- Biomedical Research (2010) Volume 21, Issue 3
Effect of addition of intrathecal midazolam to 0.5 % Bupivacaine on duration of analgesia
Shirish Chavan1, Sachin Mumbare2, Manisha Mane1Department of Anaesthesia, NDMVPS Medical College, Nashik (MH), India
2Department of PSM, NDMVPS Medical College, Nashik (MH), India
- Corresponding Author:
- Shirish Chavan
Department of Anaesthesia
NDMVPS Medical College
Agra Road, Adgaon Nashik 422003, India
Accepted date: May 27 2010
Abstract
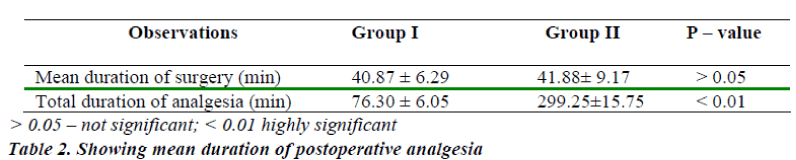
The Objectives were to compare the mean period of analgesia for intrathecal Midazolam 0.5 ml plus bupivacaine and bupivacaine alone and to monitor the side effects of intrathecal Midazolam (0.5 ml) plus bupivacaine and bupivacaine alone. A comparative study was carried out to evaluate the analgesic efficacy and side effects of intrathecal Midazolam along with 0.5% Bupivacaine in 80 ASA I and II patients, aged 16 – 60 years, undergoing surgeries below umbilicus, elective as well as emergency. They were randomly divided into two groups of 40 each (n=40). Group I (control): received Bupivacaine 0.5% heavy 3 ml + NS 0.5 ml and Group II (study): received Bupivacaine 0.5% heavy 3 ml + Midazolam 0.5 ml. All patients were haemodynamically stable and there were no serious side effects in any of the patients in both the groups. The mean duration of sensory analgesia in group I and II was 76.30 ± 6.05 minutes and 299.25 ± 15.75 minutes respectively. The difference in the mean duration of sensory analgesia in both the groups was statistically significant. (P < 0.01)
Keywords
Midazolam, bupivacaine, intrathecal, analgesia
Introduction
One of the primary aims of anaesthesia is to alleviate the patient’s pain and agony, there by permitting the performance of surgical procedures without any discomfort. Relief of postoperative pain has gained real importance in recent years considering the central, peripheral and immunological stress response to tissue injury. Any expertise acquired in this field should be extended into the postoperative period, which is the period of severe, intolerable pain requiring attention. So there is need of extended analgesia without any side effects to achieve this goal.
Intrathecal 0.5 % bupivacaine is routinely used for neuraxial blockade. Many authors have suggested the addition of 0.5 ml Midazolam to bupivacaine to extend the period of analgesia [1,2,3]. Many of these studies were based on the animal models. So this study was carried out to study the effect of addition of 0.5 ml intrathecal Midazolam to bupivacaine in humans with the objectives to compare the mean period of analgesia for intrathecal Midazolam 0.5 ml plus bupivacaine and bupivacaine alone and to compare the side effects of intrathecal Midazolam (0.5 ml) plus bupivacaine and bupivacaine alone.
Material and Methods
Type of study : Randomised Control Trial.
Study Setting: NDMVPS Medical College and Hospital, Nashik.
Study Period: January 2008 to December 2008.
Inclusion Criteria
1) ASA grade I to II, posted for operations below umbilicus, elective as well as emergency.
2) Age group – 16 to 60 years.
3) Sex: Male or Female.
4) Patients who were ready to be included in the study, and who are ready to sign written consent.
Exclusion Criteria:
1) ASA grade III, IV, V.
2) Age below 16 or above 60 years.
3) Patient who were not ready to be included.
Protocol was sent to local Ethical committee and approval was obtained. Each patient evaluated pre-anaesthetically and detail history about previous illness and drug treat ment was elicited. Thorough physical examination was carried out. Routine investigations were done. If patients fulfill inclusion criteria, they were explained about procedure and written consent was obtained from the patients. Patients were randomly divided into group I (control) and II (study).
