Review Article - Biomedical Research (2017) Volume 28, Issue 11
Diagnosis and management of orthopedic implant-associated infection: a comprehensive review of the literature
Rares Mircea Birlutiu1,2, Victoria Birlutiu1,3*, Manuela Mihalache1, Cosmin Mihalache1,4, Razvan Silviu Cismasiu2,51Lucian Blaga University of Sibiu, Faculty of Medicine Sibiu, Romania
2FOISOR Clinical Hospital of Orthopedics, Traumatology, and Osteoarticular TB Bucharest, Romania
3Department of Infectious Diseases, Academic Emergency Hospital Sibiu, Romania
4Department of Nuclear Medicine, Academic Emergency Hospital Sibiu, Romania
5Carol Davila University of Medicine and Pharmacy Bucharest, Romania
- *Corresponding Author:
- Victoria Birlutiu
Lucian Blaga” University Sibiu
Academic Emergency Hospital, Romania
Accepted date: April 4, 2017
Abstract
Orthopedic surgery of total hip and total knee arthroplasty were the most successful orthopedic surgeries of the last century. In the context of an increase number of primary and revision total hip and total knee arthroplasty performed each year, an increased risk of complication is expected. The aim of our review is to present comprehensive data form the literature regarding the diagnosis strategies (according to the international guideline for the diagnosis of biofilm infections) and treatment strategies (irrigation and debridement with retention of components, one-stage or two-stage revision) for prosthetic joint infection. A MEDLINE via PubMed, Scopus, and Web of Science search for original and review articles was performed using key terms, prosthetic joint infection, PJI, biofilm, total hip arthroplasty, total knee arthroplasty, diagnosis, and treatment. Prosthetic joint infection, still, remains the most common and feared arthroplasty complication. Recent studies have shown that the formation of biofilm by pathogens is involved in prosthetic joint infection, infections that are acquired either via an exogenous or an endogenous pathway. The diagnosis and treatment of prosthetic joint infection remains a challenge. A correct diagnosis of infection is decisive for a correct treatment of orthopedic implantrelated infections.
Keywords
Prosthetic joint infection, PJI, Total hip arthroplasty, Total knee arthroplasty, Diagnosis, Treatment.
Introduction
Orthopedic surgery of total hip and total knee arthroplasty were the most successful orthopedic surgeries of the last century, surgeries with a primary purpose of restoring the joint function of persons affected by osteoarthritis. Arthroplasty surgeries have a significant effect on the quality of life, on reducing symptoms, on regaining physical function, and on improving mobility and regaining the independence of daily routines [1]. The number of arthroplasty surgeries is increasing from year to year, in 2010, in the US 719.000 interventions of Total Hip Arthroplasties (THA) [2], and in 2012, 600.000 interventions of Total Knee Arthroplasties (TKA) were performed [3]. In Romania, according to the Romanian Arthroplasty Register, in 2014 were reported to be performed 10.179 primary surgeries of THA, and 2.619 primary surgeries of TKA. In 2015, according to the same register, 9.297 THA and 2.770 TKA primary interventions were conducted. In this context of an increase number of primary surgeries, in Romania, during 01.01.2014-01.01-2016, 1.164 hip and 167 knee revision surgeries were performed.
With an increasing number of primary and revision THA and TKA performed each year there is an expected increased risk of complication. One of the most serious complications, still, remains Prosthetic Joint Infection (PJI). Infections that can occur despite well-established hospital cleaning, disinfection policies and procedures, the conditions of prosthesis manufacturing or antibiotic prophylaxis prior to surgery, measures that are helping to reduce the rate of infection. PJI are devastating complications after arthroplasties, and are associated with an increased rate of morbidity and mortality. According to the data published in the literature, orthopedic implant-associated infection rate is between 1-9%, nearly doubling for revision surgeries. According to Montanaro et al., a rate of infections between 3.2% and 5.6% [4]. It is considered that 0.5-2% of patients develop a biofilm orthopedic implantassociated infection in the first 2 years after surgery [5-7]. Other authors have reported an average incidence rate of infection of 0.25-1% at 1 y after THA, and 0.4-2% TKA primary surgery [8]. Also, according to the Romanian Arthroplasty Register and the first annual report that has been published in October 2013, report the covers over 81.288 hip and 10.752 knee interventions performed between 2001 and 2011, early infection rate for hip arthroplasties was 1.6% and for knee arthroplasties 0%, in terms of delayed infection, the rate was 6.8% hip arthroplasties respectively 0% for knee arthroplasties. This 0% is subject to interpretation, an explanation may be the lack of reporting to the register the reasons for revision surgery of the primary total knee arthroplasty.
In terms of etiologic, most commonly isolated germ is Staphylococcus aureus, either Methicillin-resistant Staphylococcus aureus (MRSA) or Methicillin-susceptible Staphylococcus aureus (MSSA). In the US up to 46% of Staphylococcus aureus strains are methicillin-resistant strains and up to 23% of strains of Enterococci spp are vancomycinresistant Enterococci. The alarming emergence of these resistant strains may lead to increased patients’ morbidity and mortality [9]. A correct diagnosis of infection is decisive for a correct treatment of orthopedic implant-related infections. The aim of our review is to present comprehensive data form the literature regarding the diagnosis strategies (according to the international guideline for the diagnosis of biofilm infections) and treatment strategies (irrigation and debridement with retention of components, one-stage or two-stage revision) for prosthetic joint infection.
