Research Article - Journal of Parasitic Diseases: Diagnosis and Therapy (2016) Volume 1, Issue 1
Dot-Enzyme Linked Immunosorbent Assay for Diagnosis of Ovine Oesophagostomosis
Rajeshwari Gaddam*, Murthy GSS and Sudhakar Kommu
Rajeshwari Gaddam, Department of Veterinary Parasitology, College of Veterinary Science,Rajendranagar, Hyderabad-30, India
Murthy GSS, Department of Veterinary Parasitology, College of Veterinary Science, Rajendranagar,Hyderabad-30, India
Sudhakar Kommu, Department of Veterinary Parasitology, College of Veterinary Science,Rajendranagar, Hyderabad-30, India
- *Corresponding Author:
- Rajeshwari Gaddam
Department of Veterinary Parasitology, College of Veterinary Science, Rajendranagar, Hyderabad-30, India
Tel: +919441239946
E-mail: rajeshwari.g9946@gmail.com
Received Date: October 14, 2016; Accepted Date: October 18, 2016; Published Date: October 27, 2016
Abstract
Dot Enzyme Linked Immunosorbent Assay (Dot-ELISA) was standardized and evaluated to detect anti-Oesophagostomum antibodies in sera of sheep by using three different antigenic components obtained from the parasite Oesophagostomum i.e., Crude Somatic Antigen (CSA), Excretory Secretory Antigen (ESA) and Larval Antigen (LA). Dot ELISA was standardized at an antigenic concentrations of 0.06 μg CSA, 0.3 μg of ESA and 0.1 μg of LA with sera at 1:100 dilution with Phosphate buffered saline with Tween-20 (0.1% PBST), using sheep anti-rabbit antibodies with horse radish peroxidase enzyme as conjugate at 1:5000 concentration and di-amino benzedine as substrate. The sensitivity, specificity of test were 73.33%, 66.67% by using CSA; 80%, 72.22% by using ESA and as 40%, 66.67% by using LA with statistically significant p value (p<0.05). By using this test 22.01, 10.02, 5.5 were percentage positives detected respective to their antigens, crude somatic antigen, excretory secretary antigen and larval antigen.
Keywords
Dot-ELISA; ovine oesophagostomosis; excretory secretary antigens
Introduction
Oesophagostomosis is one of the prevalent and pathogenic gastrointestinal nematode infections of livestock in India (Gupta et al., 1987., Singh et al., 1997), affecting their large intestines causing nodular worm diseases and often resulting in vast economic losses due to condemnation of intestines and also resulting in poor growth rate however prepatent stages of Oesophagostomum species are primarily involved in the pathogenic effects which can’t be diagnosed by conventional methods. Microscopic examination of eggs in the faeces is not reliable as the morphology of Oesophagostomum egg is similar to all stroyngle eggs, although L3 identification obtained through copra culture of eggs is reliable but is a difficult and laborious task. Also the egg stage is detected only after patency of approximately 41-45 days, till then the prepatent stages of parasite cause major damage to the host. Therefore it is essential to detect infection at early stages; therefore there occurs a need to develop immunodiagnostic tests which are more reliable and precise in detecting the infection rather than identification of egg. Although molecular diagnosis as PCR is confirmatory with high sensitivity and specificity but it is costly. Therefore Dot-ELISA can be used as rapid, reliable and low cost immunediagnostic tool for the diagnosis of the pathogenic stages of Oesophagostomosis under field conditions.
Materials and Methods
A. Collection of Adult Worms
Intestines with knotty gut lesions or with cheesy nodular lesions along with faeces were collected from sheep (Ovis aries) slaughtered at Chengicherla slaughter house, Hyderabad, transferred to the laboratory and processed by cutting longitudinally and the worms are collected using a tissue forceps from caecum and colon and placed in beaker containing PBS (pH 7.2). The worms were washed several times to remove host material in PBS (pH 7.2) and Oesophagostomum columbianum (as per keys provided by Soulsby, 1982., Singh, 2003) worms were collected.
