- Biomedical Research (2010) Volume 21, Issue 1
Effects of Recombinant Human Erythropoietin (rHuEPO) Treatment on Plasma Insulin-like Growth Factor-I (IGF-I) and Hemoglobin Concentra-tions in Patients with Type 2 Diabetes Mellitus Associated with Neph-ropathy and Anemia of Chronic Renal Failure.
Motoi Sohmiya* and Yoko SohmiyaDepartment of Endocrinology and Metabolism, Sohmiya Clinic, Watarihashi-cho 730-1, Izumo 693-0004, Japan.
- *Corresponding Author:
- Motoi Sohmiya
Department of Endocrinology and Metabolism
Sohmiya Clinic
Watarihashi-cho 730-1
Izumo 693-0004, Japan
E-mail: motoi@sohmiya-clinic.com
Accepted date: September 28 2009
Abstract
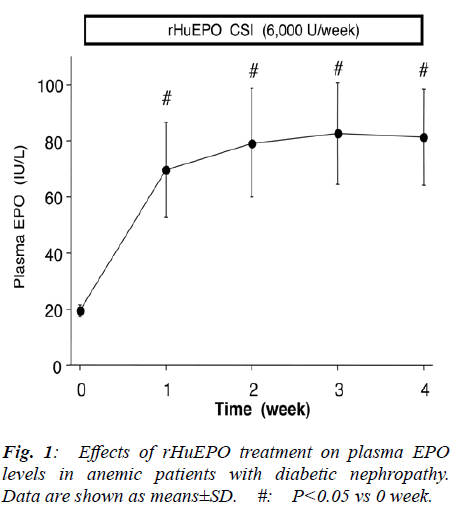
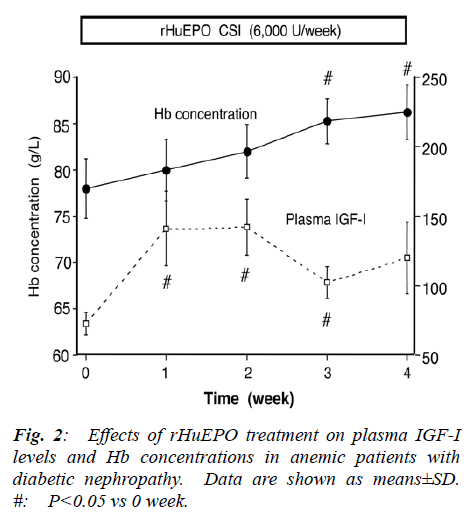
Both insulin-like growth factor-I (IGF-I) and erythropoietin (EPO) have been found to stimulate erythropoiesis, and IGF-I levels are decreased in patients with chronic renal fail-ure (CRF). Decreased IGF-I levels might contribute to the progression of anemia of CRF. However, no studies have examined the effects of rHuEPO therapy on plasma IGF-I levels and Hb concentration in predialysis diabetic patients with CRF and anemia. Therefore, we investigated the effects of rHuEPO treatment on plasma IGF-I levels and Hb concentrations in patients with diabetes and anemia of CRF. Seven patients with type 2 diabetes mellitus accompanied by advanced nephropathy (renal failure stage) were studied. The mean (±SE) age was 62.6±6.2yrs. Serum creatinine and creatinine clearance levels were 327.1±44.2 μmol/L and 0.261±0.022 ml/s, respectively. Re-combinant human EPO (rHuEPO) was subcutaneously infused into the abdomen for 4 weeks at a flow rate of 6,000 IU/2.5ml/week using a portable infusion pump. Plasma EPO levels were increased from 19.4±2.2 IU/L to 69.6±16.9 IU/L 1 week after the start of rHuEPO administration, and were maintained at a steady state. Plasma IGF-I lev-els at time 0 decreased to 72.7±8.4 μg/L compared with age-matched diabetic patients with-out nephropathy (166.0±15.5 μg/L). Plasma IGF-I levels were increased 1-3 weeks after the start of rHuEPO administration, followed by an increase in Hb concentrations 3-4 weeks after rHuEPO administration. We have therefore hypothesized that rHuEPO administration increases plasma IGF-I levels followed by Hb concentrations. These findings suggest that rHuEPO treatment has a stimulatory effect on IGF-I production, and that increases in IGF-I might be a good indica-tor of improved Hb concentrations.
