randomly selected photograph field of vision. As far as possible when you take a photo for tissue full of the entire field of vision, ensure each photo light background are the same. Application of Image-Pro Plus6.0 software to select the same tan as judge all photos positive unified standard, for each photograph analysis, it is concluded to get the accumulation of each photo positive optical density (IOD).
Quantitative real time polymerase chain reaction (QPCR) of SIRT2 and MMP-9 expression
Liver tissue samples were froze by liquid nitrogen, and used homogenizer to homogenize with buffer. Total RNA was extracted using TRIzol (Invitrogen). 1 μg of RNA and with One Step RT-PCR Kit (TaKaRa) was used to synthesize cDNA. ABI-7500 RT-PCR system (Applied Biosystems) and the SYBR Green (TaKaRa) were used to perform Q-PCR and analyze the SIRT2 gene expression. The primers of SIRT2, MMP-9 and GAPDH were showed at Table 1.
| Gene | Sequence (F; 5`- 3`) | Sequence (R; 5`- 3`) |
|---|---|---|
| SIRT2 | CCATGGCAAATTCTTCTGGCGTGT | TAGAGACTTGCACTGCACGGTTGA |
| MMP-9 | GCCTGGGTTCCCAAAAGGAG | GAGCGGAAGTCAGGGATACC |
| GAPDH | CCTTCATTGACCTCAACTAC | GGAAGGCCATGCCAGTGAGC |
Table 1. The primers of SIRT2, MMP-9 and GAPDH.
Serum levels of alanine transaminase (ALT), inflammatory factor and chemoattractant protein 1 (MCP-1) expression
The serum samples were collected form eye socket and microcentrifuged for 15 min 16,000 g at 4°C. Supernates were collected and used to analyze cytokines and MCP-1 expression. The serum of ALT, tumor necrosis factor-α (TNF- α), interleukin-1 (IL-1), interleukin-6 (IL-6), interleukin-10 (IL-10) and MCP-1 expressions were measured by using Quantikine ELISA kits (R&D Systems) according to the manufacturer’s protocol.
Western blotting of NF-κB p65 and p38MAPK
Liver tissue samples were froze by liquid nitrogen, and used homogenizer to homogenize with buffer containing lysed with Radio-Immunoprecipitation Assay (RIPA). Miscible liquids were microcentrifuged for 15 min 16,000 g at 4°C. Protein concentrations were estimated by Pierce BCA protein assay kit (BD Biosciences, San Jose, CA). Equal protein samples were loaded onto 10% SDS–polyacrylamide gel. Separated proteins were transferred into polyvinylidene fluoride (PVDF) membrane (Bio-Rad, Hercules, CA) after electrophoresis. The PVDF membrane was washed with 5% non-fat dry milk in TBS for 1-2 h, incubated with antibodies: NF-κB p65 (dilution 1/2000) and phosphorylation-p38MAPK (dilution 1/1000) overnight at 4°C. The PVDF membrane was incubated with ECL Prime reagents from GE Life Sciences (Piscataway, NJ, USA).
Statistical analysis
Data was analyzed expressed as mean ± SEM using either Student’s t test (for two groups) or one-way ANOVA (for more than two groups) and performed using the SPSS 17.0 (IBM SPSS Statistics, IBM corporation, Armonk, NY).
