Research Article - Biomedical Research (2017) Volume 28, Issue 4
Effect of qidan granule and tetrandrine on the information transmission channel of TGF-?1-smads for silicosis fibrosis
Zhang H1, Xu S1, Wu Q1, Wang W1* and Xin H21Department of Respiratory Medicine, The Second Hospital of Shandong University, China
2Department of Respiratory Medicine, Shandong Province Hospital, China
- *Corresponding Author:
- Wang W
Department of Respiratory Medicine
The Second Hospital of Shandong University, China
Accepted on September 15, 2016
Abstract
The study is aimed to investigate the effect of Qidan Granule and tetrandrine on the signal transduction pathway of TGF-β1 (Transforming Growth Factor-β1) for silicosis fibrosison in Wistar rats developed by dust injection. Silicon nodules are mainly of grade III-IV without any treatment, while those in Qidan Granule and tetrandrine groups were of grade II. Pulmonary coefficient and content of hydroxyproline in both Qidan groups and tetrandrine ones are lower, so are the expressions of TGFβ1 and Smad3 in bronchoalveolar lavage fluid and pulmonary tissue. Furthermore, expressions of Smad7 in treating groups are higher than those in the model group. Meanwhile, kidney injuries can be found in all rats of both tetrandrine groups, while not in those of other groups. It is clear that Both Qidan Granule and tetrandrine can inhibit expression of both Smad3 and TGF-β1 and promoting expression of Smad7. Qidan granules and Tetrandrine could inhibit remarkably silicotic fibrosis in rats. However, Qidan Granule is much safer without renal toxicity.
Keywords
Qidan granule, Experimental silicosis, Transforming growth factor β1, Transcription factor, Smad3, Smad7.
Introduction
More than 400,000 patients are suffering from silicosis currently, with an annual increase of 10,000~15,000. 55% of the people working in dusty environment in India suffered from this disease, while 37% in Latin American countries. In the United States, 10,000 workers of the one million who worked in the above environment were afflicted with silicosis.
Traditional treatment with tetrandrine could not be widely used because of the high price and side effects. In order to find an ideal medicine for treatment and prevention of silicosis, this experiment was set to use the Chinese herbal medicine Qidan Granule (mainly composed of root of red-rooted salvia, Radix Astragali, Rhizoma Chuanxiong, Atractylodes ovate, Tuckahoe, Hirudo, Pinellia ternate, Platycodon grandiflorum) and tetrandrine to cure the experimental silicosis on rats. Effects and molecular mechanism of the treatment for silicosis by Qidan Granule and tetrandrine were further studied through the observation of rats’ pulmonary coefficient, changes of hydroxyproline content in pulmonary tissue and expressions of TGF-β1 in bronchoalveolar lavage fluid, as well as determination of transforming growth factor β1, transcription factors Smad3 and Smad7 in pulmonary tissue.
Materials and Methods
Animal modeling and grouping
A total of 120 healthy SPF (Specific-Pathogen Free) grade Wistar rats, each weighing (180 ± 20) g, were provided by Laboratory Animal Center of Shandong University and fed according to SPF standard. The rats were randomly divided into normal control group, model group, Qidan group 1, Qidan group 2, tetrandrine group 1 and tetrandrine group 2.2% pentobarbital (0.7 ml/100 g) was injected into abdominal cavity of each rat to anaesthetize it. Epidural anaesthesia catheter with adapter was then inserted into the trachea. 20 rats, which were perfused with 1 ml physiological saline solution into tracheas at a time, were taken as normal control group.
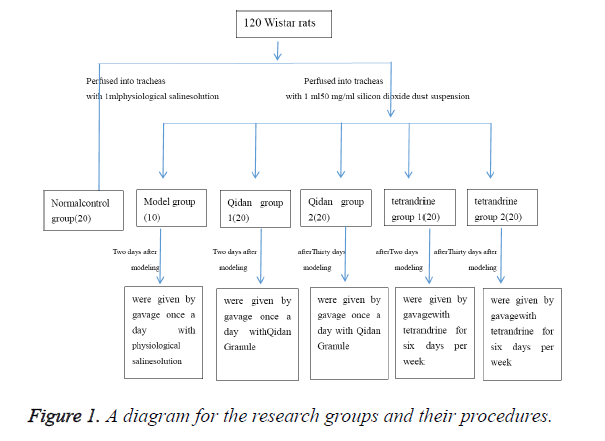
The other 100 rats were perfused into tracheas with 1 ml 50 mg/ml silicon dioxide dust suspension (produced by Sigma Company, was diluted into 50 mg/ml suspension by physiological saline solution, content of free silica ≥ 99%, <5 μm particles ≥ 99%, 8000 U/ml penicillin was added before use) and 0.25 ml air, then were rotated immediately afterwards to distribute the injection evenly in the lungs. 10 of these rats will be randomly chosen to form the model group. Two days after modeling, Qidan Granule (3,125 mg/kg, which was composed of crude extract of Chinese herbal medicine such as root of red-rooted salvia, Radix Astragali, Rhizoma Chuanxiong, Atractylodes ovate, Tuckahoe, Hirudo, Pinellia ternate, Platycodon grandiflorum, produced by preparation lab in Shandong Provincial Hospital) was given to rats of Qidan group 1 every day through administration by gavage, and tetrandrine (22 mg/kg) to those of tetrandrine group 1 six days per week. Thirty days after modeling, Qidan 2 group rats were given by gavage once a day with Qidan Granule (3,125 mg/ kg). Tetrandrine (22 mg/kg, purchased from Hangzhou Siwor Industrial & Trading Co., Ltd., was diluted by 0.2% hydrochloric acid solution) were given to rats of tetrandrine 2 for six days per week through administration by gavage. Physiological saline solution was given to rats of the normal control group every day (2 ml/rat) through administration by gavage. Three months later, drugs were stopped in tetrandrine group for one month, because of its strong renal toxicity. This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The animal use protocol has been reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) of The Second Hospital of Shandong University (The research groups and their procedures were shown in Figure 1).