Literature Search
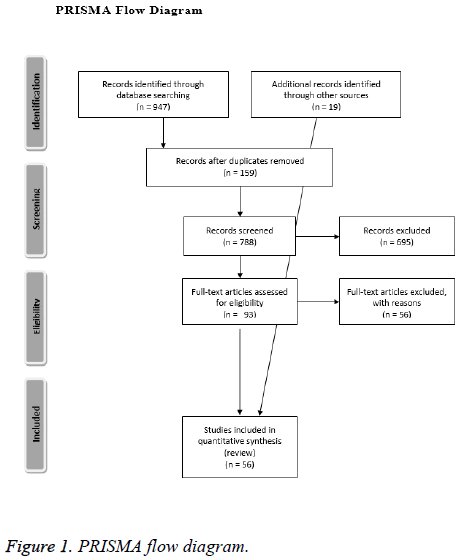
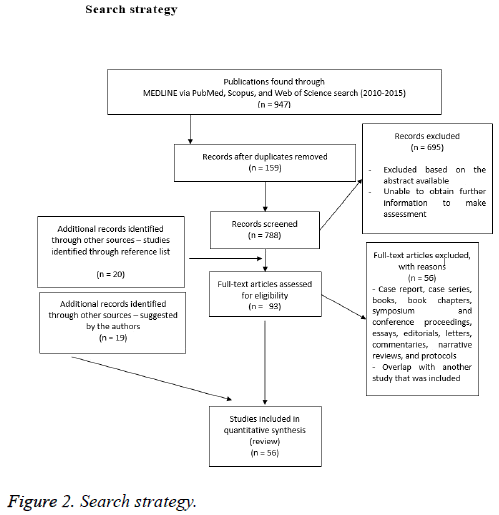
For our comprehensive review, we searched MEDLINE via PubMed (from 2010), Scopus (from 2010), and Web of Science (from 2010). The original search was conducted on January 8, 2015 and updated on December 10, 2015. No date restrictions were used for the search. Our main search terms consisted of the terms prosthetic joint infection, PJI, biofilm, total hip arthroplasty, total knee arthroplasty, diagnosis, and treatment. Our inclusion criteria were defined a priori. We included just English-language articles. Experimental, observational, and qualitative studies were considered eligible. Systematic reviews addressing our research were included. Case report, case series, books, book chapters, symposium and conference proceedings, essays, editorials, letters, commentaries, narrative reviews, and protocols were excluded. All authors, independently, screened every title and abstract identified. Consensus was reached on decisions to advance studies to full text screening, discrepancies were resolved by discussion between the authors. Full text versions of eligible studies were retrieved for detailed review. References from relevant reviews, meta-analysis, overviews of reviews and relevant international clinical guidelines were also examined by two authors (RMB, VB) to identify potential relevant articles, and included. A PRISMA 2009 Flow Diagram is provided as Figure 1. Our search strategy is included as Figure 2. A completed PRISMA checklist is included as a supplement material file 1 with this publication.
Pathogenesis
In terms of pathogenesis, either an acute or a chronic infection can be acquired primarily on two pathways: an exogenous path during the time of the surgery or in the postoperative period, and an endogenous path-a hematogenic one. Recent studies have shown that in both cases, the presence of the biofilm is involved. Thus, more than 30 years ago William "Bill" Costeron discovered how bacteria form biofilm, chronic infections occur as a result of the phenotypic changes in the lifestyle and in the structural organization of bacteria: the biofilm.
The biofilm
More than 65% of the infections are related to biofilm [8]. Each year, in the US, over 12 million cases of infections associated with biofilm are reported (BIS=Biofilm-related Infections), most common being associated with orthopedic implants [10]. The surface commonly used with orthopedic implants is made of titanium (or titanium alloy), stainless steel, cobalt chrome, different polymeric biomaterials (ceramics, hydroxyapatite or polyethylene) and polymethylmethaacrylate bone cement, surfaces that represent structures likely to be colonized and consequently bacterial biofilm is formed [8,11].
The biofilm is a structure consisting of bacterial cells (one or more microorganism species) surrounded by a matrix produced by the bacteria after their adhesion to surface of the implants. The matrix is composed of polymeric secreted compounds called Extracellular Polymeric Substance (ESP) or exopolysaccharide like: polysaccharides, proteins, acids, lipids and extracellular DNA (eDNA). The biofilm is composed of 30% bacteria and 70% matrix with protective and adhesion role [12]. Both bacteria and fungi can cause infections associated with biofilm formation [13]. The biofilm is mainly responsible for chronic infections, infections that are characterized by persistence and progression, mainly due to the inflammatory response from around the biofilm. The inflammatory response is mediated by polymorphonuclear and mononuclear cells depending on the type of predominant immune response-type 2 T helper or type 1 T helper (polarized) lymphocytes [14-16]. Reduced activity of the antibiotics in the biofilm is not fully elucidated. It is considered that a dormant state or slow growth of bacteria, the presence of different bacterial subpopulations in terms of phenotypic antibiotic resistance, gene expression and an increased stress caused by environmental conditions are contributing factors to an increased resistance to antibiotics.
There are four stages of biofilm development on orthopedic implants:
1. Primary cell adhesion: With an onset seconds after the orthopedic implant is removed from the packaging and up to 2 h after the exposure to the external environmental conditions. After placing components in their final position, fibrinogen, fibronectin and vitronectin are absorbed on the surface of the implant creating favorable conditions for biofilm development.
2. Cellular aggregation: Consists of a multilayer proliferation of bacteria and adhesion, resulting bacterial colonies, which are surrounded by a polysaccharide (polysaccharide intercellular adhesion) matrix.
3. Biofilm maturation: Reaching the stage of maturation, changes in the mobility structures of the bacteria appear (cilia, flagella) and also in the exopolysaccharides. Thus the biofilm with an increased resistance appears.
4. Cell detaching stage: At maturity, planktonic structures can be released from the mature biofilm, and the same process begins.
Classification Schemes of Prosthetic Implant- Associated Infections
The most commonly used classification of PJIs is the one proposed by Trampuz and Zimmerli [17,18], depending on the onset of symptoms after arthroplasty and defines the PJIs as early (occurring within 3 months postoperatively)-commonly caused by Staphylococcus aureus or gram-negative bacteria, delayed (3-24 months) often caused by bacteria with a lower virulence, such as coagulase-negative Staphylococci and Propionibacterium acnes and late (>24 months) caused by Staphylococcus spp., Streptococcus spp. or gram-negative bacteria. Depending on the type of infection PJIs are defined as acute hematogenous PJI (less than 3 weeks’ duration of symptoms in the context of an uneventful postarthoplasty period), early postinterventional (within 1 month after an invasive procedure) and chronic PJI. Parvizi et al. mentioned a period of 3 months after performing arthroplasty as the cut-off to determine whether the infection can be regarded as being acute or not [19].
In the 1990s, Tsukayama proposed another classification based on the time since the surgical intervention and the mode of infection, first category–positive intraopeative cultures (when the surgical revision was presumed to be for an aseptic failure), second category-early postoperative infection (<1 month after surgical intervention), third category-late chronic PJI (>1month after surgery), and forth category-acute haematogenous infection. There is also a treatment suggested based on the mode of presentation: first category-antibiotic therapy, second category-debridement and prosthetic retention, third categoryprosthetic removal, and forth category-debridement and prosthetic retention or prosthetic removal [20,21]. Issues regarding the selection of a medical, surgical treatment are discussed in management of prosthetic joint infection, below.