Keywords
Insulin-like growth factor-I, Erythropoietin, Diabetes Mellitus, Diabetic nephropathy, Anemia
Introduction
Erythropoietin (EPO) is a 34-kilodalton glycoprotein hormone and a potent regulator of erythropoiesis, that is secreted primarily from the endothelium and interstitial fibroblasts of renal tubules, and to a lesser extent from the liver in the rat [1-3]. Chronic renal failure (CRF) is as-sociated with anemia, which is accompanied by relatively decreased EPO secretions from the kidney. The anemia of CRF accompanied by diabetic nephropathy is more severe than that of glomerulonephritis [4].
Insulin-like growth factor I (IGF-I) plays an important role in erythrocyte production (5), stimulating proliferation of erythroid precursor cells in vitro [6-11]. IGF-I is required for and essential to erythroid development [12,13].
Recently, some investigations have suggested that both IGF-I and EPO play an important role in erythropoiesis in vitro [9,12,14]. The addition of IGF-I with EPO leads to enhanced heme synthesis and moderate cellular prolifera-tion in the late erythroblasts in vitro [9]. Brox et al. have suggested that anemia of CRF may present both EPO-deficient and functional IGF-I-deficient states, and that subtherapeutic doses of EPO and IGF-I cause a significant increase in hemoglobin (Hb) levels in the mouse [14]. In a human study, plasma IGF-I levels were found to be increased in patients with dialysis and kidney trans-plantation treated with recombinant human EPO (rHuEPO) therapy compared without rHuEPO therapy [15]. However, no studies have examined the effects of rHuEPO therapy on plasma IGF-I levels and Hb concen-trations in predialysis diabetic patients with CRF and anemia.
In the present study, we investigated the effects of rHuEPO treatment on plasma IGF-I levels and Hb con-centrations in diabetic patients with nephropathy and anemia of CRF.
Materials and Methods
Subjects
Seven patients with type 2 diabetes mellitus accompanied by advanced nephropathy (renal failure stage) were stud-ied. The mean (±SE) age was 62.6±6.2 yrs (4 males and 3 females). BMI was 22.5±1.9 kg/m2. HbA1c levels were 6.7±0.2%. Serum creatinine and creatinine clearance levels were 327.1±44.2 μmol/L and 0.261±0.022 ml/s, respectively. Serum albumin levels were 32±5 g/L. Hb concentrations were 78±3 g/L. Urine albumin excretions for 24 hours were 2,613±578 mg/day. Type 2 diabetes mellitus was defined by criteria for the diagnosis of dia-betes in non-pregnant adults shown in American diabetes association. Anti-glutamic acid decarboxylase (GAD) antibody was negative in all patients. All patients were treated with insulin, and none underwent hemodialysis.
Study Protocol
Recombinant human EPO (rHuEPO) (Epoetin beta, Chu-gai Pharmaceutical Co.) was subcutaneously infused into the abdomen for 4 weeks at a flow rate of 6,000 IU/2.5ml/week using a portable infusion pump (SP-3HQ, Nipro Co., Japan). Both subjective and objective symp-toms were evaluated each day. Blood pressure and body weight were measured weekly. Blood samples were also obtained weekly in the morning after overnight fasting for 4 weeks before and after the start of rHuEPO administra-tion. Plasma samples were stored at -20°C until assay. Hb concentrations as well as plasma IGF-I and plasma EPO levels were measured.
The present study was carried out in accordance with the Helsinki Declaration of 1975. Written informed consent was obtained from all subjects.
