Research Article - International Journal of Pure and Applied Zoology (2017) Volume 5, Issue 1
DISTRIBUTION PATTERN & SPECIES DIVERSITY OF SEAWEEDS AT URAN (NAVI MUMBAI), WEST COAST OF INDIA
- *Corresponding Author:
- Prabhakar R Pawar
Veer Wajekar Arts, Science and Commerce College
Mahalan Vibhag, Phunde, Uran, Raigad, Navi Mumbai, Maharashtra, India
E-mail: prpawar1962@gmail.com
Received 21st January 2017; Accepted 30th January 2017; Published 06th February 2017
Abstract
Seaweeds were collected from substations along Sheva creek, Peerwadi coast and Dharamtar creek of Uran (Raigad), Navi Mumbai, west coast of India. Seaweeds attached to the boulders, jetties, rocks on the shores, stones, pebbles, fishing nets and pneumatophores of mangrove were collected monthly from June 2013 to May 2015. A total of 19 species of seaweeds representing 16 genera, 15 families and 13 orders were identified from Uran coast. Of these, 7 species belongs each to Chlorophyta and Rhodophyta, 2 each to Cyanobacteria and Ochrophyta and 1 to Charophyta. At all study sites, maximum species diversity was recorded during pre-monsoon and post-monsoon than monsoon. Diverse species composition of seaweeds is recorded at Peerwadi coast than the Sheva creek and Dharamtar creek. Lower species diversity at Sheva creek and Dharamtar creek is attributed to the non-availability of rocky substratum for settlement and maritime activities of Jawaharlal Nehru Port Trust (JNPT). The study shows that seaweeds from Uran coast are under stress due to industrial pollution and Port operations.
Keywords
Seaweeds; Anthropogenic inputs; Jawaharlal Nehru Port; Uran; Coastal biodiversity
Introduction
‘Seaweeds’ are the multicellular macroalgae with complex differentiated thallus (Subba Rao & Mantri, 2006). They are subdivided into four different groups: brown algae (Phaeophyta), red algae (Rhodophyta), green algae (Chlorophyta), and blue-green algae (Cyanophyta) (MacArtain et al., 2007). They constitute one of the important living resources of the ocean and were found attached to the bottom on solid substrates such as rocks, dead corals, pebbles, shells and plants (Sahayaraj et al., 2014).
Seaweeds are primary producers and play a central role in coastal habitats (Harley et al., 2012). They support the coastal and marine biodiversity (Christie et al., 2009) and are the base of food chain in the oceans (Figueiredo & Creed, 2009; Barot et al., 2015). Seaweeds are the providers of ecosystem goods and services like food, medicine and storm protection (Ronnback et al., 2007).
Edible seaweeds have been shown to be high in essential vitamins and minerals. Quantification of nutritional aspects of edible seaweeds include polysaccharides (Celluloses, Hemicelluloses, Xylans, Carrageenans and Alginates), minerals (Calcium, Potassium, Magnesium, Sodium, Copper, Iron, Iodine and Zinc), polyunsaturated fatty acids (PUFA), vitamins (A, B, C and E), proteins (Biliproteins) and other major compounds (Alginic acid, Fucoidan, Laminarin, Mannitol, Porphyran, Floridoside, Pentoses and Xylose) (MacArtain et al., 2007).
Therapeutic benefits offered by seaweeds include use of algae in traditional medicines, antiviral effects (herpes viruses), anticancer effects (breast cancer), immunity, inflammation and to reduce plasma cholesterol and hypertension (Fitton, 2003). Marine algae produce biogenic compounds (such as halogenated compounds, alcohols, aldehydes, terpenoids) with pharmacological importance. Seaweeds are the only source for the production of phytochemicals such as agar (China grass), carrageenan and algin. The phytochemicals from seaweeds were used as gelling, stabilizing and thickening agents in food, pharmaceutical, confectionary, dairy, textiles, paper, paint, varnish industries.etc (Kolanjinathan et al., 2014).
A biodegradable polysaccharide extracted from seaweeds Gracilaria dura, Gelidiella acerosa and Kappaphyucus alvarezi is used for preparation of biodegradable ropes to replace synthetic ropes used in seaweed harvest and other applications. It can also replace plastic in other forms like packing material, cloths drying rope, bag handles and other home décor items (Herlekar, 2015). Furthermore, products like biodiesel, bioethanol, biobutanol and hydrogen gases were synthesized from algae (Pooja, 2014).
Seaweeds are a source of novel bioactive compounds, such as phlorotannins and certain polysaccharides, that may confer certain health-promoting properties and consumption of seaweeds has been linked to a lower incidence of chronic diseases such as cancer, hyperlipidemia, and coronary heart disease (CHD) (Brown et al., 2014). Biological importance of seaweeds as food, medicines, therapeutics, fodder, fertilizer and manure is also documented by Burtin (2003), Dhargalkar and Pereira (2005), Kandale et al., (2011), Gupta (2012), Raja et al. (2013) and Krishnamurty et al., (2015).