McPherson and colleagues popularized another classification for PJI that categorizes the type of infection and the host (similar to the Cierny-Mader staging for osteomyelitis). The classification includes three of the type of infection proposed by Tsukayama (early postoperative infection, hematogenous infection, and late chronic infection) [22-24]. The classification may assist the surgeon in identifying the severity of the infection and choose an appropriate treatment option. The system has been used in clinical practise especially in the United States and the United Kingdom (Table 1).
| Infection type | Systemic host grade | Local extremity grade |
|---|---|---|
| I: early postoperative infection (<4 postoperative weeks) | A: uncompromised | 1: uncompromised |
| II: hematogenous infection (<4 weeks duration) | B: compromised (1-2 compromising factors) | 2: compromised (1-2 compromising factors) |
| III: late chronic infection (> 4 weeks duration) | C: significant compromise (> 2 compromising factors) or one of | 3: significant compromise (>2 compromising factors) |
| -absolute neutrophil count<1000/mm3 -CD4 T cell count<100/mm3 -intravenous drug abuse -chronic active infection at another site -dysplasia or neoplasm of the immune system Compromising factors: -age>80 -immunosuppressive drugs -alcoholism -malignancy -chronic active dermatitis or cellulites -pulmonary insufficiency -chronic indwelling catheter -renal failure requiring dialysis -chronic malnutrition -systemic inflammatory disease -current nicotine use -systemic immune compromise -diabetes -hepatic insufficiency |
||
| Local extremity grade (wound) | ||
| -active infection present | ||
| >3-4 months | ||
| -multiple incision with skin bridges | ||
| -soft tissue loss from prior trauma | ||
| -subcutaneous abscess>8 cm2 | ||
| -synovial cutaneous fistula | ||
| -prior periarticular fracture or trauma about a joint | ||
| -prior local irradiation | ||
| -vascular insufficiency to extremity | ||
Table 1: Staging system for prosthetic implant-associated infections according to McPherson [23].
Definition and Diagnosis of PJIs
Both early and late infections are associated with the surgical interventins, both showing local and general symptoms, associated with changes in biomarkers levels: C-Reactive Protein (CRP), Erythrocyte Sedimentation Rate (ESR) and White Blood Cell (WBC) counts. Blood cultures and tissue cultures can be used in diagnosis. There is no generally valid definition of the infection associated with orthopedic implants but the American Society of Infectious Diseases and the working groups of the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) recommends the suspicion of an infection when: sinus tract communicating is present or is persistent and active, acute pain at the level of the prosthetic joint or whenever chronic pain after surgery, especially in the absence of periods without pain, and especially after surgery.
ESCMID recommendations are based on strength of recommendation as follows: Grade A-ESCMID strongly supports a recommendation for use, B-moderately supports, Clow support, D-not recommended; and recommendation on quality of evidence 1-data from at least one randomized study, 2-data from at least one study with good design and nonrandomized, cohort or case-control studies or from dramatic results from uncontrolled experiments, 3-data provided by the authorities based on clinical studies and clinical cases [25]. Also the American Academy of Orthopedic Surgeons (AAOS) and the Centers for Disease Control and Prevention (CDC) published guidelines to establish a consensus on the definition and diagnostic criteria for orthopedic implants associated (Tables 2 and 3).
| Strength of recommendation: |
|---|
| Grade A: ESCMID strongly supports a recommendation for use |
| Grade B: ESCMID moderately supports a recommendation for use |
| Grade C: ESCMID marginally supports a recommendation for use |
| Grade D: ESCMID supports a recommendation against use |
| Quality of evidence: |
| Level I: Evidence from at least one properly designed randomized controlled trial. |
| Level IIa: Evidence from at least one well-designed clinical trial, without randomized; from cohort or case-control analytic studies (preferably from more than one center); from multiple time series; or from dramatic results of uncontrolled experiments |
| Level III: Evidence from opinions of respected authorities, based on clinical experience, descriptive case studies. |
| aAdded index: |
| Meta-analysis or systematic review of randomized controlled trails. |
| Transferred evidence, that is, results from different patient cohorts, or similar immune-status situation. |
| Comparator group is a historical control. |
| Uncontrolled trail. |
| Published abstracts (presented at an international symposium og meeting) |
Table 2: Definition of strength and quality of recommendations [26].
| Major: |
|---|
| -Sinus tract communicating with the joint; |
| -Two positive periprosthetic cultures with phenotypically identical organisms. |
| Minor: 3 from the following 5 criteria |
| -Raised serum C-Reactive Protein (CRP) and Erythrocyte Sedimentation Rate (ESR); |
| -Raised synovial fluid White Blood Cell (WBC) count (1100-4000-20000 cell/uL) or ++ change on leukocyte esterase test strip of synovial fluid; |
| -Raised synovial fluid Polymorphonuclear neutrophil percentage PMN (more than 64%-69%); |
| -A single positive culture; |
| -Positive histological analysis of periprosthetic tissue (more than 5 neutrophils/5 high power fields at 400X. |
Table 3: Diagnostic criteria for periprosthetic joint infection-accepted by the International Consensus Meeting on PJI and by the Center for Disease Control and Prevention.
In terms of the clinical picture there are two types of infection manifestations: an acute and an oligo-symptomatic one. The acute manifestations are: fever and chills (symptoms caused by bacteremia), local Celsian signs, joint swelling or active fistula (an active sinus tract communication). When the source of infection is a haematogenous one, initially systemic manifestations dominate the clinical picture and then the local ones as in endocarditis, pneumonia or urospesis. Oligosymptomatic infections are harder to be distinguished from an aseptic loosening or in case of exceeding the life of the prosthesis, and are characterized by chronic pain, low grade fever, joint swelling, and radiographic signs of loosening.
The presence of some risk factors such as: diabetes, obesity, malnutrition, rheumatoid arthritis, immunosuppression, past surgical history, malnutrition, active liver pathology, chronic kidney disease, smoking, alcohol consumption, intravenous drug abuse, recent hospitalization, prolonged hospitalization in recovery centers and male gender, but also factors that are related to the initial surgery (surgical duration, revision surgery, absence of prophylactic antibiotics, postoperative hematoma, wound dehiscence) may suggest the diagnosis of infection [27]. Patients that are nasal carriers of Staphylococcus aureus are at increased risk for health careassociated infections, patients with an increased nasal colonization have a risk of orthopedic implant-associated infection 3 to 6 times higher than those with a low level or without nasal colonization [28].