Assays
Plasma IGF-I levels were measured by specific radioim-munoassay after acid/ethanol extraction, as previously described [16]. The minimal detectable quantity was 30 μg/L using a 50 μL plasma sample. The coefficients of variation for intra- and inter-assays were 6.3% and 7.2%, respectively. The levels of plasma IGF-I were found to be 202.8±70.6 μg/L and 198.6±91.8 μg/L in males and fe-males, respectively [16]. Plasma EPO levels were meas-ured by sensitive enzyme immunoassay (EIA), as previ-ously described [17]. The sensitivity was 0.75 IU/L using 20 μl of plasma sample. The coefficients of variation for intra- and inter-assays were 5.3% and 7.2%, respectively.
Hb concentrations, serum and urine creatinine levels, se-rum and urine albumin levels and HbA1c levels, as well as common blood cell counts (CBC) and other blood chemistry were measured by conventional methods in our laboratory. The normal ranges of Hb concentration in our laboratory were found to be 136-183 g/L and 112-152 g/L in males and females, respectively.
Statistical analysis
Statistical analysis of the data was performed by ANOVA in combination with the Student's t-test. P<0.05 was con-sidered significant. Data are expressed as the mean± SD.
Results
As shown in Fig. 1, plasma EPO levels were increased from 19.4±2.2 IU/L to 69.6±16.9 IU/L 1 week after the start of rHuEPO administration, and were maintained at a steady state. The effects of rHuEPO treatment on plasma IGF-I and Hb concentrations are shown in Fig. 2. Plasma IGF-I level at time 0 were 72.7±8.4 μg/L, lower than IGF-I levels in age-matched diabetic patients without nephropathy (166.0±15.5 μg/L). Plasma IGF-I levels were increased 1-3 weeks after the start of rHuEPO ad-ministration, followed by an increase in Hb concentra-tions 3-4 weeks after rHuEPO administration. Hb con--centrations were increased from 78±3 g/L to 86±3 g/L at 4 weeks after the start of rHuEPO administration. There were no significant changes in serum creatinine levels and blood urea nitrogen levels. Blood pressure and body weight did not change during the experimental period.
Discussion
We have hypothesized that rHuEPO administration in-creases plasma IGF-I levels, followed by Hb concentra-tions. There have been few reports regarding the con-tribution of both rHuEPO therapy and IGF-I levels to the prevention of anemia. Brox et al have described that combination therapy with EPO and IGF-I induces a large increase in Hb concentrations in the mouse (14). They have suggested that anemia of CRF may represent both an EPO- and a functional-IGF-I deficient state. In human studies, there has been no report of the effects of rHuEPO therapy on plasma IGF-I and Hb concentrations in pre-dialysis CRF patients. In the present study, plasma IGF-I basal levels were decreased in diabetic patients with anemia of CRF compared with diabetic patients without anemia and diabetic nephropathy. Plasma IGF-I levels were increased to a normal range 1 - 3 weeks after rHuEPO administration. Such increases in plasma IGF-I levels were rapid. These findings may suggest that rHuEPO has a direct stimulatory effect on erythropoiesis as well as IGF-I production in humans. Although this study was small in scale and not a placebo control study
Our results suggest that increased IGF-I levels might be a good indicator of improved Hb concentrations. Therefore, a large-scale study is needed to clarify the relationship between rHuEPO treatment and IGF-I production.
IGF-I production is affected by diabetes mellitus in vivo. Plasma IGF-I levels are decreased in streptozoto-cin-induced diabetic rats (18), and the abundance of total hepatic IGF-I mRNA decreases after streptozotocin ad-ministration [19]. Circulating IGF-I levels decrease in patients with diabetes mellitus [20]. In addition, IGF-I levels are low in patients with diabetic complications, negatively correlating with HbA1c levels [21]. In the present study, plasma glucose control was good in all subjects (data not shown).