Seaweeds are particularly useful organisms for studying diversity patterns and planning the conservation and sustainable use of inshore marine resources, and are also useful as indicators of climatic change (Van der Strate et al., 2002). Distribution and abundance of seaweeds is affected by local environmental productivity and degree of exposure to disturbances like high temperatures, desiccation stress, herbivores and competition with coastal fauna and flora (Figueiredo & Creed, 2009). The impacts of ongoing anthropogenic climate change in seaweed dominated ecosystems remain poorly understood (Harley et al., 2012).
In India, many of the rocky beaches, mudflats, estuaries, coral reefs and lagoons along the coast provide ideal habitats for the growth of seaweeds. In all, 271 genera and 1153 species of seaweeds have been enumerated till date from the Indian waters (Subba Rao and Mantri, 2006). Maritime activities of Jawaharlal Nehru Port Trust (JNPT, an international port) and other establishments affect the ecology of seaweeds from Uran coast, Navi Mumbai (Pawar, 2013).
Although many studies have been undertaken to evaluate the species diversity of seaweeds in India, no scientific studies have been carried out on the species composition of seaweeds of Uran, Navi Mumbai; hence, the present study is undertaken. Objective of the study is to evaluate the impact of anthropogenic inputs on species composition of seaweeds with respect to tidal and seasonal variability.
Materials and Methods
Study area
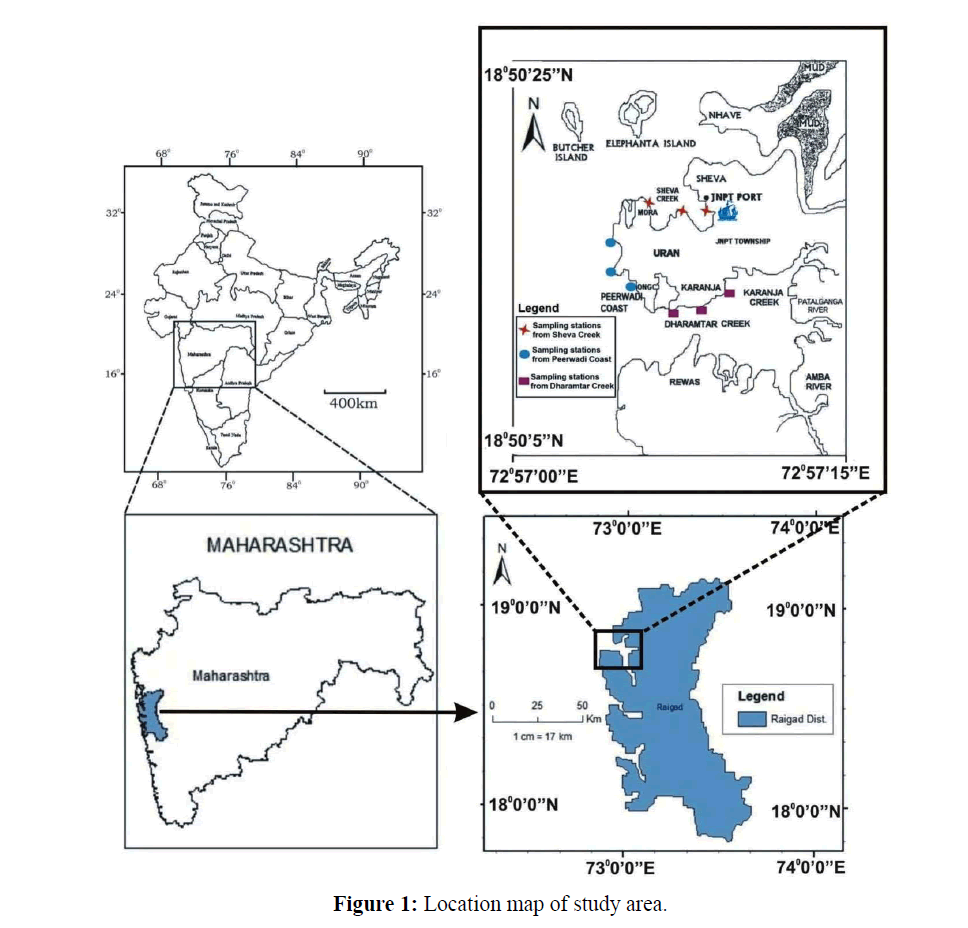
Geographically, Uran (Lat. 18º50'5'' to 18º50'20'' N and Long. 72º57'5'' to 72º57'15'' E) with the population of 23,254 is located along the eastern shore of Mumbai. Uran is included in the planned metropolis of Navi Mumbai and its port, the Jawaharlal Nehru Port (JNPT) (Figure 1).