Results
Expression of SIRT2 gene in liver tissue of sepsis mice
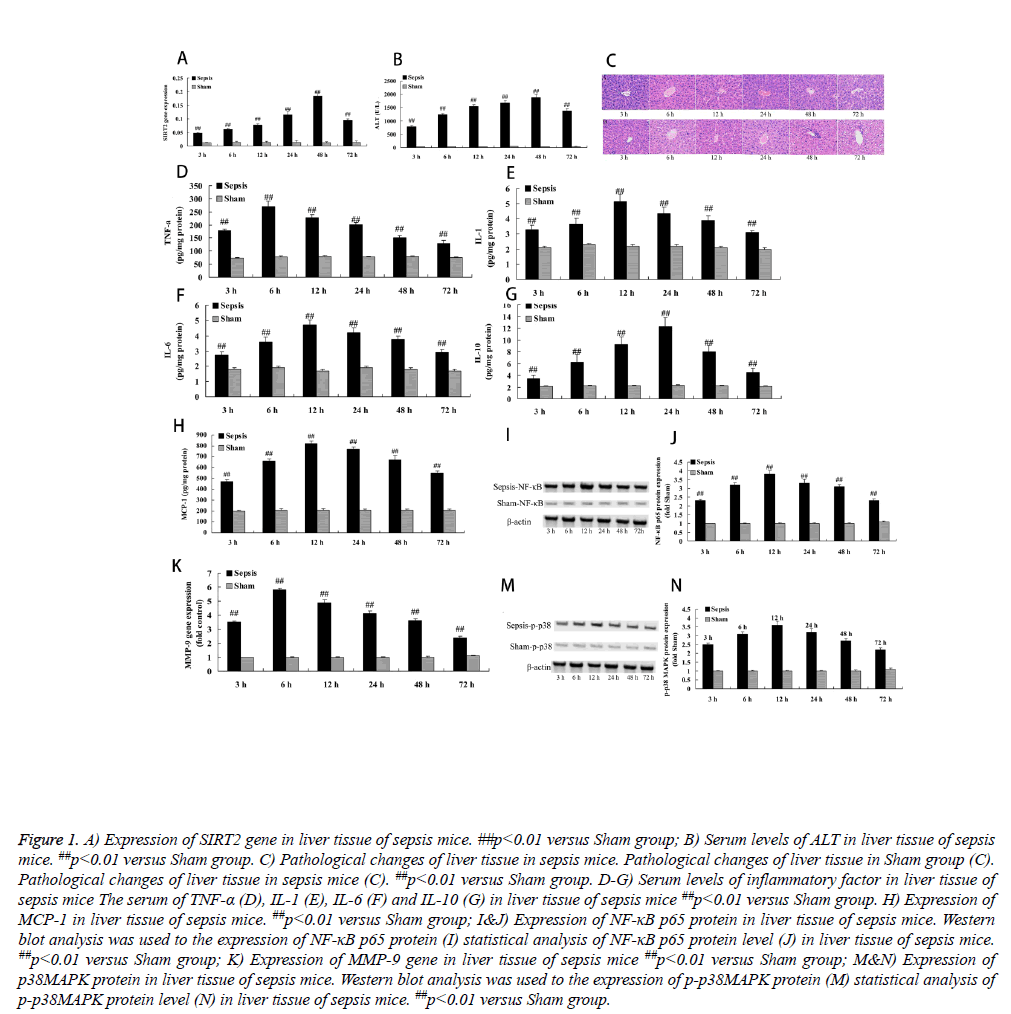
To assess the expression of SIRT2 in sepsis mice, we evaluated the expression of SIRT2 gene in liver tissue of sepsis mice. In sham group, the expression of SIRT2 gene was lower and basic similarity in different time (Figure 1A). As expected, the expression of SIRT2 gene in sepsis mice was rapidly increased since 3 h operation, and reached peak at 48 h after operation, compared with the sham group (Figure 1A).
Figure 1: A) Expression of SIRT2 gene in liver tissue of sepsis mice. ##p<0.01 versus Sham group; B) Serum levels of ALT in liver tissue of sepsis mice. ##p<0.01 versus Sham group. C) Pathological changes of liver tissue in sepsis mice. Pathological changes of liver tissue in Sham group (C). Pathological changes of liver tissue in sepsis mice (C). ##p<0.01 versus Sham group. D-G) Serum levels of inflammatory factor in liver tissue of sepsis mice The serum of TNF-α (D), IL-1 (E), IL-6 (F) and IL-10 (G) in liver tissue of sepsis mice ##p<0.01 versus Sham group. H) Expression of MCP-1 in liver tissue of sepsis mice. ##p<0.01 versus Sham group; I&J) Expression of NF-κB p65 protein in liver tissue of sepsis mice. Western blot analysis was used to the expression of NF-κB p65 protein (I) statistical analysis of NF-κB p65 protein level (J) in liver tissue of sepsis mice. ##p<0.01 versus Sham group; K) Expression of MMP-9 gene in liver tissue of sepsis mice ##p<0.01 versus Sham group; M&N) Expression of p38MAPK protein in liver tissue of sepsis mice. Western blot analysis was used to the expression of p-p38MAPK protein (M) statistical analysis of p-p38MAPK protein level (N) in liver tissue of sepsis mice. ##p<0.01 versus Sham group.
Serum levels of ALT in liver tissue of sepsis mice
To check into the expression of SIRT2 on the serum of ALT level, we inspected the serum of ALT level in this study. In sham group, there was no different in the serum of ALT level in different time, which was lower level (Figure 1B). Nevertheless, sepsis markedly induced the serum of ALT level, and reached peak at 48 h after operation, compared with the sham group (Figure 1B).