Sequential design was used for the study. All the patients were subjected to spinal analgesia with all aseptic precautions with a 23 or 24 gauge lumbar puncture needle in L3 – L4 interspace. After obtaining free and clear flow of cerebrospinal fluid, intrathecal administration of drugs was done as follows:
Group I (control): received Bupivacaine 0.5% heavy 3 ml + NS 0.5 ml
Group II (study): received Bupivacaine 0.5% heavy 3 ml + Midazolam 0.5 ml. (2.5 mg – Preservative free)
Times of onset of analgesia, upper level of sensory analgesia, time for complete motor block, duration of motor block and total duration of sensory block were noted. Any side effects were also noted.
Intraoperatively, all the patients received adequate intravenous fluids and blood loss was replaced as and when needed. PR, BP, RR were monitored every 5 minutely till the patients were shifted from operating table. Patients were watched for nausea, vomiting, itching, dryness of mouth, sweating intraoperatively.
After completion of surgery, patient was shifted to the recovery room. A person who was unknown to either of the groups observed patients, till the effect of spinal analgesia wore out. All the relevant information was recorded on a pretested, predefined, semiopen proforma. Any analgesic or sedative were withheld in the postoperative period, unless the patient complained of pain (Grade II). PR, BP and RR were recorded every one hourly till 6 hours and every 4 hourly till 24 hours. Side effects like nausea, vomiting, itching, degree of sedation and respiratory depression were noted in the postoperative period.
Evaluation of pain and pain relief was done according to McGill pain questionnaire (0 – no pain to 5 – excruciating pain). When patients complained of discomforting pain (McGill grade II) parenteral analgesic was prescribed and the total number of doses in the 24 hour period were noted.
Statistical analysis of the data was done using computers. Statistical tests such as chi-square, Z test were used wherever applicable. P<0.05 was considered to be significant.
Observations
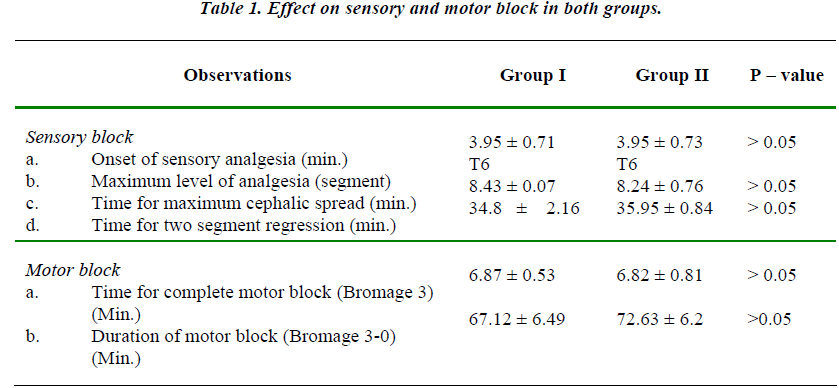
The two groups were comparable with regards to mean age, height and weight of the patients. The maximum upper level of sensory block attained was up to T6 level in both the groups and mean value of maximum upper level was comparable in both the groups. (Table 1).
There was no statistically significant difference in the time required for onset of adequate analgesia i.e. time taken to attain T10 level in the two groups.
There was no statistically significant difference in the time required for onset of complete motor block and also in the total duration of the motor block. (P > 0.05)
The mean duration of sensory analgesia in group I was 76.30 ± 6.05 min. The mean duration of sensory analgesia in group II was 299.25 ± 15.75 min. The difference in the mean duration of sensory analgesia in both the groups was statistically significant. (P < 0.01) (Table-2)
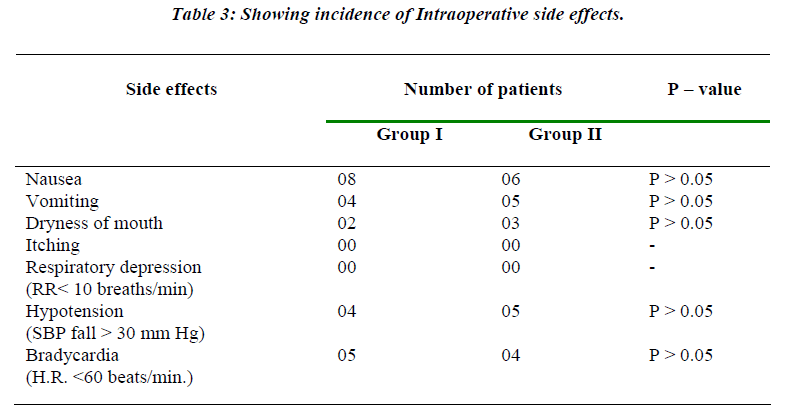
Table 3 shows the incidence of various side effects in both the groups. There was no statistical difference in the incidence of side effects in these groups.