Observation items and determination methods
Five months after modeling, the rats were weighed before being executed. 2% pentobarbital (0.7 ml/100 g) was injected to abdominal cavity of the rats to make them anaesthetized. Ten rats were randomly taken from each group, and epidural anaesthesia catheter with adapter was inserted into tracheas of these rats. A dose of 4 ml 37°C physiological saline solution was perfused slowly into each rat’s lungs, and then drawn out 30 seconds later. The procedure was repeated for three times. 2 ml bronchoalveolar lavage fluid, which was centrifuged in 1,500 rpm, 4°C, was collected. After extracting the supernatant, TGF-β1 expressions were examined according to operation procedures of ELISA (Enzyme-Linked Immuno Sorbent Assay) reagent kit (TGF-β1 ELISA reagent kit was purchased from American Alpha Diagnostic Intl). Pulmonary tissue of the other 60 rats was picked out. Connective tissue such as trachea and bronchus was removed. The pulmonary tissue was rinsed in physiological saline solution and weighed after the moisture was absorbed by filter paper. Pulmonary coefficient was calculated as: pulmonary coefficient=wet weight of the lung (mg)/body weight of the rat (g). The right lung was picked out to examine the content of hydroxyproline by using alkali hydrolysis (according to the instructions of hydroxyproline reagent kit, which purchased from Nanjing Jiancheng Bioengineering Institute.). Apex and base of the left lung were cut off, and the middle part of the left lung was antisepticised in 10% buffered formalin, paraffin embedded, then cut into 5 μm thick sections, which were processed with Hematoxylin-Eosin (HE) staining and observed under ordinary optical microscope to examine the cellular construction of silicon nodules. The silicosis was classified into 4 grades according to the 4-grade classification methods of experimental silicosis: Grade 0 with no pathological changes, Grade I cellular nodules, Grade II cellular fibrosis nodules, Grade III fibrocyte nodules and Grade IV with fibrosis silicon nodules and nodule fusion in some parts. Expressions of TGF- β1, Smad3 and Smad7 in pulmonary tissue were detected by using immunohistochemical method (according to the operation procedures of immunohistochemical reagent kit, which purchased from Wuhan Boster Biological Technology Co., Ltd.). The remaining pulmonary tissue was preserved in liquid nitrogen. The left kidney was antisepticised in 10% buffered formalin, paraffin embedded, then cut into 5 μm thick, processed by Hematoxylin-Eosin (HE) staining, and observed under ordinary optical microscope to examine the pathological changes of the renal tissue (All chemicals and their origins' were explained in Table 1).
| Chemicals | Origins |
|---|---|
| Silicon dioxide dust | Sigma Company |
| Qidan Granule | Preparation lab in Shandong Provincial Hospital |
| ELISA reagent kit | American Alpha Diagnostic Intl |
| Hydroxyproline reagent kit | Nanjing Jiancheng Bioengineering Institute |
| Immunohistochemical reagent kit | Wuhan Boster Biological Technology Co., Ltd. |
Table 1. Chemicals and their origins.
Statistical analysis
All numerical values were expressed in ± SD and calculated by SPSS11.5 statistical software. Comparison among groups was conducted by one-way analysis of variance, and the variances were analyzed by Pearson correlation analysis.
Results
Pathological examination of pulmonary diseases
Rats’ lungs in the normal control group were pink and smooth without any abnormal changes. Volume and weight of the lungs in the model group increased. The lungs became hard. On the surface and slices, there were pale millet-sized nodules, which tended to inosculate. The nodules were hard and stuck out. Bleeding spots can be observed on the lungs of both Qidan groups and tetrandrine groups. Occasionally there were pale millet nodules on the surface and slices of the lung, which, however, did not stick out, and there was no inosculation.