B. Preparation of Crude Somatic Antigen (CSA)
Three hundred worms of O. columbianum were collected and washed five times in the Phosphate Buffer Saline (PBS) and were finally homogenized in 10 mL of cool 0.15 M Phosphate Buffer Saline (pH-7.2) containing two to three drops of 25 mM Phenyl Methyl Sulfonyl Fluoride (PMSF) stored at -20°C and few drops of 24 mM Ethylene-Diamino Tetra Acetic Acid (EDTA) maintained at 4°C in a glass tissue homogenizer (Jas et al., 2010), disintegrated by sonication (Soniprep-150) at 20 kHz, 1 mA of 5 cycles each of 90 sec duration with four intervals of two seconds each on ice bath. This extract was centrifuged at 4°C at 10000 g for 30 min (slightly modified) and the supernatant was collected as the CSA. Protein concentration of CSA was found to be 3.88 mg/mL according to (Lowry et al., 1951). This crude somatic antigen was aliquoted and stored at -20°C for further use in the assay.
C. Excretory Secretory Antigen (ESA) of Adult Oesophagostomum columbianum
Adult live O. columbianum worms were collected and washed five time in Phosphate buffer saline (pH 7.2), containing antibiotics penicillin (500 IU/mL) and streptomycin (5 mg/mL). Motile worms were transferred to RPMI 1640 medium glutamine amino acid rich containing penicillin (1000 IU/100 mL) and streptomycin (1 mg/100 mL) and cultured at a concentration of approximately fifty worms per twenty mL in a culture flask at 5% carbon dioxide atmosphere at 37°C for 24 hrs. The medium was changed every 6 hrs (fresh medium was added with 2% glucose) when the color of media was turning to yellow (pH of the media shifting to acidic). Worm viability was monitored throughout this period on the basis of motility, integrity of the worms. The culture media was collected by decantation and centrifuged at 4,000 rpm for 15 min to remove gross particles to prevent clogging of filter pores and then the supernatant was filtered through a syringe filter (0.22 μm pore size) from Millipore. Then the culture medium was centrifuged at 10,000 rpm for 30 min at 4°C and the supernatant was labeled as excretory secretory antigen. Finally, the antigen obtained was concentrated by dialysis (membrane cut off, 10 kDa) against polyethylene glycol (PEG 20000 SRL, India) in 8% solution over a period of 6 hrs at 4°C. The concentrated material was added with 1 mM Phenyl Methyl Sulfonyl Fluoride (PMSF) and 1 mM Ethylene diamine tetra acetic acid (EDTA) and stored at -20°C. The protein concentration of the ESA was determined using Lowry protein estimation kit (Genei, Bangalore) was found to be 4 mg/mL. Excretory and Secretary antigen was aliquoted and stored at -20°C for further use in the assay.
D. Preparation of Larval Antigen (LA)
About 10,000 L3 larvae were collected into 1 mL (100 μL contains 1000 larvae) of cool PBS (pH 7.2), added with 5 mM PMSF, 5 mM EDTA are homogenized in a glass tissue homogenizer followed by sonication at 20 KHz, 1 mA of 5 cycles each for 90 sec duration with four intervals of 2 sec each on ice and repeated after a gap of 15 min. The disintegrated larval contents were extracted at 4°C overnight and centrifuged at 10,000 g for 30 min and the resultant supernatant was collected as the larval antigen and stored at -20°C by adding 5 mM PMSF. The protein concentration of the Larval antigen was determined using Lowry protein estimation kit (Genei, Bangalore) as 0.2 mg/mL and was aliquoted and stored at -20°C for further use in the assay.