According to the latest consensus of the Musculoskeletal Infection Society definite PJI exists when:
There is a sinus tract communicating with the prosthesis; or a pathogen is isolated by culture from at least two separate tissue or fluid samples obtained from the affected prosthetic joint; or Four of the following six criteria exist: -Elevated serum Erythrocyte Sedimentation Rate (ESR) and serum C-Reactive Protein (CRP) concentration, -Elevated synovial leukocyte count, -Elevated synovial neutrophil percentage (PMN%), - Presence of purulence in the affected joint, -Isolation of a microorganism in one culture of periprosthetic tissue or fluid, or -Greater than five neutrophils per high-power field in five high-power fields observed from histologic analysis of periprosthetic tissue at X400 magnification. (PJI may be present if fewer than four of these criteria are met).
In terms of etiology, the most common identified etiological agent is Staphylococcus aureus (21-43%), followed by coagulase-negative staphylococci (17-39%), streptococci (7-12%), Gram-negative bacilli (2-12%), Enterococcus spp. (1-8%), anaerobic bacteria (2-6%), with no identified etiologic agent (4-12%) and Propionibacterium acnes (38%), frequently associated with THA [12].
Laboratory diagnosis of PJIs
Among the serological changes in patient’s blood, an ESR greater than 30 mm/h or a CRP above 10 mg/dl could suggest an acute infection with a sensitivity of 91-97%, a specificity of 70-80% and a negative predictive value of 96%, while in the case of an chronic infections (biofilm associated infection) the usefulness of these markers greatly decreases. The latest American consensus raises a question mark regarding the references values of this parameter because of differences between laboratories in which the samples analysed and because of the changes of this references values depending on the age, gender and comorbidities of the patient, may be elevated because of other inflammatory conditions or, may be between normal references values in the context of suppressive antimicrobial therapy or low-virulence organisms; the consensus also warns on the possibility that these serological markers might be elevated up to 60 d after surgery [29]. However, a normal CRP level along with a normal ESR is suggestive of a very low probability of infection. The roles of other markers, including interleukin-1 (IL-1) and -6 (IL-6), procalcitonin, and tumor necrosis factor-alpha still remain to be clarified.
The synovial fluid study in the case of an orthopedic implantassociated infection can highlight the following changes: a WBC count of greater than 4200/uL and greater than 80% granulocytes in the case of an hip prostheses, and in the case of the knee prosthesis a WBC count of greater than 1700/uL and with gather than 65% polymorphonuclear cells-values that can be applied for a period that does not exceeds no more than 2 months after surgery; above this time line, a WBC count of more than 25000/uL is necessary in the case of an PJI [12].
The common methods of diagnosis, such as cultures, most often do not indicate the presence of a microorganism (a sensitivity between 13.4% and 94.8%) depending also on the number of samples that are taken during the surgery-ideally should be harvested at least 3 specimens, but no more than 5 [29]. Thus, according to the European guideline, ESCMID guideline for diagnosis and treatment of infections associated with biofilm, published in 2014 [25], techniques such as electron microscopy or FISH (Fluorescence in situ hybridization) probes and fluorescence microscopy can reveal the presence biofilm with a sensitivity of 80%-100% [8]; techniques that can be associated with other non-culture-based techniques of fluid and tissue sample analysis through a detection by PCR (Polymerase Chain Reaction), quantitative PCR or multiplex PCR, Matrix Assisted Laser Desorption/ Ionization-Time of Flight Mass Spectrometry (MALDI-TOF MS), pyrosequencing, and next-generation sequencing, not all of this techniques are available for routine diagnostic work in the clinical microbiological laboratory. From the microscopic techniques that can reveal the presence of biofilm can be used: optical microscopy associated with Gram stains, technique that reveals the inflammatory cells, microorganisms and the biofilm matrix (IIA) [16]. Techniques such as Confocal Laser Scanning Microscopy (CLSM) and Scanning Electron Microscopy (SEM), are the best ways to reveal the presence of the biofilm, techniques that have the disadvantage of being unable to be performed in a routine manner (BIII) [30]. In addition to these techniques, the use of sonication is a cheap method, which significantly increases the diagnosis rate [31]. Following sonication the number of colony-forming units (CFU) is reported [25]. The orthopedic implant is inserted is a sterile container together with a variable quantity of saline solution or Ringer's Lactate solution and then inserted into a sonication bath where the sonication process produces strong enough micro air bubbles that generate the detach of the biofilm from the implant surface, thereafter the sonication fluid can be cultivated either on solid of fluid culture medium and techniques like FISH (IIA) or PCR can be applied [32,33]. Studies such as those of Bouza et al. and Percival et al. have shown that through sonication or centrifugation are recovered more colony-forming units of Candida spp. than through brewing [34,35].
In Romania, from 2012 sonication began to be used at The National Institute for Infectious Diseases “Prof. Dr. Matei Bals, the only published data is from July 2012-July 2014, study in which were included 39 of orthopedic implants (21 hip prostheses, 11 knee prostheses and 7 fixation devices), 9 breast implants, and 10 other devices (e.g. central venous catheter, drainage tube) [36].
Histopathological criteria for the diagnosis of PJI vary widely; there is a consensus according to which the presence of more than 5 neutrophils/ high power fields at 400X in 5 different areas creates a significant suspicion of infection.
Imaging techniques have an adjunctive role in the diagnosis of orthopedic implant-associated infection, plain radiographs are neither sensitive nor specific, but may be helpful in monitoring serial changes over time after implantation, changes such as: radiolucency at the metal-bone interface, loosening (radiolucency at the interface between the prosthesis and the bone cement-PMMA-polymethylmethacrylate), or implant migration. Imaging techniques like Computed Tomography (CT) scan or Magnetic Resonance Imaging (MRI) may have a contribution to the diagnosis, but a limited one. Radioisotope techniques as well as positron emission tomography-computed tomography (PET-CT, PET/CT) are more sensitive but with less specificity, due to the possibility of contrast substance uptake at level of the incision site up to several months after surgery [12].
Non-specific proinflammatory markers such as C-reactive protein, procalcitonin, eritorictelor sedimentation rate, leukocytes or different cytokines, cannot distinguish between infections caused by bacteria in a planktonic or biofilm state (DIII).