Observations under optical microscope
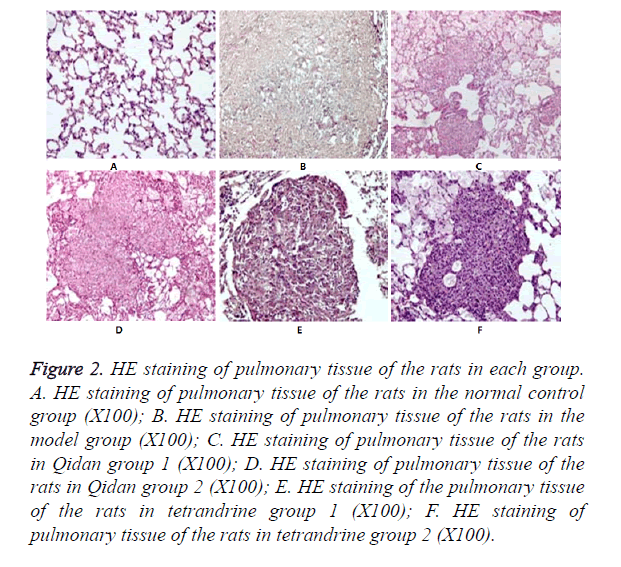
Results of HE staining: It was shown in Figure 2. that no obvious morphological changes occurred under microscope observation in the normal control group. In the model group, pulmonary tissue structures were damaged and alveolus collapsed. And there was obvious fiber hyperplasia, especially around the small blood vessels and bronchioles. A large number of silicon nodules of different sizes, mainly of Grade III~IV could be seen in the pulmonary tissue, some of which were inosculated into pieces. There was abundant collagen fiber hyperplasia in the center of the nodules, and hyalinization could be seen. Only a small number of fibroblasts and macrophages were distributed around the nodules. Silicon nodules of rats in Qidan groups and tetrandrine groups are mainly of Grade II. Scattered silicon nodules could be seen after HE staining. There was no fusion. The nodules were composed of fibroblasts, macrophages and collagen fiber. The areas of Silicon nodules were measured. It can be seen from Table 2 that both the total and Grade III~IV areas of Silicon nodules in the Qidan group 1 and 2, and tetrandrine group 1 and 2 were much lower than those in the model group. And the differences were quite significant (P<0.05). While both the total and Grade II~IV areas of Silicon nodules in Qidan 1 and 2, and tetrandrine 1 and 2 were higher than those in the normal control group, with significant differences (P<0.05).
Figure 2. HE staining of pulmonary tissue of the rats in each group. A. HE staining of pulmonary tissue of the rats in the normal control group (X100); B. HE staining of pulmonary tissue of the rats in the model group (X100); C. HE staining of pulmonary tissue of the rats in Qidan group 1 (X100); D. HE staining of pulmonary tissue of the rats in Qidan group 2 (X100); E. HE staining of the pulmonary tissue of the rats in tetrandrine group 1 (X100); F. HE staining of pulmonary tissue of the rats in tetrandrine group 2 (X100).
| Group | Quantity of rats | Total aeras | Grade I | Grade II | Grade III | Grade IV |
|---|---|---|---|---|---|---|
| Control group | 10 | 0.00 ± 0.00△ | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00△ | 0.00 ± 0.00△ |
| Model group | 10 | 3482.25 ± 307.98* | 0.00 ± 0.00 | 0.00 ± 0.00 | 1860.88 ± 329.51 * | 1621.36 ± 195.56* |
| Qidan group 1 | 10 | 1704.65 ± 134.71△* | 796.84 ± 210.17△* | 807.07 ± 166.59△* | 100.73 ± 40.21△* | 0.00 ± 0.00△ |
| Qidan group 2 | 10 | 1693.88 ± 148.43△* | 775.39 ± 169.47△* | 811.55 ± 187.37△* | 106.94 ± 38.59△* | 0.00 ± 0.00△ |
| Terandrine group 1 | 10 | 1720.12 ± 123.38△* | 818.97 ± 173.41△* | 791.93 ± 154.88△* | 109.21 ± 28.93△* | 0.00 ± 0.00△ |
| Terandrine group 2 | 10 | 1739.804 ± 102.68△* | 839.45 ± 169.03△* | 786.60 ± 161.39△* | 113.74 ± 28.40△* | 0.00 ± 0.00△ |
| Note: Compared with the model group, △P<0.05; and compared with the normal control group, *P<0.05. | ||||||
Table 2. The areas of Silicon nodules of rats in each group (um2, x ± S).
Pulmonary coefficient and content of hydroxyproline
It can be seen from Table 3 the pulmonary coefficient and content of hydroxyproline in the normal control group, Qidan group 1 and 2, and tetrandrine group 1 and 2 were much lower than those in the model group. And the differences were quite significant (P<0.05) while the pulmonary coefficient in Qidan 1 and 2, and tetrandrine 1 and 2 were higher than those in the normal control group, with significant differences (P<0.05). However, the content of hydroxyproline in Qidan group 1 and 2, and tetrandrine 1 and 2 were not significantly different from normal control group.
| Group | Quantity of rats | Pulmonary coefficient | P | Content of hydroxyproline (mg/g) | P |
|---|---|---|---|---|---|
| Control group | 10 | 3.61 ± 0.38△ | 0.0001 | 1.89 ± 0.27△ | 0.0001 |
| Model group | 10 | 13.27 ± 2.19* | 0.0001 | 3.90 ± 1.46* | 0.0001 |
| Qidan group 1 | 10 | 5.61 ± 1.11*△ | 0.0001/0.246 | 2.20 ± 0.26△ | 0.0001/0.977 |
| Qidan group 2 | 10 | 5.82 ± 1.06*△ | 0.0001/0.039 | 2.61 ± 0.50△ | 0.003/0.572 |
| Terandrine group 1 | 10 | 5.91 ± 1.13*△ | 0.0001/0.001 | 2.67 ± 0.56△ | 0.006/0.462 |
| Terandrine group 2 | 10 | 5.94 ± 1.05*△ | 0.0001/0.044 | 2.66 ± 0.47△ | 0.009/0.384 |
| Note: Compared with the normal control group, *P<0.05; and compared with the model group, ΔP<0.05. | |||||
Table 3. Pulmonary coefficient and content of hydroxyproline of rats in each group (P<0.05).