Pathological changes of liver tissue in sepsis mice
These results of HE Dyeing showed that cellular structure of liver tissue was normal and clearness, these were no seep inflammatory cells of hepatic sinus and portal area in sham group (Figure 1C). Meanwhile, sepsis mice obviously appeared seep inflammatory cells after operation at 3 h, compared with the sham group (Figure 1C).
Serum levels of inflammatory factor in liver tissue of sepsis mice
To investigate the expression of SIRT2 on inflammatory factor, we measure the serum of TNF-α, IL-1, IL-6 and IL-10 in our study. In sham group, the serum of TNF-α, IL-1, IL-6 and IL-10 maintained lower levels and basic similarity in different time (Figure 1D). However, the serum of TNF-α, IL-1, IL-6 and IL-10 in sepsis mice were rapidly enhanced since 3 h operation, the serum TNF-α level reached peak at 6 h after operation, the serum IL-1 and IL-6 level reached peak at 12 h after operation, and the serum IL-10 level reached peak at 24 h after operation, compared with the sham group (Figures 1D-1G).
Expression of MCP-1 in liver tissue of sepsis mice
To detect the expression of SIRT2 on the serum of MCP-1 level, we measured the serum of MCP-1 level in this study. Results demonstrated that the serum of MCP-1 level were very similar and lower level in different time of sham group (Figure 1H). As shown in Figure 1, the serum of MCP-1 level was memorably increased in sepsis mice since 3 h operation and reached peak at 12 h after operation, compared with the sham group (Figure 1H).
Expression of NF-κB p65 protein in liver tissue of sepsis mice
We further investigated whether SIRT2 is involved in NF-κB p65 protein expression. There was no significant difference the NF-κB p65 protein expression between different times of sham group, which was very low level (Figures 1I and 1J). In addition, the NF-κB p65 protein expression was observably improved and reached peak at 12 h after operation in sepsis mice, compared with the sham group (Figures 1I and 1J).
Expression of MMP-9 gene in liver tissue of sepsis mice
We evaluated investigated whether SIRT2 is involved in MMP-9 gene expression. We found no significant the MMP-9 gene expression in different time of sham group (Figure 1K). However, the MMP-9 gene expression was quickly improved since 3 h after operation and reached peak at 6 h after operation in sepsis mice, compared with the sham group (Figure 1K).
Expression of p38MAPK protein in liver tissue of sepsis mice
To investigate whether SIRT2 is involved in p38MAPK protein in liver tissue of sepsis mice, phosphorylation-p38MAPK (pp38) was analyzed using western blotting. Therefore, western blot analysis indicated that the p-p38 protein expression was low level and basic similarity in different time of sham group (Figure 1M). Predictably, the p-p38 protein expression was markedly promoted and reached peak at 12 h after operation in sepsis mice, compared with the sham group (Figure 1N).
Discussion
Sepsis as systemic inflammatory response syndrome caused by infections is an extremely complex process, there are several mechanisms involved in regulating, involves a large number of changes in gene expression profile [14]. The role of Epigenetic mechanisms gets more and more attention of people in the pathogenesis of sepsis in recent years. Think of epigenetic mediated inflammatory related changes in gene activity plays an important role in the pathogenesis of sepsis [15]. Because of various factors to break the body steady start SIRS in the body, the body produces a large amount of inflammatory medium into the start stage, the strength of this period’s inflammatory response is important for the prognosis of sepsis [16]. When the body coming into the second phase change period (adaptation), also named endotoxin tolerance period, with a large amount of pro-inflammatory genes suppressed, and antiinflammatory gene activation [17]. After safely through this stage, the third stage of stationary phase will come. The strength of the start-up stage decided the duration and prognosis of the phase change stage, change phase stages involved in inhibition of pro-inflammatory genes, antiinflammatory genes, metabolic activation of genes [18]. In our study, we found that the expression of SIRT2 gene and the serum of ALT level in sepsis mice was rapidly increased since 3 h operation, and reached peak at 48 h after operation, compared with the sham group.