Synovial biomarkers
Deirmengian et al. evaluated 43 biomarkers that could potentially be diagnostic for PJI a small subset of representative aseptic and PJI samples, of these 16 were selected 16 biomarkers evaluated on a larger subset, the biomarkers were: human a-defensin 1-3 (a-defensin), IL-1a, IL-1, IL-6, IL-8, IL-10, IL-17, granulocyte colony-stimulating factor (G-CSF), Vascular Endothelial Growth Factor (VEGF), CRP, neutrophil elastase 2 (ELA-2), lactoferrin, Neutrophil Gelatinase-Associated Lipocalin (NGAL), resistin, thrombospondin, and bactericidal/permeability-increasing protein (BPI). Five biomarkers (a-defensin, ELA-2, BPI, NGAL, and lactoferrin) correctly predicted the diagnosis as defined by the Musculoskeletal Infection Society criteria, biomarkers that had a sensitivity of 100% (95% CI: 88%-100%) and a specificity of 100% (95% CI: 94%-100%) with AUC values of 1.000. In addition eight biomarkers (IL-8, CRP, resistin, thrombospondin, IL-1b, IL-6, IL-10, and IL-1a) demonstrated AUC values of greater than 0.9 [37].
Leukocyte Esterase (LE) is an enzyme secreted by activated neutrophils as a response to infections. Applying synovial fluid to a simple urine strip test and reading the results for LE is a reliable predictor of prosthetic joint infection (sensitivity=81%-93%; specificity=87%-100%). If the result of the LE test is ++, it is equivalent to synovial white blood cell count threshold for diagnosing prosthetic joint infection and is considered a minor by the International Consensus Meeting on PJI and by the Center for Disease Control and Prevention criterion for diagnosis of prosthetic joint infection. LE is a fast and inexpensive test. As a technical problem, if the aspirated sample is bloody, which occurs in about 33% of the cases, centrifugation of the aspirate at 6600 revolutions per minute for 2-3 min can help separate out red blood cells from the synovial fluid and make the colorimetric test accurate and therefore feasible. Elevated synovial level of protein and glucose and several types of antibiotics may interfere with LE results [38].
α-defensin is a peptide secreted into the synovial fluid by human cells. Its antimicrobial effect is via attachment to the pathogen`s cell wall. The concentration of α-defensin in synovial fluid is measured with an immunoassay test. The cutoff positive value is 5.2 mg/L or 4.8 μg/ml [37]. The test is not affected by bloody aspirates, antibiotic therapy, or systemic inflammatory diseases, and has sensitivity and specificity of 100% [37,38].
Harvesting samples of biological material that should be sent to the clinical microbiological laboratory to detect biofilm infections
In the case of infections associated with the surgical site, biopsy tissues are considered the most reliable samples to reveal biofilm. Using a swab to collect a sample of the biofilm from the wound surface, is an inappropriate method (DII) due to contamination with skin flora, the strong adherence of the biofilm to epithelium, and due to the growth of anaerobic bacteria in the depth of the surgical site/deep tissues. In the absence of the possibility of obtaining a biopsy from the debrited wound, it is recommended to obtain biological material on a cotton swab from the surface layers, swab that may help to establish or to provide some information regarding the choice of the antibiotic therapy (AII) [39,40].
In the case of a suspected orthopedic implant-associated infection, synovial fluid should be sampled and sent to the laboratory for work-ups. If microbiological results confirm the presence of infection, lavage and debridement surgery is indicated (AIII). Intraoperative sampling is recommended and includes obtain biopsies from representative periprosthetic tissue and removal of the device/prosthesis or modular parts of it (for example inlay, screws, head, liner)-the implant should be submitted to sonicatin followed by conducting cultures of the sonication fluid (BII) [25]. The biopsies should be as large as possible (up to 1 cm3).
Biofilm antimicrobial susceptibility testing
Routine tests to determine the susceptibility to antibiotics either by disc diffusion, or by micro dilution methods, are methods performed on bacteria that have a planktonic mode of growth or Candida spp. Assessing the bacterial susceptibility to antibiotics-as Susceptible, Intermediate and Resistant (S-I-R) and the Minimum Inhibitory Concentration (MIC), in most countries from Europe is by using the EUCAST (The European Committee on Antimicrobial Susceptibility the Testing) breakpoints. Biofilm-growing microorganisms are more resistant to antibiotics, break-points concentrations are not established so far, so that the S-I-R system cannot be used to predict the therapeutic success in treating biofilm infections. Biofilm infections that associate blood-stream infections originated from biofilm infections can be treated with antibiotics based on the results of routine antibiotic susceptibility testing (AIII) [16,41-44].
Tests that evaluate the susceptibility of biofilm growing bacteria also have been developed, tests that are included in the Calgary device. The Calgary Biofilm Device (CBD) consists of a two-part reaction vessel, the top component is a lid that has 96 pegs and that is sealed on the top so that the pegs can be removed without opening the vessel and allowing possible contamination, the biofilm-growing bacteria are exposed to different antibiotic concentrations in order to determine the biofilm eradication concentration. However, the results of these tests have not yet led to the prediction of reliable test models [41,45-47].
Management of Prosthetic Joint Infection
Surgery-related biofilm infections can be prevented by administration of prophylactic perioperative antibiotics (AI) [48]
There are studies clearly showed that the use of antibiotic-impregnated materials, such as PMMA/bone cement, often with gentamicin, but also with tobramycin or vancomycin, reduce the rate of infection of prosthesis-associated biofilm infections. (AI) [49,50].
The treatment of PJI consists of both general and local antibiotic therapy, associated with surgical intervention. There are mainly seven treatment methods: debridement and implant retention (DAIR), one or two stage revision arthroplasty with re-implantation, implant retrieval, arthrodesis, massive antibiotic therapy and final option-amputation [12].
The emergence of an infection in the first 3 weeks postoperatively, when biofilm is not fully formed, without any signs of loosening, fistula or an abscess, infection that is caused microorganisms that are susceptible to antibiotics with action on the biofilm an DAIR procedure can be performed, associated with mobile component exchange and intravenous antibiotics for at least six weeks after surgery (or 2 weeks of intavenous antibiotics+10 weeks of per of antibiotics), procedure that has a success rate of approximately 70-80% (less in the case of Staphylococcus infection). Rifampicin exhibits an activity on staphylococci and fluoroquinolones on Gram-negative bacilli, antibiotics that must be administered in association with other antibiotics to prevent rapid onset of resistant strains. This strategy has a AII recommendation [7,51-54].