Expressions of TGF-β1 in bronchial lavage fluid of the rats
In Table 4 expressions of TGF-β1 in bronchial lavage fluid in the normal control group, Qidan 1 and 2, and tetrandrine 1 and 2 were lower than those in the model group, with significant differences (P<0.05). Expressions of TGF-β1 in bronchial lavage fluid in the model group, Qidan group and tetrandrine group were higher than those in the normal control group, with significant differences (P<0.05).
| Group | Quantity of rats | TGF-β1 | P |
|---|---|---|---|
| Control group | 10 | 0.314 ± 0.0268△ | 0.0001 |
| Model group | 10 | 0.468 ± 0.0322* | 0.0001 |
| Qidan group 1 | 10 | 0.372 ± 0.0197△* | 0.0001/0.047 |
| Qidan group 2 | 10 | 0.380 ± 0.0270△* | 0.0001/0.020 |
| Terandrine group 1 | 10 | 0.373 ± 0.0360△* | 0.0001/0.006 |
| Terandrine group 2 | 10 | 0.379 ± 0.0407△* | 0.0001/0.029 |
| Note: Compared with the normal control group *P<0.05, and compared with the model group, △P<0.05. | |||
Table 4. Expressions of TGF-β1 in BALF of rats in each group (x ± s).
Expressions of TGF-β1, Smad3 and Smad7 in the pulmonary tissue
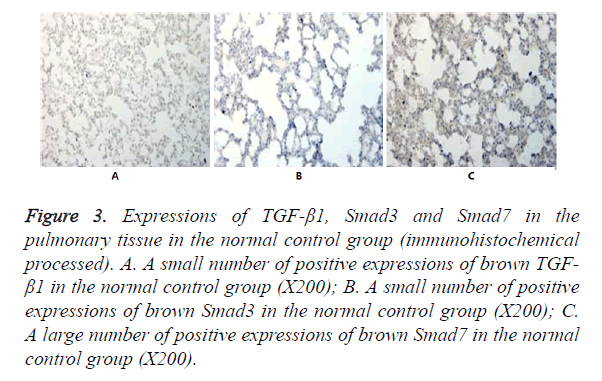
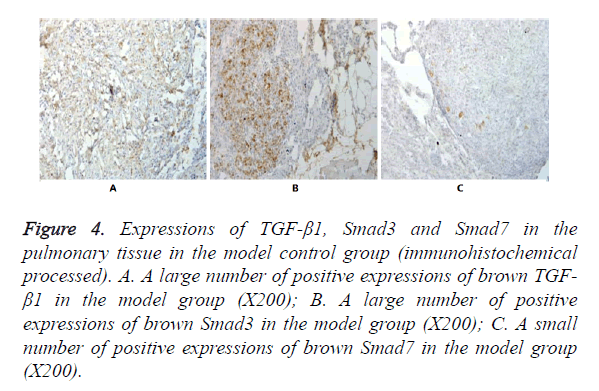
In Figures 3-8, it was show that a large number of brown TGF- β1 and Smad3, as well as a small number of Smad7 positive expressions appeared in the pulmonary tissue of the model group after immunohistochemical process. A small number of brown TGF-β1 and Smad3, as well as a large number of Smad7 positive expressions appeared in Qidan group 1 and 2, and tetrandrine group 1 and 2. TGF-β1 was localized in the cytoplasm of alveolus macrophages, epithelial cells, endothelial cells and fibroblasts. Smad7 and Smad3 were localized in the cytoplasm of alveolus macrophages, fibroblasts and epithelial cells.
Figure 3. Expressions of TGF-β1, Smad3 and Smad7 in the pulmonary tissue in the normal control group (immunohistochemical processed). A. A small number of positive expressions of brown TGF- β1 in the normal control group (X200); B. A small number of positive expressions of brown Smad3 in the normal control group (X200); C. A large number of positive expressions of brown Smad7 in the normal control group (X200).
Figure 4. Expressions of TGF-β1, Smad3 and Smad7 in the pulmonary tissue in the model control group (immunohistochemical processed). A. A large number of positive expressions of brown TGF- β1 in the model group (X200); B. A large number of positive expressions of brown Smad3 in the model group (X200); C. A small number of positive expressions of brown Smad7 in the model group (X200).
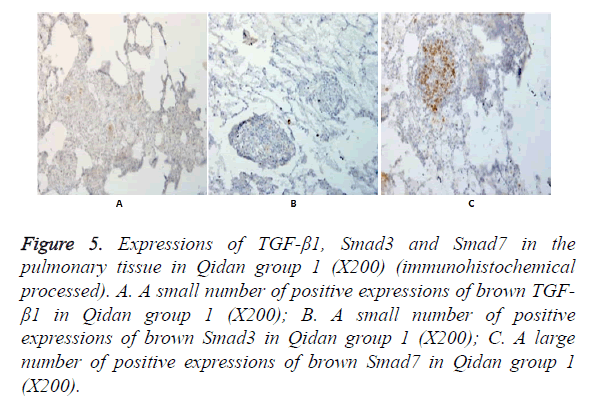
Figure 5. Expressions of TGF-β1, Smad3 and Smad7 in the pulmonary tissue in Qidan group 1 (X200) (immunohistochemical processed). A. A small number of positive expressions of brown TGF- β1 in Qidan group 1 (X200); B. A small number of positive expressions of brown Smad3 in Qidan group 1 (X200); C. A large number of positive expressions of brown Smad7 in Qidan group 1 (X200).