E. Lab Animals and Raising of Hyper Immune Serum (HIS)
Three New Zealand white rabbits (Oryctolagus cuniculus) of 2 kg and of nearly one year age, screened free of gastrointestinal parasites were selected for raising hyper immune serumagainst adult worm crude somatic antigen (CSA), excretory secretory antigens (ESA) and larval antigen (LA) of O. columbianum. These were maintained in the rabbit house College of Veterinary Science, Rajendranagar, dewormed, fed ad-libitum and given adequate clean drinking water. For raising HIS (Permission was accorded by Ethical committee bearing Ref: 698/CPCSEA dated 01/10/2002 F. No. 25/60/2010-AWD/Veterinary college/ Hyderabad). 500 μg of crude somatic antigen mixed along with equal volume of Freund’s Complete Adjuvant was subcutaneously injected in to rabbit one. Similarly 500 μg of ESA mixed along with equal volume of Freund’s Complete Adjuvant subcutaneously injected in to second rabbit and 500 μg of Larval antigen mixed along with equal volume of Freund’s Complete Adjuvant subcutaneously injected in to third rabbit. Four booster doses each containing 500 μg of crude somatic antigen, excretory secretory antigen and larval antigen mixed along with equal volume of Freund’s Incomplete Adjuvant were administered subcutaneously to the respective rabbits at weekly intervals. The animals were tested by the puncture of marginal ear vein on 27th day of 1st injection and checked for antibody response by agar gel precipitation test (DID-Double Immuno Diffusion). Blood was collected by cardiac puncture on the sixth day of last booster injection. Serum was separated under sterile conditions and complement inactivation was done in water bath at 56ºC for 30 min. Sera were tested for the presence of antibodies using corresponding antigen with DID. The sera containing antibodies for respective antigens was made into aliquots and stored at -20°C for further usage in the assay. The immune precipitin reactions between antigens with respective hyper immune sera developed in rabbits were detected by Double Imuno Diffusion Test (Figures 1-3).
F. Standardization of Dot-ELISA
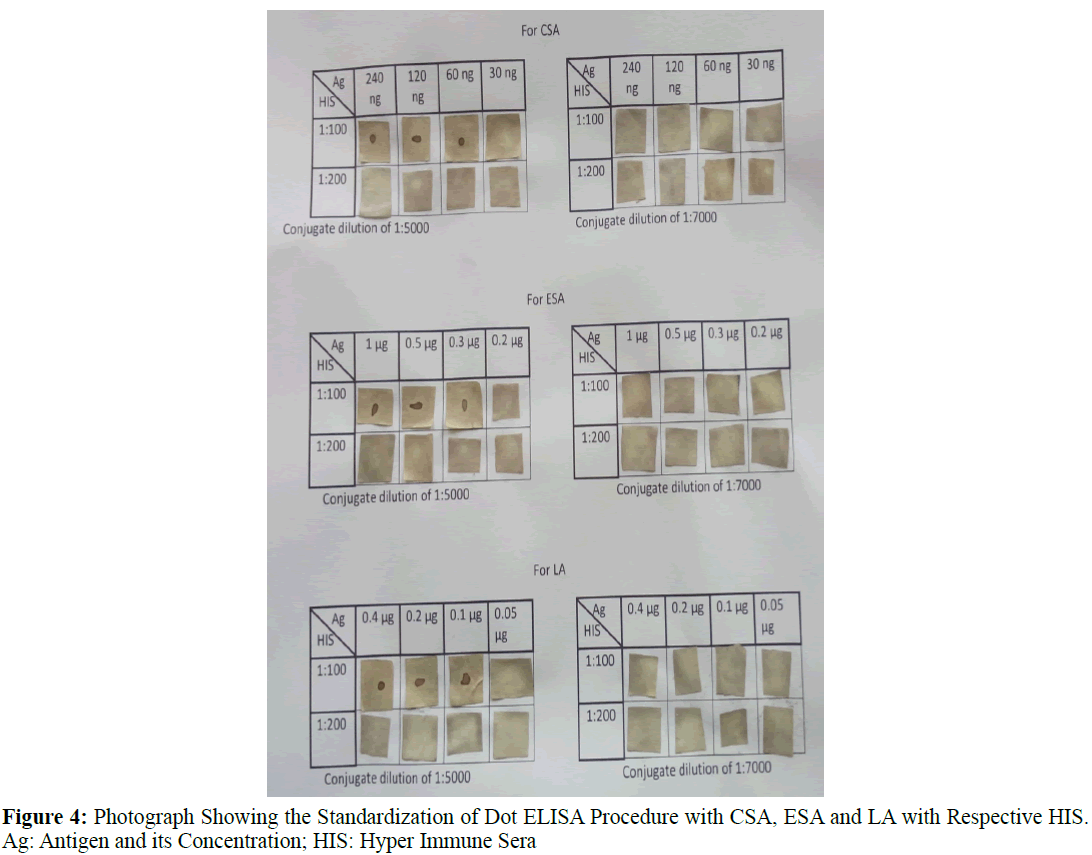
Dot-ELISA was standardized by charging 2 μL of different dilutions of the antigens (3.85 mg/mL, 1.925 mg/mL, 0.9625 mg/mL, 0.481 mg/mL, 0.240 mg/mL, 0.120 mg/mL, 0.06 mg/mL, 0.03 mg/mL for CSA and 4 mg/mL, 2 mg/mL, 1 mg/mL, 0.5 mg/ mL, 0.3 mg/mL, 0.25 mg/mL for Excretory secretary antigens, 0.200 mg/mL, 0.1 mg/mL, 0.05 mg/mL, 0.025 mg/mL of LA was coated on to nitrocellulose (NC) membrane of 1 cm2 which was previously washed in autoclaved double distilled water and dried at room temperature. The strips were kept in the incubator at 37°C for 1 hr. NCM was blocked with 2% gelatin. The serum samples (HIS and negative controls) at different dilutions (1:50, 1:100, 1:200 and 