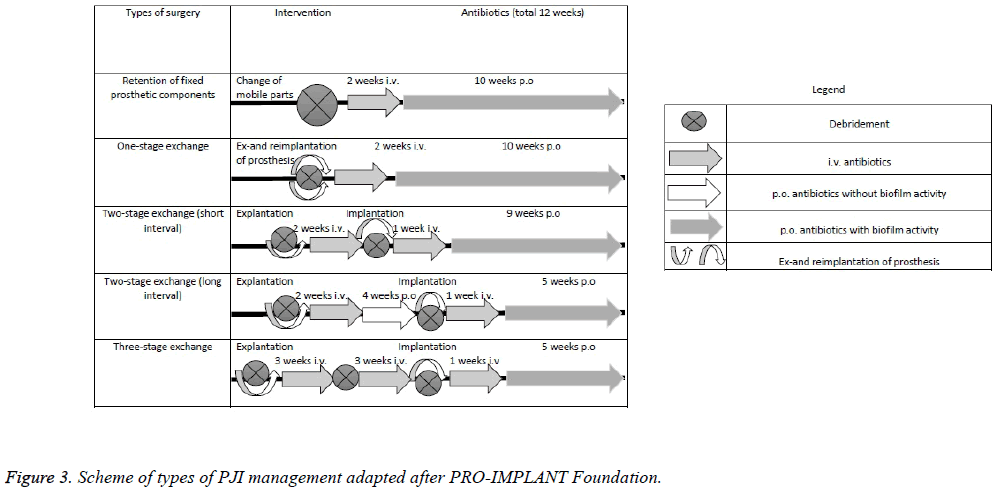
If the infection occurs after this period of 3 weeks from surgery, after a thorough debridement, prosthetic implant removal is mandatory [51]. There are two types of revision surgeries: one stage revision/exchange (when there is no active fistula or abscess, and the etiologic agent is susceptible to antibiotics; explanation and implantation is performed and antibiotic therapy is recommended: 2 weeks of intravenous antibiotics followed by 10 weeks of per antibiotics) or two stage revision/exchange (in cases with resistant bacteria strains and presence of fistula or abscess; the first step is lavaje and debridement followed by removal of the implant components with the possibility of implantation of an antibioticimpregnated polymethylmethacrylate bone-cement spacers, followed by a brief pause of 2-4 weeks (without antibiotics) or one, long, of 6-8 weeks (in this case it is recommended to 6 weeks without antibiotic, and 2 with antibiotics)-BII-and phase 2 consists of the implantation of prosthesis followed by 6 weeks of antibiotic treatment in case of a short pause, or variable duration in the case of long pauses [12,55]. According to the PRO-IMPLANT Foundation the two-stage exchange can also be performed as an two-stage exchange with short interval (debridement+explanation → 2 weeks of i.v. antibiotics → debridement+implantation → 1 week of i.v. antibiotics+9 weeks of p.o antibiotics) or as an two-stage exchange with long interval (debritment+explanation → 2 weeks of i.v. antibiotics +4 weeks of p.o antibiotics → debridement+implantation → 1 week of i.v. antibiotics+5 weeks of p.o antibiotics). Also according to the PRO-IMPLANT Foundation there is the possibility of performing a three-stage exchange (debritment +explanation → 3 weeks of i.v. antibiotics → debritment → 3 weeks of i.v. antibiotics → debridment+implantation → 1 week of i.v. antibiotics+5 weeks of p.o antibiotics) (Table 4 and Figure 3).
| Antibiotic | Class | Spectrum | Mechanism of action | Side effects | Serum half-time | Remarks |
|---|---|---|---|---|---|---|
| Rifampicin | Rifamycin | Gram-positive and -negative bacteria | Bactericidal Inhibition of bacterial RNA synthesis | Nausea, gastrointestinal disturbances, hepatotoxicity, thrombocytopenia, rash, red discolorationof urine, flu-like symptoms | t1/2:4h | Should be used in combination with other drugs due to the rapid emergency of bacterial resistance |
| Daptomycin | Lipopetide | Gram-positive bacteria including MRSA, VRSA, VRE, and PRSP. Log- and stationary-phase of bacteria | Bactericidal Insertion of hydrophobic tail into cell membrane, resulting in membrane depolarization and cell death | Nausea, vomiting, diarrhoea, hypertension and hypotension, myopathy, neuropathy, urethritis, anemia, hypokalemia, arthralgia | t1/2:9h | |
| Linezolid | Oxazolidinones | Gram-positive bacteria including MRSA, MSSA, CoNS, and enterococci includingVRE. Good tissue distribution and bioavailability | Bacteriostatic Binds to the bacterial 23S ribosomal RNA of the 50S subunit, thus preventing the formation of a functional 70S complex. Production by MSSA and MRSA | Nausea, vomiting, diarrhoea, thrombocytopenia, myelosuppression, reversible optic neuritis, irreversible peripheral neuropathy, serotonin syndrome | t1/2:6h | |
| Tigecycline | Glycylcylines | Active against Gram-positive bacteria (including VRE and MRSA), Gram-negative bacilli, and anaerobes | Bacteriostatic Binds 30S bacterial ribosomal subunit and prevents binding of tRNA to the mRNA ribosome complex | Nausea, vomiting, diarrhoea, sore mouth and throat, dysphagia, vitamin B complex deficiency, dental abnormalities, hepatotoxicity | t1/2:42h | |
| Minocycline | Tetracyclines | Active against Gram-positive bacteria (including VRE and MRSA), Gram-negative bacilli including Neisseria meningitidis and anaerobes | Bacteriostatic Binds 30S bacterial ribosomal subunit and prevents binding of tRNA to the mRNA ribosome complex | Nausea, vomiting, diarrhoea, sore mouth and throat, dysphagia, vitamin B complex deficiency, dental abnormalities, hepatotoxicity, vestibular disturbances with dizziness, tinnitus, and impaired balance-especially in women | t1/2:15h | |
| Vancomycin | Glycopeptides | Gram-positive bacteria. MRS | Bactericidal Inhibits bacterial cell wall formation Interferes with peptidoglycan synthesis | Tinnitus, deafness (reversible on cessation of drug), nephrotoxicity, maculopapular rash (with rapid i.v. infusion) | t1/2:8h |
Table 4: Presumptive antibiotics that are considered to be active on biofilm.
Suppressive long term per os antibiotics therapy may be taken into account for cases where the retrieval of the prosthetic components cannot be performed. Possible indications include poor general condition, cases where the retrieval of components leads to poor functional results and patient desire. The purpose of long-term antibiotic therapy is asymptomatic functioning of the prosthesis and not eradication of the infection. Promising results were reported by Rao et al. that approximately 86% of patients on a medium fallow-up [56].
What about Genetics?