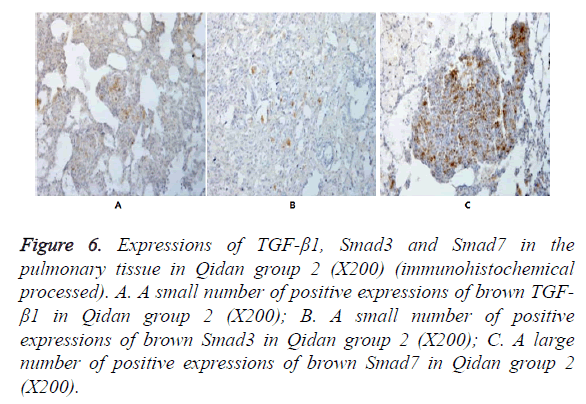
Figure 6. Expressions of TGF-β1, Smad3 and Smad7 in the pulmonary tissue in Qidan group 2 (X200) (immunohistochemical processed). A. A small number of positive expressions of brown TGF- β1 in Qidan group 2 (X200); B. A small number of positive expressions of brown Smad3 in Qidan group 2 (X200); C. A large number of positive expressions of brown Smad7 in Qidan group 2 (X200).
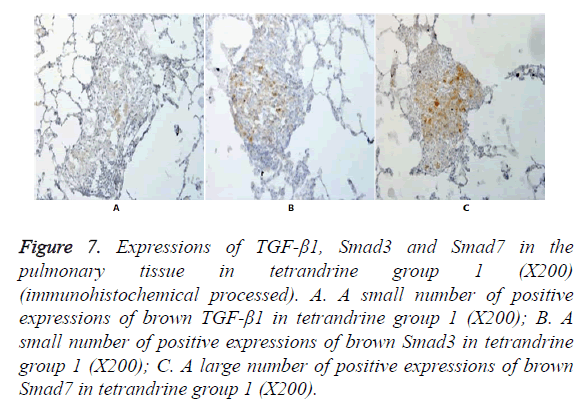
Figure 7. Expressions of TGF-β1, Smad3 and Smad7 in the pulmonary tissue in tetrandrine group 1 (X200) (immunohistochemical processed). A. A small number of positive expressions of brown TGF-β1 in tetrandrine group 1 (X200); B. A small number of positive expressions of brown Smad3 in tetrandrine group 1 (X200); C. A large number of positive expressions of brown Smad7 in tetrandrine group 1 (X200).
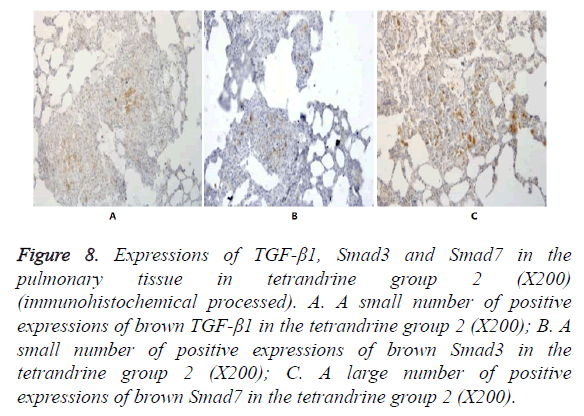
Figure 8. Expressions of TGF-β1, Smad3 and Smad7 in the pulmonary tissue in tetrandrine group 2 (X200) (immunohistochemical processed). A. A small number of positive expressions of brown TGF-β1 in the tetrandrine group 2 (X200); B. A small number of positive expressions of brown Smad3 in the tetrandrine group 2 (X200); C. A large number of positive expressions of brown Smad7 in the tetrandrine group 2 (X200).
In Table 5 expressions of TGF-β1 and Smad3 in the pulmonary tissue in the normal control group, both Qidan groups and both tetrandrine groups were lower than those in the model group, with significant differences (P<0.05). Expressions of TGF-β1 and Smad3 in the pulmonary tissue in the model group, Qidan group 1 and 2 and tetrandrine group 1 and 2 were higher than those in the normal control group, with significant differences (P<0.05).
| Group | Quantity of rats | TGF-β1 | P | Smad3 | P | Smad7 | P |
|---|---|---|---|---|---|---|---|
| Control group | 10 | 167.05 ± 3.23△ | 0.0001 | 176.63 ± 2.11△ | 0.0001 | 137.59 ± 1.76△ | 0.0001 |
| Model group | 10 | 150.13 ± 3.21* | 0.0001 | 156.25 ± 3.95* | 0.0001 | 196.79 ± 3.42* | 0.0001 |
| Qidan group 1 | 10 | 158.88 ± 3.97△* | 0.0001/0.0001 | 165.89 ± 3.07△* | 0.0001/0.0001 | 164.01 ± 4.55△* | 0.0001/0.0001 |
| Qidan group 2 | 10 | 156.28 ± 3.17 | 0.0001/0.0001 | 164.15 ± 2.96△* | 0.0001/0.0001 | 169.55 ± 4.73△* | 0.0001/0.0001 |
| Terandrine group 1 | 10 | 156.53 ± 3.84△* | 0.009/0.0001 | 165.35 ± 3.14△* | 0.0001/0.0001 | 167.49 ± 5.97△* | 0.0001/0.0001 |
| Terandrine group 2 | 10 | 155.99 ± 3.62△* | 0.016/0.0001 | 163.63 ± 3.34△* | 0.001/0.0001 | 169.59 ± 4.88△* | 0.0001/0.0001 |
| Note: Compared with the model group, ΔP<0.05; and compared with the normal control group, *P<0.05. | |||||||
Table 5. TGF-β1, Smad3 and Smad7 protein grey value in the pulmonary tissue of the rats in each group (x ± s).