1:400) were allowed to react with the antigen loaded 100 μL/strip for 1 hr at 37°C followed by washing the NC strips for 6 times in 0.1% PBST (pH 7.2). The NC membranes were treated with goat anti rabbit IgG HRP conjugate at different dilutions (1:500, 1:1000, 1:2000, 1:3000 and 1:5000) followed by washing 4 times each for 10 min. Subsequently, the Ab and conjugate treated antigen coated Nitrocellulose membrane strips were finally treated with di-amino benzidine in the dark for 2-3 min. The dark brown spot appeared on the NC strip indicated as positive reaction. The reaction was stopped with double distilled water. Irrespective of the intensity of the color the reaction was considered as positive (Figure 4).
Results
A. Dot-ELISA for Detection of Anti Oesophagostomum Antibodies in Affected Sheep
The Dot-ELISA was optimized to estimate the concentration of antigen and serum by checkerboard titration. All tests were performed two times to eliminate variations in results if any. Gelatin was used as blocking agent rather than BSA as it was mentioned that blocking with BSA inhibits the action of rabbit anti sheep HRPO conjugate by the manufacture.
A nitrocellulose membrane (BIO-RAD, Germany) of size 1 cm2 was cut, washed in double distilled water, dried and put into a plastic tissue culture plates. Antigen (2 μL) was dotted on to individual strip and air dried for 30 min. The unadsorbed antigen was removed by washing the strips with PBST (sterile PBS (pH 7.2) containing 0.1% Tween-20). The uncoated inactive sites on the strips were blocked with PBST containing 2% gelatin by incubating for 1 hr at 37°C with constant shaking followed by washing the strips for three times in PBST. The strips were incubated in 1:100 dilutions of sera in PBST for 1 hr at room temperature with constant shaking followed by washing for three times in PBST as described earlier to remove unbound antibodies in the sample serum. Rabbit anti-sheep- IgG-HRPO (Jackson immune research, USA) was used as secondary antibody at a dilution of 1:5,000 in PBS (pH 7.2) containing Tween-20 (0.1%) as per the manufactures instructions followed by incubation at room temperature for 1 hr in the dark with constant shaking and subsequent washing as before to remove unbound conjugate. Freshly prepared substrate (3 mg of 3.3' di-amino benzidine (DAB) (SRL, India) in 5 mL of 50 mM tris buffer (pH 7.4), 0.001 g of cobalt chloride and 30 μL of H2O2) solution was added and the strips were dispensed and incubated for 15-20 min at 37°C in dark under constant shaking. The reaction was stopped by washing the strips in double distilled water. The positive reaction was indicated by the development of brown colored spot. When the reaction was complete, the strips were washed in distilled water and allowed to dry. Once dried, the strips were stored in the dark.