The Uran coast is tide-dominated and the tides are semidiuranal. The average tide amplitude is 2.28 m. The flood period lasts for about 6-7 h and the ebb period lasts for about 5 h. The average annual precipitation is about 3884 mm and temperature range is 12-36°C, whereas the relative humidity remains between 61% and 86% and is highest in the month of August.
Sampling procedures
The present study was carried out for a period of two years, i.e., from June 2013 to May 2015. Three study sites namely Sheva Creek, site I (Lat.18º50'20'' N and Long. 72º57' 5'' E), Peerwadi coast, site II (Lat.18º 50' 10'' N and Long. 72º57' 1'' E) and Dharamtar Creek, site III (Lat.18º48' 03'' N and Long. 72º58' 31'' E) separated approximately by 10 km were selected along the coast.
The study sites were visited monthly during spring low tides from June 2013 to May 2015 and seaweed samples were collected from the intertidal region. As per seaweed collection procedures documented by Rath and Adhikary (2006), seaweeds attached to the boulders, jetties, rocks on the shores, stones, pebbles, fishing nets and pneumatophores of mangrove were collected by scrapping and preserved in 4% formaldehyde-seawater solution. Microphotographs of the seaweeds were taken with Cannon EOS1100D digital camera and samples were identified following the standard taxonomic keys of Kaliaperumal et al. (1995), Dhargalkar & Kavlekar (2004), Krieg (2005), Bhavanath Jha et al. (2009), Edwards et al. (2012) and Manisseri et al. (2012).
Results and Discussion
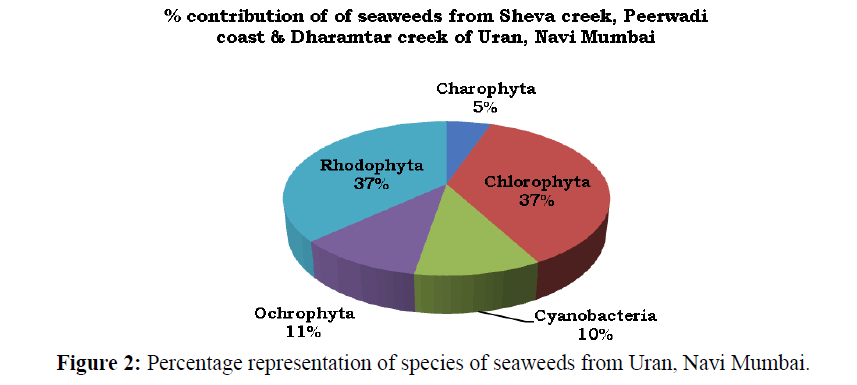
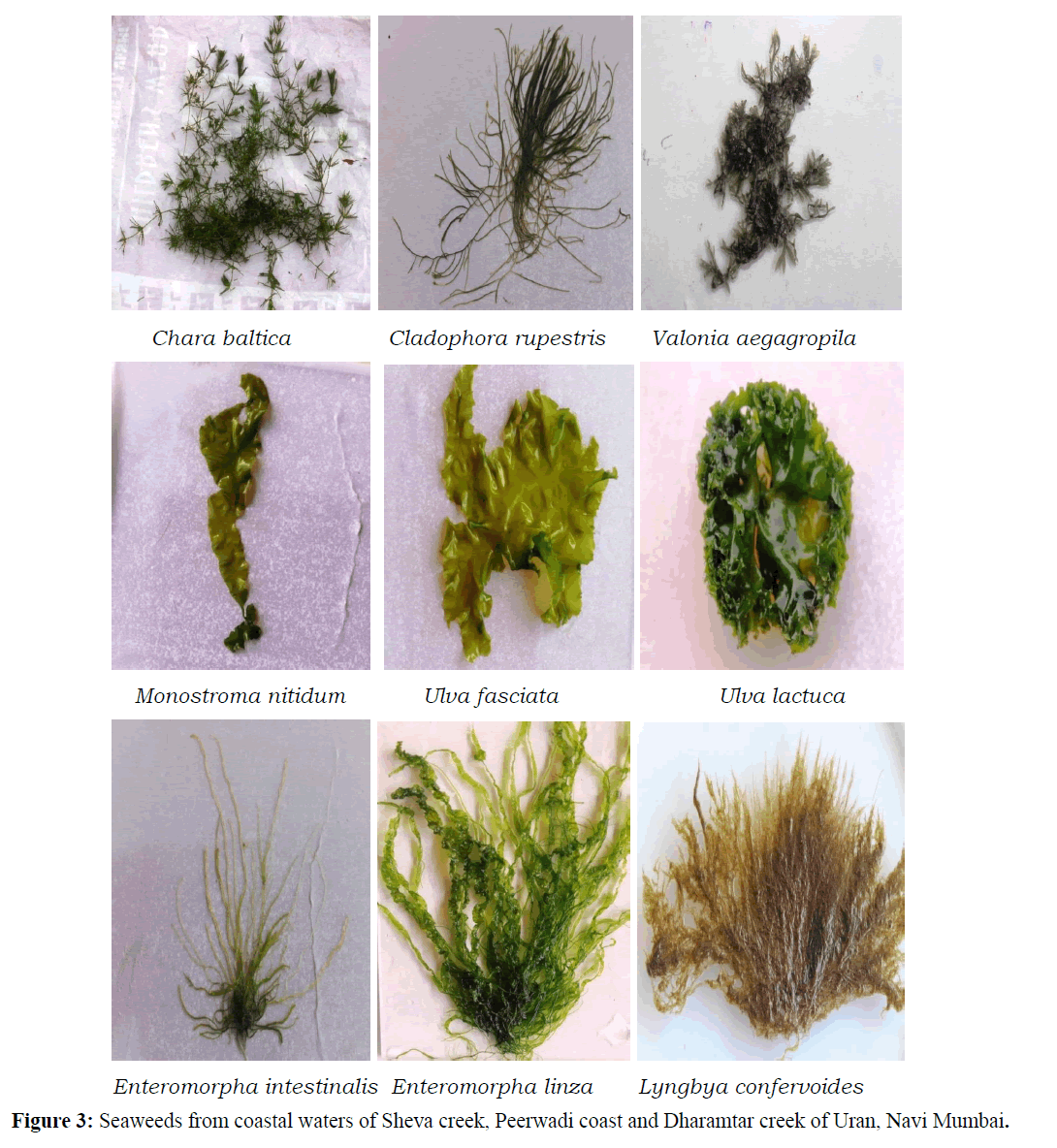
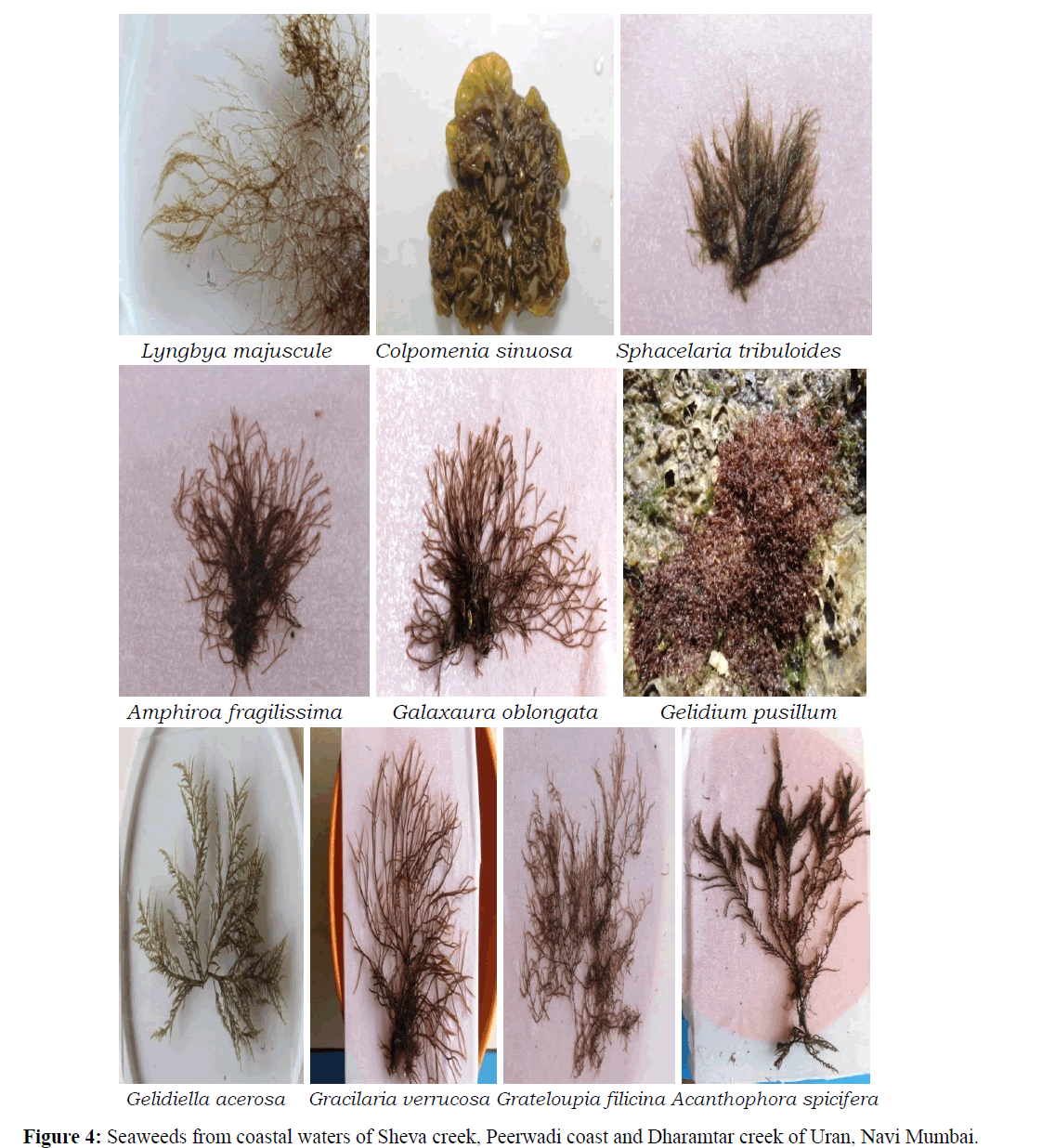
A total of 19 species of seaweeds representing 16 genera, 15 families and 13 orders were recorded from Uran coast (Table 1) and (Figures 3 and 4). Of these, 7 species belongs each to Chlorophyta and Rhodophyta, 2 each to Cyanobacteria and Ochrophyta and 1 to Charophyta. Varied diversity of seaweeds belonging to Charophyta, Chlorophyta, Cyanobacteria, Ochrophyta and Rhodophyta is recorded from three sites. Of the recorded species, 36.84% belongs each to Chlorophyta and Rhodophyta, 10.53% each to Cyanobacteria and Ochrophyta, and 5.26% to Charophyta (Figure 2).