Studies have shown that during sepsis, inflammatory cell infiltration, besides macrophage is the main cell of throughout the wound healing process, monocyte chemoattractant protein-1 (MCP-1) abnormally elevated can stimulate macrophages release large amounts of lysosomal enzymes, and by producing oxygen free radicals and pro-inflammatory factor aggravating inflammation of the organization [19]. SIRT2 agonist resveratrol, inhibit nicotinamide processing nucleus pulposus cells [20]. When the SIRT2 is activated, the TNF-α induction of nucleus pulposus cells express lower MCP-1 expression; When SIRT2 is inhibited, the TNF-α induce nucleus pulposus cells’ MCP-1 expression increasing [21]. Therefore, confirmed that SIRT-2 have the function of downregulate chemokines MCP-1 in TNF-α induce nucleus pulposus cells [21,22]. We have previously shown seep inflammatory cells was surged and the serum of TNF-α, IL-1, IL-6, IL-10 and MCP-1 were observably increased and reached peak at 6 or 12 h after operation in sepsis mice. These results hinted that SIRT2 might promote inflammatory and MCP-1 in sepsis mice. Our results were the opposite of those of other studies. For example, Lee et al. suggested that SIRT2 can inhibit lipopolysaccharide-induced inflammation in macrophages [23]. Lin et al. also indicated that SIRT2 suppresses inflammatory responses and MCP-1 in collagen-induced arthritis [24]. In acute and chronic inflammation, SIRT-2 suppresses inflammatory responses, but SIRT family and acetylation can unduly inhibits inflammation, increases a mass of immunoreactions in sepsis mice [25-27]. Several study showed that the plentiful expression of SIRT-2 inducts programmed necrosis through RIP1-RIP3 [20,28], which SIRT-2 may promotes inflammation and immunoreactions in sepsis mice. The viewpoint and specific mechanisms deserve attention and are explained in further study.
SIRT2 as a group of histone deacetylase with NAD+dependent, participate in a number of acetylation of histone and transcription factors process [29]. Histone acetylation is an important epigenetic mechanisms, it through the control of gene transcription to determine the intercellular and intracellular signal transmission, which will affect the pathophysiologic process of the individual. SIRT2 will causes the acetylation of P300’s K314 K315, K310 loci, after the implementation of P65 gene transcription regulation, and then adjust the activity of NF-κB depends inflammatory genes. In numerous pathways of SIRT2 inhibit inflammation, most notably is the inhibition of SIRT2 to the NF-κB signaling pathway [30]. The NF-κB exists in many kinds of cells, and it is the focal point of the cells’ multiple signaling pathways, and plays an extremely important role in the occurence and development of inflammation. In the stillness of the cells, the NF-κB normally exists in the form of p65 dimers [14]. Connecting to the inhibitory protein IκB, in a state of inactivation, stay in the cytoplasm, when activated, IκB with phosphorylation, ubiquitination and proteasome degradation, dissociation combine with the NF-κB, after the activated, p65 entered into the nucleus, the switching control of downstream genes including gene transcription of inflammatory factors such as IL-1, TNF-α, IL-8 and, IL-6 [31]. We observed that NF-κB p65 protein expression and the MMP-9 gene expression were rapidly accumulatived and reached peak at 12 h or 6 h, respectively, after operation in sepsis mice.
SIRT2 inhibition of p38 MAPK signaling pathways-MAPKs are serine/threonine protein kinase in cells, the article consists of three parallel signaling pathways, are respectively ERK signaling pathway, JNK pathway, p38MAPK pathway, participate in a variety of inflammatory factor signal transduction [32]. P38MAPK kinase reaction information transfer process can be described as: After cells stimulation, make MAPK kinase kinase (MAPKKK) activation through certain link [33]. Activated MAPKKK activate MAPK kinase (MAPKK), the latter through double loci p38MAPK phosphorylation activation [34]. Many stimulus such as cytokines, pathogenic microorganism products, changes in the physical and chemical properties of extracellular fluid, extracellular hypertonic, etc. all can activate p38 MAPK pathway, causing a series of related inflammatory response [35]. Our results identify the p-p38 protein expression of sepsis mice was enhanced and reached peak at 12 h after operation.
In summary, this study demonstrates that SIRT2 can increase the serum of ALT level, inflammatory response, the MMP-9 gene expression in sepsis mice. Together, our results identify that SIRT2 intensifies sepsis through mediation of NF-κB and p38 MAPK pathway. Our study may provide a new molecular way for protection effect of SIRT2 against sepsis.