B. Performance of Assay
Sensitivity and Specificity of Dot-ELISA
The sensitivity and specificity of Dot-ELISA was measured by standardized Dot-ELISA procedure described as earlier. The sensitivity of Dot-ELISA is measured by screening 15 sera samples collected from sheep which were later found positive for Oesophagostomosis with their nodules present on intestine on post slaughter examination. The sensitivity was measured by using the formula:
Sensitivity=True positive/True Positive+False Negative × 100
The specificity of laboratory Dot-ELISA was measured by screening 18 sera samples of newly born lambs reared intensively in confined area away from their dams and were found negative for the clinical symptoms of Oesophagostomosis or any other helminthic ova in their feaces. The specificity thus arrived was measured by the formula:
Specificity=True negatives/True negatives+False Positives × 100
The overall sensitivity of Dot-ELISA was determined as 73.33% (11 out of 15 true positives), 80% (12 out of 15) and 40% (6 out of 15) with Crude somatic antigen, Excretory and secretary antigen and Larval antigen respectively (Table 1) and specificity was 66.67% (12 out of 18 negative sera),72.22% (13 out of 18 negative sera) and 66.67% (12 out of 18 negative sera) in Crude somatic antigen, Excretory and secretary antigen and Larval antigen respectively (Table 2).
| Sl.no | Antigen | No. of known positive sera samples | No. of positives detected by Dot-ELISA |
Dot-ELISA negative (False negative) | Sensitivity Percentage |
|---|---|---|---|---|---|
| 1 | CSA | 15 | 11a | 4 | 73.33 |
| 2 | ESA | 15 | 12a | 3 | 80 |
| 3 | LA | 15 | 6a | 9 | 40 |
Sensitivity: True positive/True Positive+False Negative ×100
The values superscripted above with similar alphabets are not significantly different (P ≤ 0.05)
Table 1: Sensitivity of Dot-ELISA in the Detection of Anti Oesophagostomum columbianum Antibodies by Using CSA, LA & ESA
| Sl.no | Antigen | No. of known negative sera samples | No. of negatives detected by Dot-ELISA |
Dot-ELISA positive (False positive) | Specificity percentage |
|---|---|---|---|---|---|
| 1 | CSA | 18 | 12a | 6 | 66.67 |
| 2 | ESA | 18 | 13a | 5 | 72.22 |
| 3 | LA | 18 | 12a | 6 | 66.67 |
Specificity: True negatives/True negatives+False positives × 100
The values superscripted above with similar alphabets are not significantly different (P ≤ 0.05)
Table 2: Specificity Studies of Dot-ELISA in the Detection of Anti Oesophagostomum columbianum Antibodies by Using CSA, LA & ESA
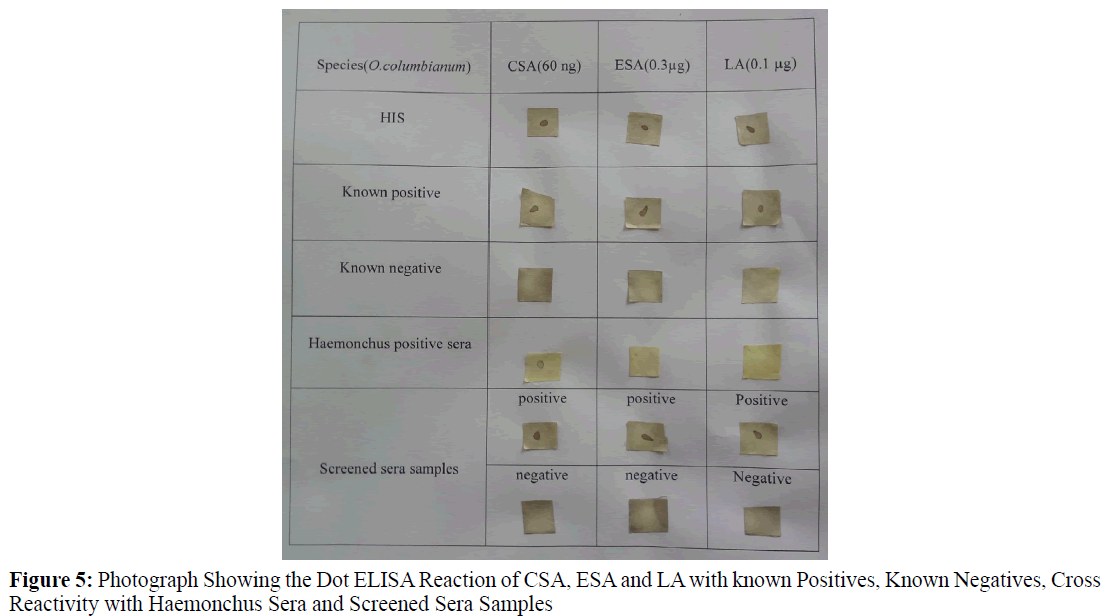