Plasma IGF-I levels decrease in patients with CRF [22,23], making plasma IGF-I a good indicator of the nu-tritional status of CRF patients [22]. Plasma IGF-I lev-els in CRF patients are lower than those in normal sub-jects and can be improved by nutritional therapy. Therefore, the increase in plasma IGF-I levels in the pre-sent study and the improvement of nutritional status due to rHuEPO administration might contribute in part to the increases in plasma IGF-I levels. However, it is un-known whether rHuEPO has a direct stimulatory effect on improvements in nutritional status.
Plasma IGF-I levels might be affected by the GH status in patients with chronic renal failure. The GH paradoxical response was observed in patients with CRF. rHuEPO administration might affect hypothalamus and pituitary function in patients with chronic renal failure [24-26]. We could not assess GH response by GH stimulation tests during rHuEPO administration. However, there has been no report that rHuEPO has a direct stimulating effect on GH secretion.
We administered rHuEPO by means of continuous subcu-taneous infusion (CSI). This method could maintain con-stant plasma EPO levels, which we believe is the physio-logical state. Intravenous injection resulted in transient increases in plasma EPO levels, producing non physio-logical conditions. We could not carry out a comparative study of CSI and SC injections. Therefore, a further com-parative study is needed.
In summary, we found that rHuEPO administration in-creases plasma IGF-I levels followed by Hb concentra-tions. It also appears that rHuEPO may have a stimula-tory effect on IGF-I production. These findings suggest that decreased IGF-I levels might play a role in the pro-gression of renal anemia in diabetic patients with renal failure and that increases in IGF-I might be a good indi-cator of improved Hb concentrations.
Acknowledgments
This work was supported in part by grants from the Renal Anemia Foundation, Japan.
References
- Clemons GK, Fitzsimmons SL, DeManincor D. Im-munoreactive erythropoietin concentration in fetal and neonatal rats and the effects of hypoxia. Blood 1986;68: 892-899.
- Kurtz A, Eckardt KU, Neumann R, et al. Site of erythropoietin formation. Contrib Nephrol 1989; 76: 14-23.
- Koury ST, Koury MJ, Bondurant MC, Caro J, Graber SE. Quantitation of erythropoietin-producing cells in kidneys of mice by in situ hybridization: correla-tion with hematocrit, renal erythropoietin mRNA, and serum erythropoietin concentration. Blood 1989;74: 645-651.
- Ishimura E, Nishizawa Y, Okuno S, et al. Diabetes mellitus increases the severity of anemia in non-dialyzed patients with renal failure. J Nephrol 1998;11: 83-68.
- Shih LY, Huang JY, Lee CT. Insulin-like growth factor I plays a role in regulating erythropoiesis in patients with end-stage renal disease and erythrocytosis. J Am Soc Nephrol 1999;10: 315-322.
- Akahane K, Tojo A, Urabe A, Takaku F. Pure erythropoietic colony and burst formations in se-rum-free culture and their enhancement by insulin-like growth factor I. Exp Hematol 1987;15: 797-802.
- Ratajczak J, Zhang Q, Pertusini E, et al. The role of insulin (INS) and insulin-like growth factor-I (IGF-I) in regulating human erythropoiesis. Studies in vitro under serum-free conditions--comparison to other cytokines and growth factors. Leukemia 1998;12: 371-381.
- Sanders MR, Lu H, Walker F, Sorba S, Dainiak N. The Raf-1 protein mediates insulin-like growth fac-tor-induced proliferation of erythroid progenitor cells. Stem Cells 1998;16: 200-207.
- Muta K, Krantz SB, Bondurant MC, W ickrema A. Distinct roles of erythropoietin, insulin-like growth factor I, and stem cell factor in the development of erythroid progenitor cells. J Clin Invest 1994;94: 34-43.
- Merchav S.The haematopoietic effects of growth hor-mone and insulin-like growth factor-I. J Pediatr En-docrinol Metab 1998;11: 677-685.