Expressions of Smad7 in the pulmonary tissue in the normal control group, Qidan group 1 and 2 and tetrandrine group 1 and 2 were higher than those in the model group, with significant differences (P<0.05). Expressions of TGF-β1 in the pulmonary tissue in the model, Qidan and tetrandrine groups were lower than those in the normal control group, with significant differences (P<0.05). Expressions of TGF-β1, Smad3 and Smad7 in Qidan group 1 and 2 were not significantly different from those in both tetrandrine groups. Through the correlation analysis, it was shown that significant positive correlation existed between TGF-β1 and Smad3 (r=0.745, P<0.01), while significant negative correlation existed between TGF-β1 and Smad7 (r=-0.771).
Pathological examination of kidneys under optical microscope observation
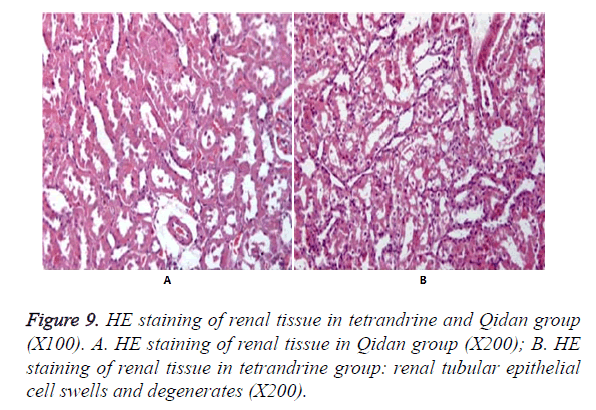
Figure 9 shows no obvious pathological changes in the normal control group, model group and Qidan groups. But swelling and degeneration were detected in renal tubular epithelial cells in tetrandrine groups. Granular degeneration appeared in some parts. And a small amount of vacuole degeneration existed. There was inflammatory infiltration in renal interstice.
Discussion
Silicosis is a respiratory disease caused by inhalation of silica dust, which leads to diffusion of pulmonary fibrosis [1,2]. It is the result of a series of complex cellular, inflammatory and immunological reactions to the silicon dioxide particles inhaled. The main pathological change is the pulmonary fibrosis caused by excessive collagen synthesis. No effective treatment has been developed by now. The Tetrandine (TET), also known as tetrandrine, is one of the bisbenzylisoquinoline alkaloid extracted from menispermaceae plant, Fourstamen stephania root. Scholars have confirmed that Tetrandrine can inhibit the release of TNF α [3,4] and prevent the synthesis of collagen, thus can be used to cure silicosis [5]. However, when intravenous injection of tetrandrine reaches the toxic dose it can cause irritation to some parts of the tissue and necrosis of liver, kidney and lymphatic tissue. The long-term oral usage may cause poison to liver, kidney and adrenal gland which will lead to the degeneration and necrosis of parenchymal cells, even the focal necrosis and secondary inflammatory reaction. Therefore, the clinical application of this medicine is limited. In this study, the tetrandrine group was taken as control group, and the results showed that tetrandrine could be effective to inhibit the development of silicosis fibrosis but was poisonous to the kidney.
From the perspective of traditional Chinese medicine, the pathogenesis of silicosis is taken as deficiency of vital energy (dyspnea, becoming serious after exercises or with sweat), deficiency of Yin (cough, less phlegm, dry pharynx and thirsty) and stagnation of phlegm (feeling stuffy, cannot breathe, chest pain, low fever, cyanosis, dark red tongue or with ecchymosis, thread and rapid pulse or rolling and rapid pulse, plosive and wheeze sound in the lung). Therefore, the therapy from traditional Chinese medical point of view is benefiting vital energy, activating blood circulation to dissipate blood stasis, clearing away the heat and reducing the phlegm.
Many researches on the pharmacology and components of certain Chinese medicine indicate that Salvia miltiorrhiza can reduce the production of oxygen radicals, protect the endothelial cells, improve micro-circulation and cure the metabolism obstacles caused by cell bleeding and hypoxia [6,7]. It can also inhibit the formation and reproduction of fibroblasts [8,9] and increase the immunity [10,11]. Radix Astragali can promote the non-specifically immune and cellular immunity [12], while has anti-bacterial, anti-fatigue and anti-aging effects. In the protection effects of ligustrazine against rats’ pulmonary fibrosis caused by bleomycin, Zhao et al. [13] revealed that the damaging mechanism of free radicals was of great importance in the formation of pulmonary fibrosis. The ligustrazine can not only directly remove the free radicals that have cytotoxicity, but also inhibit the fibroblast dividing and proliferating, thus can prevent tissue injury and pulmonary fibrosis. Qidan Granule is composed of crude extract of various Chinese herbals, such as root of red-rooted salvia, Radix Astragali, Rhizoma Chuanxiong, etc. Xin et al. [14] had studied the curative effects of Chinese medicine on pulmonary experimental fibrosis of rats. It was shown that the Qidan Granule can inhibit the expressions of TGF-β1 and TNF-α. It was satisfactory that the clinical curative effects of Qidan Granule on pulmonary fibrosis examined by Xin et al. [14,15]. Furthermore, Mossman and Churg [16] believed that pulmonary interstitial fibrosis was quite the same as silicosis, i.e., pulmonary interstitial fibrosis was the basic pathological change.