Three sera samples collected from animals positive for Haemonchus contortus (confirmed during post mortem) were used to check the cross reactivity, out of which 2 sera cross reacted to Crude somatic antigen of O. columbianum but not to Excretory and secretary antigen, Larval antigen (Figure 5).
By using Dot-ELISA to detect specific anti Oesophagostomum antibodies, out of 218 sera samples screened 48 were positive indicating 22.01% infection with Crude somatic antigen, 22 were positive indicating 10.92% infection with Excretory and secretary antigen and 12 were positive indicating 5.5% of infection with Larval antigen (Table 3).
| S. No. | Antigen | No. sera screened |
Positives sera detected | Negative sera detected | Per cent positive infection detected | Per cent negative infection |
|---|---|---|---|---|---|---|
| 1. | CSA | 218 | 48a | 170 | 22.01 | 77.98 |
| 2. | ESA | 218 | 22b | 196 | 10.02 | 89.908 |
| 3. | LA | 218 | 12c | 206 | 5.5 | 94.5 |
The values superscripted are significantly different a>b>c (P ≤ 0.05). CSA: Crude Somatic Antigen; ESA: Excretory Secretory Antigen; LA: Larval Antigen
Table 3: Detection of Anti Oesophagostomum columbianum Antibodies in Sheep Sera Samples by Dot-ELISA.
Discussion
Indirect ELISA tests for the serodiagnosis of human oesophagostomosis and caprine oesophagostomosis which required 5 μg of antigen per well for diagnosis as per (Bashir et al., 2011) but Dot- ELISA needed very low concentration of antigens (less than 5 μg of antigen per strip) i.e., 60 ng of Crude somatic antigen, 0.3 μg of Excretory and secretary antigen and 0.1 μg of Larval antigen was sufficient to diagnose.
The Dot-ELISA using Crude somatic antigen detected 11 positive cases out of 15 known positive sheep sera indicating an overall sensitivity of 76.74%, Excretory and secretary antigen detected 12 positive cases among 15 known positive sera showing an overall sensitivity of 80.48 per cent and with Larval antigen 6 positive sera were detected as positive of known 15 positive sera indicating sensitivity of 40 per cent Sensitivity of other serological test as indirect ELISA using Crude somatic antigen was 80.2% as recorded by (Bashir et al., 2011), 96% as recorded by (Jas et al., 2010). The overall sensitivity of Dot-ELISA was lower than indirect ELISA which might be due to difference in detection methods of using optical density helping in quantitative evaluation of indirect-ELISA but visibility of brown spot is positive disregarding the intensity of colour developed in Dot-ELISA as a qualitative test.