| Class | Order | Family | Binomial Name |
|---|---|---|---|
| Division: Charophyta | |||
| Charophyceae | Charales | Characeae | Chara baltica (A. Bruzelius,1824) |
| Division: Chlorophyta | |||
| Ulvophyceae | Cladophorales | Cladophoraceae | Cladophora rupestris (L.) Kutzing 1843) |
| Ulvophyceae | Cladophorales | Valoniaceae | Valonia aegagropila (C. Agardh, 1823) |
| Ulvophyceae | Ulotrichales | Gomonticeae | Monostroma nitidum (Wittrock,1866) |
| Ulvophyceae | Ulvales | Ulvaceae | Ulva fasciata (Delile, 1813) |
| Ulvophyceae | Ulvales | Ulvaceae | Ulva lactuca (L.) 1753 |
| Ulvophyceae | Ulvales | Ulvaceae | Enteromorpha intestinalis (L.) Nees, 1820 |
| Ulvophyceae | Ulvales | Ulvaceae | Enteromorpha linza (L.) J. Agardh, 1883 |
| Division: Cyanobacteria | |||
| Cyanophyceae | Oscillatoriales | Oscillatoriaceae | Lyngbya confervoides (C. Agardh ex Gomont, 1893) |
| Cyanophyceae | Oscillatoriales | Oscillatoriaceae | Lyngbya majuscule (Harvey ex Gomont) 1892 |
| Division: Ochrophyta | |||
| Phaeophyceae | Ectocarapales | Scytosiphonaceae | Colpomenia sinuosa (Derbes & Solier, 1851) |
| Phaeophyceae | Sphacelariales | Sphacelariaceae | Sphacelaria tribuloides (Meneghini, 1840) |
| Division: Rhodophyta | |||
| Florideophyceae | Corallinales | Corallinaceae | Amphiroa tribuloides (Meneghini, 1840) |
| Florideophyceae | Nemaliales | Galaxauraceae | Galaxaura oblongata (J.V.Lamouroux, 1816) |
| Florideophyceae | Gelidiales | Gelidiaceae | Gelidium pusillum (Stackhouse) Le Jolis, 1863 |
| Florideophyceae | Gelidiales | Gelidiellaceae | Gelidiella acerosa (Fosskal) Feldmann & G. Hamel, 1934 |
| Florideophyceae | Gracilariales | Gracilariaceae | Gracilaria verrucosa (Hudson) Papenfuss, 1950 |
| Florideophyceae | Halymeniales | Halymeniaceae | Grateloupia filicina (C. Agardh, 1822) |
| Florideophyceae | Ceramiales | Rhodomelaceae | Acanthophora specifera (M.Vahl)Borgesen,1910 |
Table 1: Species of seaweeds recorded from Uran, Navi Mumbai.
At Uran coast, maximum species diversity of seaweeds was recorded during pre-monsoon and post-monsoon than monsoon periods. Higher species composition of seaweeds was also noted during post-monsoon than premonsoon and monsoon. Maximum diversity of seaweeds recorded during pre-monsoon and post-monsoon is attributed to favorable environmental conditions with respect to variables like temperature, salinity, dissolved oxygen and nutrients (Table 2). Results of the study are in agreement with earlier reports of Rao et al. (2011) in the Bhimili coast, east coast of India, Reddy et al. (2014) in seaweed resources of India, Pariera and Almeida (2014) from Goa coast, Naik et al. (2015) in Karwar Bay, and Rodde and Sable (2015) from Malvan and Kunakeshwar in Sindhudurg District of Maharashtra.