References
- Chen X, Wang Y, Luo H, Luo Z, Liu L, Xu W, Zhang T, Yang N, Long X, Zhu N, Xie H, Liu J. Ulinastatin reduces urinary sepsis’ related inflammation by upregulating Ilα 10 and downregulating TNF-α levels. Mol Med Rep 2013; 8: 29-34.
- Kawai Y1, Cornell TT, Cooley EG, Beckman CN, Baldridge PK, Mottes TA, Luckritz KE, Plomaritas KS, Meade JM, Odetola FO, Han YY, Blatt NB, Annich GM. Therapeutic plasma exchange may improve hemodynamics and organ failure among children with sepsis-induced multiple organ dysfunction syndrome receiving extracorporeal life support. Pediatr Crit Care Med 2015; 16: 366-374.
- Trzeciak S, Glaspey LJ, Dellinger RP. Randomized controlled trial of inhaled nitric oxide for the treatment of microcirculatory dysfunction in patients with sepsis. Crit Care Med 2014; 42: 2482-2492.
- Zhang K, Mao X, Fang Q, Jin Y, Cheng B, Xie G, Li H, Yu L, Zhu T, Wang H, Liu X, Zhang Y, Jin Y, Zhang N, Lou T, Fang XM. Impaired long-term quality of life in survivors of severe sepsis : Chinese multicenter study over 6 years. Anaesthesist 2013; 62: 995-1002.
- Zimmerman JJ, Ringer TV. Inflammatory host responses in sepsis. Crit Care Clin 1992; 8: 163-189.
- Nagata N, Saijo M, Kataoka M. Pathogenesis of fulminant monkeypox with bacterial sepsis after experimental infection with West African monkeypox virus in a cynomolgus monkey. Int J Clin Exp Pathol 2014; 7: 4359-4370.
- Han Y, Zhan Y, Hou G, Li L. Cyclin-dependent kinase 9 may as a novel target in downregulating the atherosclerosis inflammation (Review). Biomed Rep 2014; 2: 775-779.
- Yahiaoui B, Taibi A, Ouaissi A. A Leishmania major protein with extensive homology to silent information regulator 2 of Saccharomyces cerevisiae. Gene 1996; 169: 115-118.
- Vaquero A, Scher MB, Lee DH, Sutton A, Cheng HL, Alt FW, Serrano L, Sternglanz R, Reinberg D. SirT2 is a histone deacetylase with preference for histone H4 Lys 16 during mitosis. Genes Dev 2006; 20: 1256-1261.
- Wang F, Nguyen M, Qin FX, Tong Q. SIRT2 deacetylates FOXO3a in response to oxidative stress and caloric restriction. Aging Cell 2007; 6: 505-514.
- Nie H, Li Y, Wang C, Chen X, Liu B, Wu D, Ying W. SIRT2 plays a key role in both cell cycle regulation and cell survival of BV2 microglia. Int J Physiol Pathophysiol Pharmacol 2014; 6: 166-171.
- Pérez Estrada C, Covacu R, Sankavaram SR, Svensson M, Brundin L. Oxidative stress increases neurogenesis and oligodendrogenesis in adult neural progenitor cells. Stem Cells Dev 2014; 23: 2311-2327.
- Kim HS, Vassilopoulos A, Wang RH. SIRT2 maintains genome integrity and suppresses tumorigenesis through regulating APC/C activity. Cancer Cell 2011; 20: 487-499.
- Rothgiesser KM, Erener S, Waibel S, Lüscher B, Hottiger MO. SIRT2 regulates NF-1ß dependent gene expression through deacetylation of p65 Lys310. J Cell Sci 2010; 123: 4251-4258.
- Lappas M, Mitton A, Lim R, Barker G, Riley C, Permezel M. SIRT1 is a novel regulator of key pathways of human labor. Biol Reprod 2011; 84: 167-178.
- Kim MJ, Kim DW, Park JH. PEP-1-SIRT2 inhibits inflammatory response and oxidative stress-induced cell death via expression of antioxidant enzymes in murine macrophages. Free Radic Biol Med 2013; 63: 432-445.
- Zhu J, Luo C, Wang P, He Q, Zhou J, Peng H. Saikosaponin A mediates the inflammatory response by inhibiting the MAPK and NF-1B pathways in LPS-stimulated RAW 264.7 cells. Exp Ther Med 2013; 5: 1345-1350.
- Perl M, Chung CS, Garber M, Huang X, Ayala A. Contribution of anti-inflammatory/immune suppressive processes to the pathology of sepsis. Front Biosci 2006; 11: 272-299.