Dot-ELISA using Crude somatic antigen gave 6 positive reactions with 18 known negative sera for oesophagostomosis indicating 66.67% specificity, using excretory and secretary antigen gave 5 positive results out of 18 known ovine negative sera indicating 72.22% of specificity, whereas Larval antigen reacted positively with 6 out of 18 known oesophagostomosis negative sera indicating 66.67% B specificity. Specificity of other serological test as indirect ELISA using crude somatic antigen is 21.42% as recorded by (Bashir et al., 2011), 73.9% as recorded by (Jas et al., 2010). The overall specificity of Dot-ELISA was lower than that of indirect ELISA given by Jas which might be due to difference in detection methods of using optical density helping in quantitative evaluation of indirect-ELISA but Dot–ELISA is a qualitative test.
In the present study, per cent positives of sheep sera detected were 22.01 (48 out of 218), 10.02 (22 out of 218) per cent and 5% (12 out of 218) using crude somatic antigen, Excretory and secretary antigen and Larval antigen in Dot-ELISA. The per cent of sheep sera detected positive by using crude somatic antigen is more than the per cent of sheep sera detected positive by using Excretory and secretary antigen which may be due to cross reactivity of crude somatic antigen with Haemonchus positive sera as reported by (Arunkumar and Sangaran, 2009) due to sharing of some common antigenic determinants between Oesophagostomum and Haemonchus. Larval antigen is least effective in diagnosis which might be due to presence of least antigenic determinants responsible for immunoglobulin development.
Conclusion
Therefore, it is concluded that, O. columbianum antigens were useful for the serodiagnosis of Ovine Oesophagostomosis by Dot-ELISA. Excretory and secretary antigen was most effective in diagnosis rather than Larvaland Crude somatic antigen. This test helps in mass screening and a huge number of samples can be screened in short duration. Further characterization and purification of these antigens might improve the sensitivity and specificity of the assay. Very low antigen concentration is enough to diagnose Oesophagostomosis by Dot- ELISA (i.e., less than 5 μg of antigen per strip) rather than indirect ELISA.
References
- Arunkumar, S., Sangaran, A. (2009). An immunological analysis on sharing of antigens amongst gastro-intestinal nematodes using inhibition ELISA. Indian J SciTechnol,2(12).
- Arunkumar, S. (2012). Antigenic characterization of excretory/secretory antigen of Oesophagostomum columbianum using western blotting. J Biotech, 1, 28-31.
- Bashir, A.L., Chisti, M.Z., Ahmad, F., Hidayatullah, T. (2011). Serodiagnosis of Oesophagostomum columbianum infection in Goats using indirect ELISA. Vet World,4(11): 503-506.
- Boctor, F.N., Steck, M.J., Peter, J.B., Kamal, R. (1987).Simplification and standardization of Dot-ELISA for human schistosomiasis. JParasitol,73(3), 589-592.
- Gupta, R.P., Yadav, C.L., Choudhri, S.S. (1987). Epidemiology of gastrointestinal nematodes of sheep and goat in Haryana. India VetParasitol,24, 117-127.
- Jas, R., Ghosh, J.D., Das, K. (2010). Diagnosis of Oesophagostomum columbianum infection in goat by indirect enzyme linked immunosorbent assay. Helminthologia,47(2), 83-87.
- Lowry, O.H., Rosebrough, N.J., Farr, A.B., Randall, R.J.(1951). Protein measurement with the Folin-phenol reagent. J BiolChem,193, 265.
- Roldan, W., Cornejo, W., Espinjo, Y. (2006). Evaluation of the dot enzyme-linked immunosorbent assay in comparision with standard ELISA for the immunodiagnosis of human toxocariasis. Mem InstOswaldo Cruz,101(1), 71-74.
- Singh, D., Swarnakar, C.P., Khan, F.A., Srivatsava, C.P., Bhagwan, P.S.K.(1997). Epidemiology of ovine gastrointestinal nematodes at an organized farm in Rajasthan, India. Small Ruminant Res,26: 31-37
- Singh, K.S.(2003). Veterinary Helminthology. ICAR Publishedpp: 354-366.
- Snedecor, G.W.,Cochrran, W.G.(1989).“Statistical methods” (8thedn.)Lowa State University Press, Ames, IA.
- Soulsby, E.J.L. (1982). Helminths, arthropods and protozoa of domesticated animals (7thedn.) BailliereTindall, London.pp:186-191.