| Sr. No. | Species Name | Pre-monsoon (February to May) |
Monsoon (June to September |
Post-monsoon (October to January |
|---|---|---|---|---|
| 1 | Chara baltica (A. Bruzelius,1824) |
+ | + | + |
| 2 | Cladophora rupestris (L.) Kutzing 1843) |
- | - | + |
| 3 | Valonia aegagropila (C. Agardh, 1823) |
+ | - | - |
| 4 | Monostroma nitidum (Wittrock,1866) | - | + | + |
| 5 | Ulva fasciata (Delile, 1813) |
- | + | + |
| 6 | Ulva lactuca (Linnaeus, 1753) |
- | + | + |
| 7 | Enteromorpha intestinalis (L.) Nees, 1820 |
- | - | + |
| 8 | Enteromorpha linza (L.) J. Agardh, 1883 |
- | - | + |
| 9 | Lyngbya confervoides (C. Agardh ex Gomont, 1893) |
+ | + | + |
| 10 | Lyngbya majuscule (Harvey ex Gomont, 1892) |
+ | + | + |
| 11 | Colpomenia sinuosa (Derbes & Solier, 1851) |
+ | - | + |
| 12 | Sphacelaria tribuloides (Meneghini, 1840) | + | + | + |
| 13 | Amphiroa tribuloides (Meneghini, 1840) |
- | - | + |
| 14 | Galaxaura oblongata (J.V.Lamouroux, 1816) | + | - | + |
| 15 | Gelidium pusillum (Stackhouse) Le Jolis, 1863 |
+ | + | + |
| 16 | Gelidiella acerosa (Fosskal) Feldmann & G. Hamel, 1934 | + | - | + |
| 17 | Gracilaria verrucosa (Hudson) Papenfuss, 1950 |
- | - | + |
| 18 | Grateloupia filicina (C. Agardh, 1822) |
- | + | + |
| 19 | Acanthophora specifera (M.Vahl)Borgesen,1910 |
+ | + | + |
Table 2: Seasonal distribution of seaweeds from Uran, Navi Mumbai.
During present study, diverse species composition of seaweeds was recorded at Peerwadi coast than Sheva creek and Dharamtar creek. This is correlated with the presence of rocky upper littoral zone which provide suitable substratum and appropriate habitat for seaweeds. Also at site II, comparatively less number of Container Freight Stations and other port related establishments are present (Pawar, 2015). Similar results were reported by Barot et al. (2015) at Okha coast, western India.
At Sheva creek has extensive mudflats with little rocky substratum. Jawaharlal Nehru Port Trust (JNPT) and other establishments are located in the stretch of Sheva creek. Also, Gharapuri Island (Elephanta caves) is present on the north side of the creek. The creek receives wastes and effluents from Asia’s largest industrialized zone namely Thane Belapur industrialized area along with the Navi Mumbai Urban area. Maritime activities of JNPT and nonavailability of rocky and coral substratum might be related to minimum density of seaweeds at Sheva creek (Pawar, 2013). Similar results on species diversity of seaweeds were reported by Rath and Adhikary (2006) in Orissa, east coast of India and Satheesh and Wesley (2012) in the Kudankulam coastal waters, south-eastern coast of India.
Dharamtar creek is the confluence of Karanja creek, Patalganga River and Amba River. Intertidal region of Dharamtar creek is with rocky and coral substratum towards the Dronagiri Mountain and remaining part of the creek is dominated by the marshy areas and mud flats. The rocky substratum of Dharamtar creek supports moderate species diversity of seaweeds. This is attributed to the substantial amount of waste water received from petrochemical complex and other industries that opens into Dharamtar creek (Pawar and Kulkarni, 2007).
At Uran coast, species composition of Chlorophyta and Rhodophyta has dominated the Cyanobacteria, Ochrophyta and Charophyta. This is correlated with the topography of the coast which is made up primarily of rocks of green basalt, which are glossy black above and covered by calcareous surfaces. Results of the study are in agreement with earlier reports of Kerswell (2006), Domettila et al. (2013), Karthick et al. (2013), Reddy et al. (2014), Sahayraj et al. (2014) and Valanja and Jamdhade (2014).
Seaweeds are known to be vulnerable to physical and chemical changes in the marine environment (Harley et al. 2012). Sahoo et al. (2003) reported that large-scale aquacultural activities in Chilika Lake, Orissa have resulted in eutrophication which has changed its floristic composition. Satheesh and Wesley (2012) noted that seaweeds are under threat in developing countries, where they are being disturbed by a variety of human activities. Gradual disappearance of marine algae along Visakhapatnam coast by disturbances created by discharge of effluents, environmental factors, tidal waves and cyclones is reported by Krishnamurty et al. (2015).