From recent data available from cancer research studies, scientists realized that enhancing patient’s immune system to fight against infections, on one hand, may be more efficacious than the use of high toxicity systemic drugs. The use of immunotherapy, either by the use of vaccines or agents that target microorganisms, might be a promising way to battle against infections. Combining the data from cancer research and the one from the human genome project, genetic susceptibility to prosthetic joint infection in being revealed. This data suggests that the polymorphisms of C allele and genome type C/C for mannose binding lectin MBL-single nucleotide polymorphisms SNP, genotype A/A for MBL-54 SNP increases the risk of prosthetic joint infection. G allele and genotype G/G for MBL-550 SNP reduces the risk for prosthetic joint infection in the Caucasian population [27].
Conclusions
The diagnosis and management of orthopedic implantsassociated infections still remain a problem. Prosthetic joint infection still remains the most common and feared arthroplasty complication. Despite the scientific progress in the last years, the incidence of infections is increasing, both related to an increase number of primary interventions and to the emergence of drug resistant microorganisms. There still are a lot of questions without available answers. Is it recommended to use systemic antibiotics or just local antibiotics for treating infections? Can we trust bacterial cultures for bacteria that grow in colonies? Should we associate sonication regularly? Should we sacrifice bone vascularization through remaining after extraction of an infected implant? Because we know that the biofilm develops on surfaces. The presence of the "3 weeks window" is the key point in time where either we won the battle for the "surface" or we lost it. Long-term antibiotic therapy is necessary?
The existence of adapted protocols for managing biofilm infections and the new diagnostic methods, improved the rate of infection eradication, without a 100% certainty that we have eradicated it. Well-equipped treatment centers for diagnosis and multidisciplinary teams (orthopedic surgeon, infection diseases specialist, and microbiologist) are mandatory to offer the chance of an orthopedic implant-associated biofilm infections well-management.
References
- Douglas RO, Elie FB, Anthony RB, Daniel L, Werner Z, James MS. Executive summary: diagnosis and management of prosthetic joint infection: Clinical Practice Guidelines by the Infectious Diseases Society of America. Clin Infect Dis 2013; 56: 1-10.
- CDC. Center for Disease Control and Prevention 2010.
- Cram P. Physicians weekley 2013.
- Montanaro L, Speziale P, Campoccia D, Ravaioli S, Cangini I. Scenery of Staphylococcus implant infections in orthopedics. Future Microbiol 2011; 6: 1329-1349.
- Stoodley P, Kathju S, Hu F, Erdos G, Levenson J, Mehta N. Molecular and imaging techniques for bacterial biofilms in joint arthroplasty infections. ClinOrthopRelat Res 2005; 437: 31-40.
- Johannsson B, Taylor J, Clark CR, Shamsuddin H, Beekmann SE. Treatment approaches to prosthetic joint infections: results of an Emerging Infections Network survey. DiagnMicrobiol Infect Dis 2010; 66: 16-23.
- Cataldo MA, Petrosillo N, Cipriani M, Cauda R, Tacconelli E. Prosthetic joint infection: recent developments in diagnosis and management. J Infect 2010; 61: 443-448.
- Gbejuade HO, Lovering AM, Webb JC. The role of microbial biofilms in prosthetic joint infections. ActaOrthop 2015; 86: 147-158.
- Kapadia BH, Berg RA, Daley JA, Fritz J, Bhave A. Periprosthetic joint infection. Lancet 2016; 387: 386-394.
- Wolcott RD, Ehrlich GD. Biofilms and chronic infections. JAMA 2008; 299: 2682-2684.
- Rochford ET, Richards RG, Moriarty TF. Influence of material on the development of device-associated infections. ClinMicrobiol Infect 2012; 18: 1162-1167.
- Domizia S. Infections after total joint arthroplasty. CRC Course during the 16th EFORT Congresss Prague 2015.
- Ramage G, Robertson SN, Williams C. Strength in numbers: antifungal strategies against fungal biofilms. Int J AntimicrobAgents 2014; 43: 114-120.
- Høiby N. A personal history of research on microbial biofilms and biofilm infections. Pathog Dis 2014; 70: 205-211.
- Hoiby N, Bjarnsholt T, Givskov M, Molin S, Ciofu O. Antibiotic resistance of bacterial biofilms. Int J Antimicrob Agents 2010; 35: 322-332.
- Bjarnsholt T, Jensen P, Fiandaca M, Pedersen J, Hansen C, Andersen C. Pseudomonas aeruginosa biofilms in the respiratory tract of cystic fibrosis patients. PediatrPulmonol 2009; 44: 547-558.
- Zimmerli W, Trampuz A, Ochsner PE. Prosthetic-joint infections. N Engl J Med 2004; 351: 1645-1654.
- Trampuz A, Zimmerli W. Diagnosis and treatment of implant-associated septic arthritis and osteomyelitis. Curr Infect Dis Rep 2008; 10: 394-403.
- Parvizi J, Jacovides C, Antoci V, Ghanem E. Diagnosis of periprosthetic joint infection: the utility of a simple yet unappreciated enzyme. J Bone Joint Surg Am 2011; 93: 2242-2248.
- Tsukayama DT, Estrada R, Gustilo RB. Infection after total hip arthroplasty. A study of the treatment of one hundred and six infections. J Bone Joint Surg Am 1996; 78: 512-523.
- Tsukayama DT, Goldberg VM, Kyle R. Diagnosis and management of infection after total knee arthroplasty. J Bone Joint Surg Am 2003; 1: 75-80.
- McPherson EJ, Tontz W, Patzakis M, Woodsome C, Holtom P. Outcome of infected total knee utilizing a staging system for prosthetic joint infection. Am J Orthop (Belle Mead NJ) 1999; 28: 161-165.
- McPherson EJ, Woodson C, Holtom P, Roidis N, Shufelt C. Periprosthetic total hip infection: outcomes using a staging system. ClinOrthopRelat Res 2002; 8-15.
- Cierny G, Mader JT, Penninck JJ. A clinical staging system for adult osteomyelitis. ClinOrthopRelat Res 2003; 7-24.
- Hoiby N, Bjarnsholt T, Moser C, Bassi GL, Coenye T. ESCMID guideline for the diagnosis and treatment of biofilm infections 2014. ClinMicrobiol Infect 2015; 21: 1-25.
- Ullmann A, Cornely O, Donnelly J, Akova M, Arendrup M, Arikan-Akdagli S. ESCMID* guideline for the diagnosis and management of Candida diseases 2012: developing European guidelines in clinical microbiology and infectious diseases. ClinMicrobiol Infect 2012; 18: 1-8.