- Miyagawa S, Kobayashi M, Konishi N, Sato T, Ueda K. Insulin and insulin-like growth factor I support the pro-liferation of erythroid progenitor cells in bone marrow through the sharing of receptors. Br J Haematol 2000;109: 555-562.
- Boyer SH, Bishop TR, Rogers OC, et al. Roles of erythropoietin, insulin-like growth factor 1, and uni-dentified serum factors in promoting maturation of pu-rified murine erythroid colony-forming units. Blood 1992;80: 2503-2512.
- Sawada K, Krantz SB, Dessypris EN, Koury ST, Saw-yer ST. Human colony-forming units-erythroid do not require accessory cells, but do require direct interaction with insulin-like growth factor I and/or insulin for erythroid development. J Clin Invest 1989;83: 1701-1709.
- Brox AG, Zhang F, Guyda H, Gagnon RF. Subthera-peutic erythropoietin and insulin-like growth factor-1 correct the anemia of chronic renal failure in the mouse. Kidney Int 1996;50: 937-943.
- Malyszko J, Wolczynski S, Zbroch E, et al. Insulin-like growth factor system components in relation to erythropoietin therapy and bone metabolism in dialyzed patients and kidney transplant recipients. Nephron 2002;90: 282-289.
- Yamamoto H, Sohmiya M, Oka N, Kato Y. Effects of aging and sex on plasma insulin-like growth factor I (IGF-I) levels in normal adults. Acta Endocrinologica (Copenh) 1991;124: 497-500.
- Sohmiya M, Kato Y. Molecular and electrical hetero-geneity of circulating human erythropoietin (EPO) measured by sensitive enzyme immunoassay. Eur J Clin Invest 2000;30: 344-349.
- Maes M, Underwood LE, Ketelslegers JM. Low se-rum somatomedin-C in insulin-dependent diabetes: evidence for a postreceptor mechanism. Endocrinology 1986;118: 377-382.
- Yang H, Scheff AJ, Schalch DS. Effects of streptozoto-cin-induced diabetes mellitus on growth and hepatic insulin-like growth factor I gene expression in the rat. Metabolism 1990;39: 295-301.
- Tan K, Baxter RC. Serum insulin-like growth factor I levels in adult diabetic patients: the effect of age. J Clin Endocrinol Metab 1986;63: 651-655.
- Garay-Sevilla ME, Nava LE, Malacara JM, et al. Ad-vanced glycosylation end products (AGEs), insulin-like growth factor-1 (IGF-1) and IGF-binding protein-3 (IGFBP-3) in patients with type 2 diabetes mellitus. Diabetes Metab Res Rev 2000;16: 106-113.
- Lu F, Li P, Zheng F, Zhang Z, Tomino Y. Serum insu-lin-like growth factor 1 level and nutritional assessment in nondialytic patients with chronic renal failure. Kid-ney Blood Press Res 2002;25: 116-119.
- Besbas N, Ozaltin F, Coskun T, et al. Relationship of leptin and insulin-like growth factor I to nutritional status in hemodialyzed children. Pediatr Nephrol 2003;18: 1255-1259.
- Diez JJ, Iglesias PL, Sastre J, Gomez-Pan A, et al. Influence of erythropoietin on paradoxical responses of growth hormone to thyrotropin-releasing hormone in uremic patients. Kidney Int 1994;46: 1387-1391.
- Tokgoz B, Utas C, Dogukan A, Oymak O, Kelestimur F. Influence of long term erythropoietin therapy on the hypothalamic-pituitary-thyroid axis in patients under-going capd. Ren Fail 2002;24: 315-323.
- Diez JJ, Sastre J, Iglesias P, et al. Growth hormone responses to pituitary and hypothalamic stimuli in CAPD patients treated with recombinant human erythropoietin. Adv Perit Dial 1992;8: 340-345.