This study has shown that the mean duration of analgesia is extended if midazolam is added to the bupivacaine, without increasing the side effects.
Discussion
In 1987, Goodchild and Serrao reported that benzodiazepines might have analgesic effects at spinal cord level in animals [1]. Analgesic efficacy of intrathecal Midazolam in humans has been demonstrated recently. The δ selective opioid antagonist Naltrindole suppresses antinociceptive effect of intrathecal Midazolam, suggesting that intrathecal Midazolam is involved in release of an endogenous opioid acting at spinal δ receptors.
Demonstration of benzodiazepine stereospecific binding sites in the spinal cord and brain, it seems likely that there exists a neurotransmission system involving benzodiazepine like peptides interacting with receptors in spinal cord [4,5]. This evidence points to possible spinal mechanism, which may be site of action of Midazolam in producing analgesia and interruption of somatosympathetic reflexes [7].
The present study shows that addition of 0.5 ml midazolam to 3 ml of 0.5% bupivacaine does not alter the onset of adequate analgesia and maximum upper level of sensory block, time to reach maximum level of block (dermatome) and duration of motor block.
Many authors have studied the analgesic effects of midazolam plus Bupivacaine. M.H.Kim and Y.M.Lee observed that there was statistically significant difference in the analgesic effect of midazolam plus Bupivacaine as compared with Bupivacaine alone in haemorrhoidectomy patients. [8]
Similar findings were also reported earlier [9.10]. Intrathecal midazolam produces post operative analgesia without prolonging motor block. [6,11] Whereas Tucker A. P. et al reported that the use of midazolam is as safe as bupivacaine [12]
So this study has shown that addition of 0.5 ml midazolam (2.5 mg, preservative free) to bupivacaine significantly increases the period of analgesia without increase in the side effects.
References
- Goodchild CS, Serrao JM. Intrathecal midazolam in the rat: evidence for spinally mediated analgesia. Br J Anaesth 1987; 59: 1563-1570.
- Rigoli M.; Epidural analgesia with benzodiazepines Pharmacological basis of Anaesthesiology, Clinical Pharmacology of new analgesics and anaesthetics, Edition, Tiengo M. and Cowins M.J; New York, Raven press. 69-76.
- Whitman JG, Niv D, Loh L, and Jack RD: Depression of nociceptive reflexes by intratechal benzodiazepines in dog. Lancet. 1982; 2: 1465.
- Mohler H; okada T; Benzodiazepine receptors: demonstration in the central nervous system, Science, 1977; 198: 849-851;
- Schoch P, Richards JG, Haring P, Takacs B, Stahli C, Staehelin T, et al. Co-localization of GABA receptors and benzodiazepine receptors in the brain shown by monoclonal antibodies. Nature 1985; 314: 168- 171.
- Valentine JM, Lyons G, Bellamy C. The effect of intrathecal midazolam on postoperative pain. Eur J Anaesthesiol 1996; 13: 589-593.
- Niv D; Whitwam J.G; Loh L. Depression of nociceptive sympathetic reflexes by intrathecal administration of Midazolam. British Journal of An- aesthesia, 1983; 53: 542-547.
- Kim MH, Lee YM. Intrathecal midazolam increases the analgesic effects of spinal blockade with bupivacaine in patients undergoing haemorrhoidectomy. British Journal of Anaesthesia, 2001; 86: 177-179.
- Prakash S, Gupta A et al. The effect of intrathecal midazolam 2.5 mg with hyperbaric Bupivacaine on postoperative pain relief in patients undergoing orthopaediac surgery. Internet Journal of Anaesthesiology. 2007; 14 2:
- Agrawal N et al. Effect of intrathecal midazolam Bupivacaine combination on postoperative analgesia, Indian Journal of Anaesth 2005; 49 (1): 37-39.
- Batra YK, Jain K, Chari P et al. Addition of intrathecal midazolam to bupivacaine produces better postoperative analgesia without prolonging recovery. Int J Clin Pharmacol Ther 1999; 37 (10): 519-527.
- Tucker AP, Lai C, Nadeson R, Goodchild CS. Intrathecal midazolam I: a cohort study investigating safety. Anesth Analg. 2004; 98: 1512-1520.