Fibrosis is a slowly dynamic process and a progressing pathological change, which involves a variety of factors, such as cells, cell factors, Extracellular Matrix (ECM), etc., and is a complex procedure of multi-interaction and multi-adjustment between sections. The accumulation of ECM is the basic pathology of silicosis. Many evidences show that among cell factors regulating ECM metabolism, TGF-β, which has been most studied, is related to ECM accumulation the most closely, and widely recognised as the curative target in tissue fibrosis treatment [17,18]. There are five kinds of TGF-β isomers, among which TGF-β1, TGF-β2 and TGF-β3 express in mammals. Their biological characteristics are basically the same, with a homology of 60%-80%. And TGF-β1 is the most important one. TGF-β1 is a cell factor that can lead to fibrosis, regulate and control the proliferation and differentiation of cells, and participate in the repair and fibrosis of tissue [19,20]. It is also a strong degradation inhibitor of extracellular matrix that can restrain the collagenase and matrix metalloproteinase from degrading the enzymes synthesis of extracelluar matrix to reduce the degradation of collagen. Meanwhile, it can promote the synthesis of TIMP-1 to produce a variety of extracelluar matrix by fibroblasts. This shows that TGF-β1 plays an important role in the regulation of cell proliferation and differentiation, the formation of extracelluar matrix, and the construction of tissue. Therefore, we choose TGF-β1 as the curative indicator of the drug treatment for silicosis. It is shown in this experiment that: pulmonary coefficient and the content of hydroxyproline in Qidan group 1 and 2, and tetrandrine group 1 and 2 are lower than those in the model group, with significant differences (P<0.05). Therefore, it is indicated that the collagen syntheses of pulmonary tissue in both Qidna groups and both tetrandrine groups are inhibited. Expressions of TGF-β1 in the pulmonary tissue and BALF are higher in the model group. But expressions of TGF-β1 in the pulmonary tissue and BALF in the treatment groups are lower than those in the model group. So it is further proved that TGF- β1 can promote the formation of fibrosis, and Qidan Granule and tetrandrine can delay the progress of silicosis development by inhibiting the expressions of TGF-β1. When the potential TGF-β 1 is activated and combined with type I and II receptors, it can start the extracelluar information transmission through transcription factors Smads. It is known that there are 10 species of Smad protein. Smad2 and Smad3 can make activation and phosphorylation of TGF-βRI. Smad6 and Smad7 can block the phosphorylation of Smad2 and Smad3 to inhibit the information transmission of TGF-β1. There are more and more evidences to confirm that Smad3 plays a more important role in the information transmission of TGF-β1 than Smad2.
Kobayashi et al. [21] used TGF-β1 to stimulate the pulmonary fibroblasts that were infected by Smad2-siRNA and Smad3- siRNA, and found that collagen synthesis of pulmonary fibroblasts cured by Smad3-siRNA, expressions of α-smooth muscle action (α-SMA) and the contraction of α-SMA did not differ from those in the control group. On the contrary, TGF-β1 can increase the collagen synthesis of pulmonary fibroblasts cured by Smad2-siRNA, expressions of α-SMA and contraction of α-SMA. Therefore, it is indicated that TGF-β1 transfers information through Smad3 to cause the activation and collagen synthesis of fibroblasts. Smad7 is of some value in the treatment for pulmonary fibrosis. It can prevent TGF-β1 from inducing the pulmonary fibroblasts to differentiate into myofibroblasts. So we choose Smad3 and Smad7 as observation indicators to make further study on the molecular mechanism of the inhibition of fibrosis by Qidan Granule and tetrandrine through TGF-β1. This study shows that, expressions of Smad3 in the model group are higher than those in the normal control group, Qidan 1, 2, and tetrandrine 1, 2, while expressions of Smad7 are lower than those in the normal control group, Qidan 1, 2, and tetrandrine 1, 2. Correlation analysis shows that significant positive correlation exists between TGF-β1 and Smad3 (r=0.745, P<0.01), while significant negative correlation exists between TGF-β1 and Smad7 (r=0.745, P<0.01). Therefore, Qidan Granule and tetrandrine can block the information transmission channel of TGF-β1 through the inhibition of Smad3 expressions and the promotion of Smad7 expressions to inhibit the formation of fibrosis and delay the progress of silicosis development.
Meanwhile, the results show that pulmonary coefficient, content of hydroxyproline and expressions of TGF-β1, Smad3 and Smad7 in Qidan group 1 and 2 do not differ significantly from those in tetrandrine groups. This may be related to the special pathogenesis of silicosis, which as respiratory disease caused by inhalation of dissociated silica dust, is different from other pulmonary interstitial fibrosis, and leads to diffusion of pulmonary fibrosis. It is the result of a series of complex cellular, inflammatory and immunological reactions when inhale silicon dioxide particles, which continuously stimulates the fibroblasts to accelerate the formation of collagen fiber. Qidan Granule and terandrine can inhibit the formation of fibrosis and delay the progress of silicosis development by blocking the information transmission channel of TGF-β1, even grade II-III silicon nodules have already formed. There is no significant difference compared with earlier medical treatments.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (No. 81473485).