- Parvizi J, Haddad FS. Periprosthetic joint infection: the last frontier. Bone Joint J 2015; 97: 1157-1158.
- Bode LKJ, Wertheim H, Bogaers D, Vandenbroucke-Grauls C, Roosendaal R, Troelstra A. Preventing surgical-site infections in nasal carriers of Staphylococcus aureus. N Engl J Med 2010; 362: 9-17.
- http://www.aaos.org/AAOSNow/2011/Nov/clinical/clinical1/?ssopc=1
- Malic S, Hill KE, Hayes A, Percival SL, Thomas DW. Detection and identification of specific bacteria in wound biofilms using peptide nucleic acid fluorescent in situ hybridization (PNA FISH). Microbiology 2009; 155: 2603-2611.
- Sorli L, Puig L, Torres-Claramunt R, Gonzales A, Alier A, Knobel H. The relationship between microbiology results in the second of a two-stage exchange procedure using cement spacer and the outcome after revision total joint replacement for infection: the use of sonication to aid bacteriological analysis. J Bone Join Surg Br 2012; 94: 249-253.
- Trampuz A, Osmon DR, Hanssen AD, Steckelberg JM, Patel R. Molecular and antibiofilm approaches to prosthetic joint infection. ClinOrthopRelat Res 2003; 69-88.
- Trampuz A, Piper KE, Jacobson MJ, Hanssen AD, Unni KK. Sonication of removed hip and knee prostheses for diagnosis of infection. N Engl J Med 2007; 357: 654-663.
- Bouza E, Alcala L, Munoz P, Martin-Rabadan P, Guembe M, Rodriguez-Creixems M. Can microbiologists help to assess catheter involvement in candidaemic patients before removal? ClinMicrobiol Infect 2013; 19: 29-35.
- Percival S, Kite P, Eastwood K, Murga R, Carr J, Arduino M. Tetrasodium EDTA as a novel central venous catheter lock solution against biofilm. Infect Control HospEpidemiol 2005; 26: 515-519.
- Daniela T, Raluca M, Olga D, Vlad P, Rodica M, Olivera L. Sonication-further progress in the microbiological diagnosis in implant-associated infections. BMC Infectious Diseases 2014; 14: 37.
- Deirmengian C, Kardos K, Kilmartin P, Cameron A, Schiller K, Parvizi J. Diagnosing periprosthetic joint infection: has the era of the biomarker arrived? ClinOrthopRelat Res 2014; 472: 3254-3262.
- Enayatollahi MA, Parvizi J. Diagnosis of infected total hip arthroplasty. Hip Int 2015; 25: 294-300.
- Percival SL, Hill KE, Williams DW, Hooper SJ, Thomas DW. A review of the scientific evidence for biofilms in wounds. Wound Repair Regen 2012; 20: 647-657.
- Lipsky B, Berendt A, Cornia P, Pile J, Peters E, Armstrong D. Executive summary: 2012 Infectious Diseases Society of America clinical practice guideline for the diagnosis and treatment of diabetic foot infections. Clin Infect Dis 2012; 54: 679-684.
- Doring G, Flume P, Heijerman H, Elborn JS; Consensus Study Group. Treatment of lung infection in patients with cystic fibrosis: current and future strategies. J Cyst Fibros 2012; 11: 461-479.
- Kizilbash QF, Petersen NJ, Chen GJ, Naik AD, Trautner BW. Bacteremia and mortality with urinary catheter-associated bacteriuria. Infect Control HospEpidemiol 2013; 34: 1153-1159.
- Hoiby N, Johansen HK, Ciofu O, Jensen PO, Bjarnsholt T. Foreign body infections-biofilms and quorum sensing. UgeskrLaeger 2007; 169: 4163-4166.
- Rodríguez-Tudela JL, Arendrup MC, Cuenca-Estrella M, Donnelly JP, Lass-Florl C. EUCAST breakpoints for antifungals. Drug News Perspect 2010; 23: 93-97.
- Moskowitz S, Emerson J, McNamara S, Shell R, Orenstein D, Rosenbluth D. Randomized trial of biofilm testing to select antibiotics for cystic fibrosis airway infection. PediatrPulmonol 2011; 46: 184-192.
- Moskowitz S, Foster J, Emerson J, Burns J. Clinically feasible biofilm susceptibility assay for isolates of Pseudomonas aeruginosa from patients with cystic fibrosis. J ClinMicrobiol 2004; 42: 1915-1922.
- Waters V, Ratjen F. Standard versus biofilm antimicrobial susceptibility testing to guide antibiotic therapy in cystic fibrosis. Cochrane Database Syst Rev 2012; 11: 009528.
- Song Z, Borgwardt L, Hoiby N, Wu H, Sørensen T, Borgwardt A. Prosthesis infections after orthopedic joint replacement: the possible role of bacterial biofilms. Orthop Rev (Pavia) 2013; 5: 65-71.
- Parvizi J, Saleh KJ, Ragland PS, Pour AE, Mont MA. Efficacy of antibiotic-impregnated cement in total hip replacement. ActaOrthop 2008; 79: 335-341.
- Marschall J, Lane M, Beekmann S, Polgreen P, Babcock H. Current management of prosthetic joint infections in adults: results of an Emerging Infections Network survey. Int J Antimicrob Agents 2013; 41: 272-277.
- Osmon D, Berbari E, Berendt A, Lew D, Zimmerli W, Steckelberg J. Diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis 2013; 56: 1-25.
- Zhao X, Drlica K. Restricting the selection of antibiotic-resistant mutant bacteria: measurement and potential use of the mutant selection window. J Infect Dis 2002; 185: 561-565.
- Vilchez F, Martinez-Pastor J, Garcia-Ramiro S, Bori G, Macule F, Sierra J. Outcome and predictors of treatment failure in early post-surgical prosthetic joint infections due to Staphylococcus aureus treated with debridement. ClinMicrobiol Infect 2011; 17: 439-444.
- Parvizi J, Gehrke T, Chen AF. Proceedings of the International Consensus on Periprosthetic Joint Infection. Bone Joint J 2013; 95: 1450-1452.
- Sendi P, Zimmerli W. Antimicrobial treatment concepts for orthopaedic device-related infection. ClinMicrobiol Infect 2012; 18: 1176-1184.
- Rao N, Crossett LS, Sinha RK, Le Frock JL. Long-term suppression of infection in total joint arthroplasty. ClinOrthopRelat Res 2003; 55-60.