JNPT and Container Freight Stations (CFS) have affected the mangrove biodiversity and incidences of coastal pollution occur because of leakage/discharge of transporting materials along with industrial effluents (Pawar and Kulkarni, 2007; Pawar, 2013). Since no earlier reports are available on taxonomy and species diversity of seaweeds from Uran coast, data presented here can be taken as a baseline data in knowing the status of seaweeds from Uran coast and effect of industrial development on it and for a better management of marine algae.
Conclusion
This study shows that species of Chlorophyta and Rhodophyta have dominated the Cyanobacteria, Ochrophyta and Charophyta. Activities of Jawaharlal Nehru Port (JNPT) and Container Freight Stations (CFS) will affect the species diversity of seaweeds from Uran coast in near future. Present information on species diversity of seaweeds would be helpful as a baseline data for further monitoring of anthropogenic inputs on species diversity of seaweeds from Uran coast.
References
- Megha, B., Nirmal Kumar, J. I. and Kumar, R. N., 2015. Seaweed Species Diversity in Relation to Hydro Chemical Characters of Okha Coast, Western India. Int. J. Recent Res. and Review. 3: 16-28.
- Bhavanath, J., Reddy, C. R. K., Thakur, M. C. and Umamaheswara, R., 2009. Seaweeds of India-The Diversity and Distribution of Seaweeds of the Gujarat Coast. Springer Dordrecht Heidelberg London New York pp: 216.
- Brown, E. M., Philip, J. A., Pamela, J. M., Chris, IR. G., Sonja, N., Conall, R. S. and Emeir, M. M., 2014. Seaweed and human health. pp: 1-12.
- Burtin, P. 2003. Nutritional Value of Seaweeds. Electron. J. Environ. Agric. Food. Chem. 2: 1-6.
- Christie, H., Norderhaug, K. M. and Fredriksen, S., 2009. Macrophytes as habitat for fauna. Mar. Ecol. Prog. Ser. 396: 221–33.
- Dhargalkar, V. K. and Devanand, K., 2004. Seaweeds - a field manual. National Institute of Oceanography, Dona Paula, Goa – 403 004 pp: 42.
- Dhargalkar, V. K. and Neelam, P., 2005. Seaweed: Promising Plant of the Millennium. Science and Culture. pp: 60-66.
- Domettila, C., Brintha, T. S. S., Sukumaran, S. and Jeeva, S., 2013. Diversity and distribution of seaweeds in the Muttom coastal waters, south-west coast of India. Biodiversity Journal. 4: 105-110.
- Edwards, M., Hannifffy, D., Heesch, S., Hernandez-Kantun, J., Moniz, M., Queguineur, B., Ratcliff, J., Soler-Vila, A., and Wan, A., 2012. Macroalgae Fact-sheets. pp: 40.
- Figueiredo, M. A. O. and Creed, J. C., 2009. Marine Algae and Plants. In: Kleber Del Claro, Paulo S. Oliveira & Victor Rico-Gray Eds, Tropical Biology and Conservation Management. Botany Vol. IV. Encyclopedia of Life Support Systems. Eolss Publishers Co. Ltd; Oxford, United States. pp: 1-7.
- Fitton, H. J., 2003. Brown Marine Algae-A Survey of Therapeutic Potentials. Alternative & Complementary Therapies, pp: 29-33.
- Gupta, R., 2012. Red Algae’s and its Medicinal and Therapeutic Uses. Int. J. Sci. Inventions Today. 1: 27-31.
- Harley, C. D. G., Kathryn, M. A., Kyle, W. D., Jennifer P. J., Rebecca, L. K., and Theraesa, A. C., 2012. Effects of Climate Change on Global Seaweed Communities. J. Phycol.
- Herlekar, I., 2015. Biodegradable ropes from seaweed extracts. Curr. Sci. 108: 2140.
- Kaliaperumal, N., Kalimuthu, S. and Ramalingam, J. R., 1995. Economically Important Seaweeds. ICAR Special Publication No. 62, Central Marine Fisheries Research Institute, Indian Council of Agricultural Research, Post Box No. 1603, Cochin - 682014, India. pp: 44.
- Ajit, K., Meena, A. K., Rao, M. M., Panda, P., Mangal, A. K., Reddy, G. and Ramesh, B., 2011. Marine algae: An Introduction, Food value and Medicinal uses. J. Pharmacy Research. 4: 219-221.
- Karthick, P., Mohanraju, R., Ramesh, C. H. and Murthy, K. N., 2013. Distribution and diversity of seaweeds in North and South Andaman Island. Seaweed res. Utiln. 35: 8-16.
- Kerswell, A. P., 2006. Global Biodiversity Patterns of Benthic Marine Algae. Ecology. 87: 2479–2488.
- Kolanjinathan, K., Ganesh, P. and Saranraj, P., 2014. Pharmacological Importance of Seaweeds: A Review. World J. Fish and Marine. Sci. 6: 01-15.
- Krieg, K., 2005. Field Guide to Oregon’s Rocky Intertidal. PISCO’s Online Taxonomic Database. pp: 126.