- Agarwal A, Sharma V, Tewari R, Koul N, Joseph C, Sen E. Epigallocatechin-3-gallate exhibits anti-tumor effect by perturbing redox homeostasis, modulating the release of pro-inflammatory mediators and decreasing the invasiveness of glioblastoma cells. Mol Med Rep 2008; 1: 511-515.
- Narayan N, Lee IH, Borenstein R, Sun J, Wong R, Tong G, Fergusson MM, Liu J, Rovira II, Cheng HL, Wang G, Gucek M, Lombard D, Alt FW, Sack MN, Murphy E, Cao L, Finkel T. The NAD-dependent deacetylase SIRT2 is required for programmed necrosis. Nature 2012; 492: 199-204.
- Jarvis JN, Meintjes G, Bicanic T, Buffa V, Hogan L, Mo S, Tomlinson G, Kropf P, Noursadeghi M, Harrison TS. Cerebrospinal fluid cytokine profiles predict risk of early mortality and immune reconstitution inflammatory syndrome in HIV-associated cryptococcal meningitis. PLoS Pathog 2015; 11: e1004754.
- Inoue T, Hiratsuka M, Osaki M. SIRT2, a tubulin deacetylase, acts to block the entry to chromosome condensation in response to mitotic stress. Oncogene 2007; 26: 945-957.
- Lee AS, Jung YJ, Kim D. SIRT2 ameliorates lipopolysaccharide-induced inflammation in macrophages. Biochem Biophys Res Commun 2014; 450: 1363-1369.
- Lin J, Sun B, Jiang C, Hong H, Zheng Y. Sirt2 suppresses inflammatory responses in collagen-induced arthritis. Biochem Biophys Res Commun 2013; 441: 897-903.
- McCall CE, El Gazzar M, Liu T, Vachharajani V, Yoza B. Epigenetics, bioenergetics, and microRNA coordinate gene-specific reprogramming during acute systemic inflammation. J Leukoc Biol 90: 439-446, 2011.
- McCall CE, Yoza B, Liu T, El Gazzar M. Gene-specific epigenetic regulation in serious infections with systemic inflammation. J Innate Immun 2010; 2: 395-405.
- Eskandarian HA, Impens F, Nahori MA, Soubigou G, Coppée JY, Cossart P, Hamon MA. A role for SIRT2-dependent histone H3K18 deacetylation in bacterial infection. Science 2013; 341: 1238858.
- Sun L, Wang H, Wang Z. Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell 2012; 148: 213-227.
- Jing E, Gesta S, Kahn CR. SIRT2 regulates adipocyte differentiation through FoxO1 acetylation/deacetylation. Cell Metab 2007; 6: 105-114.
- Lo Sasso G, Menzies KJ, Mottis A, Piersigilli A, Perino A, Yamamoto H, Schoonjans K, Auwerx J. SIRT2 deficiency modulates macrophage polarization and susceptibility to experimental colitis. PLoS One 2014; 9: e103573.
- Li Y, Dai D, Lu Q, Fei M, Li M, Wu X. Sirt2 suppresses glioma cell growth through targeting NF-1B -miR-21 axis. Biochem Biophys Res Commun 2013; 441: 661-667.
- Li Y, Matsumori H, Nakayama Y, Osaki M, Kojima H, Kurimasa A, Ito H, Mori S, Katoh M, Oshimura M, Inoue T. SIRT2 down-regulation in HeLa can induce p53 accumulation via p38 MAPK activation-dependent p300 decrease, eventually leading to apoptosis. Genes Cells 2011; 16: 34-45.
- Lim SJ, Lee YJ, Lee E. p38MAPK inhibitor SB203580 sensitizes human SNU-C4 colon cancer cells to exisulind-induced apoptosis. Oncol Rep 2006; 16: 1131-1135.
- Noel JK, Crean S, Claflin JE, Ranganathan G, Linz H, Lahn M. Systematic review to establish the safety profiles for direct and indirect inhibitors of p38 Mitogen-activated protein kinases for treatment of cancer. A systematic review of the literature. Med Oncol 2008; 25: 323-330.
- Palanivel K, Kanimozhi V, Kadalmani B. Verrucarin A alters cell-cycle regulatory proteins and induces apoptosis through reactive oxygen species-dependent p38MAPK activation in the human breast cancer cell line MCF-7. Tumour Biol 2014; 35: 10159-10167.