References
- Jalloul AS, Banks DE. The health effects of silica exposure. In: Rom WN, ed. Environmental and occupational medicine. Philadelphia, PA: Lippincott Williams & Wilkins 2007; 4: 365-387.
- Greenberg MI, Waksman J, Curtis J. Silicosis: a review. Dis Mon 2007; 53: 394-416.
- Chen L, Chen L, Lv Y, Cui Z, Bei G, Qin G, Zhou J, Ge T. Tetrandrine ameliorates cognitive ampairment via inhibiting astrocyte-derived S100B activation in a rat model of chronic cerebral hypoperfusion. Neurol Res 2013; 35: 614-621.
- Lin TY, Tseng SH, Li SJ, Chen JC, Shieh JS, Chen Y. Tetrandrine increased the survival rate of mice with lipopolysaccharide-induced endotoxemia. J Trauma 2009; 66: 411-417.
- He Y, Liu B, Miao Q. Inhibition of mRNA expression of silicotic collagen gene by tetrandrine. Zhonghua Yu Fang Yi Xue Za Zhi 1995; 29: 18-20.
- Hung YC, Wang PW, Pan TL. Functional proteomics reveal the effect of Salvia miltiorrhiza aqueous extract against vascular atherosclerotic lesions. Biochim Biophys Acta 2010; 1804: 1310-1321.
- Han JY, Horie Y, Miura S, Akiba Y, Guo J, Li D, Fan JY, Liu YY, Hu BH, An LH, Chang X, Xu M, Guo DA, Sun K, Yang JY, Fang SP, Xian MJ, Kizaki M, Nagata H, Hibi T. Compound Danshen injection improves endotoxin-induced microcirculatory disturbance in rat mesentery. World J Gastroenterol 2007; 13: 3581-3591.
- He S, Yang Y, Liu X, Huang W, Zhang X, Yang S, Zhang X. Compound Astragalus and Salvia miltiorrhiza extract inhibits cell proliferation, invasion and collagen synthesis in keloid fibroblasts by mediating transforming growth factor-beta/Smad pathway. Br J Dermatol 2012; 166: 564-754.
- Zhang M, Cao SR, Zhang R, Jin JL, Zhu YF. The inhibitory effect of salvianolic acid B on TGF-beta1-induced proliferation and differentiation in lung fibroblasts. Exp Lung Res 2014; 40: 172-185.
- Liang XY, Li HN, Yang XY, Zhou WY, Niu JG, Chen BD. Effect of Danshen aqueous extract on serum hs-CRP, IL-8, IL-10, TNF-alpha levels, and IL-10 mRNA, TNF-alpha mRNA expression levels, cerebral TGF-beta1 positive expression level and its neuroprotective mechanisms in CIR rats. Mol Biol Rep 2013; 40: 3419-3427.
- Wong CK, Tse PS, Wong EL, Leung PC, Fung KP, Lam CW. Immunomodulatory effects of yun zhi and danshen capsules in health subjects--a randomized, double-blind, placebo-controlled, crossover study. Int Immunopharmacol 2004; 4: 201-211.
- Jian J, Wu Z. Influences of traditional Chinese medicine on non-specific immunity of Jian Carp (Cyprinus carpio var. Jian). Fish Shellfish Immunol 2004; 16: 185-191.
- Zhao J, Dai H, Zhang YJ. Research of ligustrazine protection over rats’ pulmonary interstitial fibrosis caused by bleomycin. Med J Chinese People's Liberation Army 2005; 30: 455.
- Xin HT, Song XJ, Xue SH, Mu XY, Wang J, Jin CJ, Hang C. The Infection of Qidan Granule to TGF-β and TGF-α expressions in rats’ pulmonary interstitial fibrosis. Journal of Capital Medical University 2004; 25: 49-52.
- Xin HT, Liu QH, Zhang X, Hang C, Jin CJ, Zhang JP. Clinic study of Qidan Granule therapy of pulmonary interstitial fibrosis. Shandong Medicine 2002; 42: 20-21.
- Mossman BT, Churg A. Mechanisms in pathogenesis of asbestosis and silicosis. Am J Respir Crit Care Med 1998; 157: 1666-1680.
- Coker PK, Laurent GJ, Shahzeidi S, Lympany PA, du Bois RM, Jeffery PK, Mc Anulty RJ. Transforming Growth Factors-β1, -β2, and -β3 stimulate fibroblast procollagen production in vitro but are differentially expressed during bleomycin-induced lung fibrosis. Am J Pathol 1997; 150: 981-991.
- Zhao Y, Shah DU. Expression of transforming growth factor- beta type I and type II receptors is altered in rat lungs undergoing bleomycin-induced pulmonary fibrosis. Exp Mol Pathol 2000; 69: 67-78.
- Fernandez IE, Eickelberg O. The impact of TGF-b on lung fibrosis. From targeting to biomarkers. Proc Am Thorac Soc 2012; 9: 111-116.
- Letterio JJ, Roberts AB. Regulation of immune responses by TGF-beta. Annu Rev Immunol 1998; 16: 137-161.
- Kobayashi T, Liu X, Wen FQ. Smad3 Mediates TGF-Beta1-Induced Collagen Gel Contraction by Human Pulmonary Fibroblasts. Biochem Biophys Res Commun 2006; 339: 290-295.