- Krishnamurty, V. S., Chennubhotla, K. S. R. and Kaliaperumal, N., 2015. Effect of some adverse factors on the diversity and distribution of marine macro algae along the Indian coasts. Seaweed Res. Utiln. 36: 5-11.
- MacArtain, P., Christopher I. R. G., Mariel, B., Ross, C. and Ian R. R., 2007. Nutritional Value of Edible Seaweeds. Nutrition Reviews. 65: 535-543.
- Manisseri, M. K., Geetha, A. and Rao, G. S., 2012. Common Seaweeds and Seagrasses of India. Herbarium Vol.1. Central Marine Fisheries Research Institute Indian Council of Agricultural Research Kochi- 682 018, Kerala, India. pp: 47.
- Naik, U. G., Beligiriranga, V. and Shivakumar B. Haragi. 2015. Seaweeds of Karwar Bay, Arabian Sea, West Coast of India-a Diversity Profile. Int. J. Sci. Nat. 6: 728-732.
- Pariera, N. & M. R. Almeida. 2014. A preliminary checklist of marine algae from the coast of Goa. Indian J. Mar. Sci. 43: 655-665.
- Pawar, Prabhakar R. 2013. Monitoring of impact of anthropogenic inputs on water quality of mangrove ecosystem of Uran, Navi Mumbai, west coast of India. Mar. Poll. Bull. 75:291–300.
- Pawar, P. R., 2015. Monitoring of Pollution Using Density, Biomass and Diversity Indices of Macrobenthos from Mangrove Ecosystem of Uran, Navi Mumbai, west coast of India. J. Bioremed. Biodeg. 6:299.
- Pawar, P. R. and Balasaheb G. K., 2007: Assessment of water quality in Karanja creek Dist-Raigad, Maharashtra, West coast of India. J. Ecophysiol. Occup. Hlth. 7:61–72.
- Pooja, S. 2014. Algae used as Medicine and Food-A Short Review. J. Pharm. Sci. & Res. 6: 33-35.
- Raja, A., Vipin, C. and Aiyappan, A., 2013. Biological importance of Marine Algae- An overview. Int. J. Curr. Microbiol. App. Sci. 2: 222-227.
- Rao, K. S., Murty, P. M. and Narasimha Rao, G. M., 2011. Seasonal Studies on Marine algae of the Bhimili Coast, East Coast of India. J. Algal Biomass Utln. 2: 69-82.
- Rath, J. and Adhikary, s. p., 2006. Marine Macro-algae of Orissa, East Coast of India. Algae. 21: 49-59.
- Reddy, C. R. K., Rao S. P. V., Ganesan, M., Eswaran, K., Zaidi, S. H. and Mantri, V. A., 2014. The Seaweed Resources of India. In: A. T. Critchely, M. Ohno and D.B. Largo edn. World Seaweed Resources in DVD format, ETI Information Services Ltd., Wokingham, Berkshire, UK. pp: 25.
- Rode, Surekha and Sabale, A., 2015. Diversity of Seaweeds from Malvan and Kunakeshwar in Sindhudurg District of Maharashtra. Ind. J. Appl. Res. 5: 413-415.
- Ronnback, P., Kautsky, N., Pihl, L., Troell, M., Soderqvist, T. and Wennhage, H., 2007. Ecosystem goods and services from Swedish coastal habitats: identification, valuation, and implications of ecosystem shifts. Ambio. 36: 534–44.
- Sahayaraj, K., Rajesh, S., Asha, A., Rathi, J. M. and Patric, R., 2014. Distribution and diversity assessment of the marine macroalgae at four southern districts of Tamil Nadu, India. Ind. J. Geo-Mar. Sci. 43: 607-617.
- Debasish, S., Nivedita, S. and Dinabandhu, S., 2003. A Critical Survey of Seaweed Diversity of Chilika Lake, India. Algae. 18: 1-12.
- Satheesh, Sathianeson & Wesley, S. G., 2012. Diversity and distribution of seaweeds in the Kudankulam coastal waters, South-Eastern coast of India. Biodiversity Journal. 3: 79-84.
- Rao, S. P. V. and Vaibhav, A. M., 2006. Indian seaweed resources and sustainable utilization: Scenario at the dawn of a new century. Curr. Sci. 91: 164-174.
- Valanju, N. M. and Jamdhade, V. M., 2014. Study of Marinemacro-Algal Biodiversity at Kurli-Kasop Beach, Ratnagiri Dist of Maharashtra. Bionano Frontier. 7: 172-176.
- Van der S, H. J., Boele-Bos, S. A., Olsen, J. L., van de, Z. L. and Stam, W. T., 2002. Phylogeographic studies in the tropical seaweed Cladophoropsis membranacea Chlorophyta, Ulvophyceae reveal a cryptic species complex. Journal of Phycology 38: 572-582.