- Biomedical Research (2009) Volume 20, Issue 1
Cytogenetic study on genotoxicity of antitumor-antibiotic Mitomycin C
Yogesh A Sontakke* and R R FulzeleDepartment of Anatomy, J N Medical College, Wardha- 442004, MS, India.
- *Corresponding Author:
- Yogesh A Sontakke
Department of Anatomy, Jawaharlal Nehru Medical College
Sawangi (M), Wardha, M.S., India.
e-mail: dryogeshas@rediffmail.com
Accepted date: October 26 2008
Abstract
Mitomycin C (MMC) is antitumor-antibiotic, currently in use for treatment of various solid tumors. The effect of different dosages of MMC was tested in human lymphocyte culture after exposure for different duration. The exposure of 0.05μg/ml MMC for 24 hours was not sufficient to induce mitotic inhibition and chromosomal aberrations. The cells exposed to concentration 2.0μg/ml and above for last 24 hours showed complete inhibition of mitotic activity. Low concentration exposure for long duration inhibits mitosis rather than exposure to high concentration for short duration. Mitotic indexes in after exposure of MMC for same duration were decreased significantly with the increase in the dose of MMC. The highest frequency of chromosomal aberrations was observed in cultures treated with 0.5μg/ml MMC for the last 24 hours of culture. MMC did not induce chromosomal aberrations randomly; which more commonly affected chromosome 1, 9 and 16. Most commonly affected was chromosome 9 followed by the involvement of chromosome 1 and least was the involvement of chromosome 16. The non random interchange breakages induced in these chromosomes were significantly at secondary constriction region i.e., site of paracentric heterochromatin, most commonly that of chromosome 9.
Keywords
Mitomycin C, chromosomal aberrations, heterochromatin, genotoxicity
Introduction
The ideal antitumor drug should produce maximum mitotic inhibition of tumor cells with minimum side effects. To achieve this, the dose of the drug in the lowest possible concentration should be administered. Maximum chemotherapeutic drugs are first studied in animals but cytogenetic studies involving culture of human cells in which action of drug can be assessed in multiplying human cells. Mitomycin C (MMC) is a chemically reactive antitumor-antibiotic derived from Streptomyces caespitosus [1]. MMC had been in clinical use for more than 30 years. MMC has proved effective in the frontline treatment of many solid tumors such as superficial bladder cancer [2], gastric carcinoma [3], pancreatic neoplasias [4], anal carcinomas and esophageal carcinomas [5]. It is also used in combinations with other anti-tumor agents in palliative treatment of many advanced cancers or cancers that have become resistant to other forms of therapy [6]. The activity of this agent in solid tumors and its enhanced effectiveness against hypoxic cells those are resistant to radiation [7]. This increased interest in MMC and investigations of new approaches that increasing its effectiveness.
The usual side effect of currently practiced dose of MMC is myelosuppression which is dose dependent and increases with dosage >10 to 20 mg/m2. This toxicity is dose limiting [3,8]. There is need of further evaluation to find new dose regimen for MMC to enhance its therapeutic effects and minimize side effects.
The primary action of MMC has been considered to inhibit the deoxyribonucleic acid (DNA) synthesis. Very few workers have studied the cytogenetic effects of MMC on human chromosomes in lymphocyte culture. There were many short comings of these Cytogenetic studies. Some of them have used very few samples for testing effects of MMC on chromosomes; some have used crude method for identification of chromosomes while some have used other DNA damaging drugs simultaneously with MMC [9-13]. This indicated that there was need of further evaluation of MMC actions on chromosomes in lymphocyte culture to overcome all the short comings of previous studies. Hence present study was undertaken to provide the basis for evaluating antitumor effects MMC with regards to its effects on specific sites and specific regions of chromosomes of human lymphocytes.
Materials and Methods
Preheparizined syringe was used to collect 3 ml venous blood from each one of the 100 healthy individuals. 100 samples so collected were grouped in to group A (40 samples), group B (20 samples), group C (20samples) and group D (20 samples) and processed in following manner.
Each sample of peripheral blood was cultured in RPMI1640medium (Himedia) supplemented with 10% FBS (Himedia) and 10 μg PHA/ml (Geni) and incubated at 37°C for 72 hrs. Group A was not exposed to MMC, Group B was exposed to six different concentrations (subgroup B1-B6: 0.05, 0.1, 0.5, 1.0, 2.0, 5.0 μg/ml med-ium respectively) of MMC (Sigma, USA) for 24 hours of culture, Group C was exposed to two different concentra-tions (subgroup C1 and C2: 0.05, 0.1μg/ml respectively) for whole 72 hours. Each sample of group D, after 24 hrs of incubation, was exposed to two different concentra-tions (subgroup D1 and D2: 0.5, 1.0μg/ml respectively) for 1 hour. After 25th hr, cells were washed with RPMI 1640 medium thrice to obtain a MMC free cell suspension and cells were resuspended in fresh medium and reincub-ated for remaining period. One hr prior to harvesting 100 μL Colchicine (Himedia) (0.25 μL/ml) was added to each culture tube. Cells were centrifuged at 1000rpm for 10min. Cells were suspended in 5ml of prewarmed (37°C) 0.075M KCl (hypotonic solution) and incubated at 37°C for 10min. Cells were resuspended in 5ml of chilled fixative (methanol: glacial acetic acid: 1: 3). The fixative was removed by centrifugation and the procedure was repeated thrice. To prepare slides 2-3drops of fixed cell suspension were dropped on clean slides and air dried. After treatment of 0.1% Trypsin-EDTA for 12-20 seco-nds, slides were stained in 5%Giemsa solution in phosph-ate buffer (pH 6.8) for 4-5min. Slides were screened using Olympus CX31(Japan) microscope and microphotograp-hed. Data was analyzed using S.P.S.S. version 14.0 soft-ware.
Observations and Results
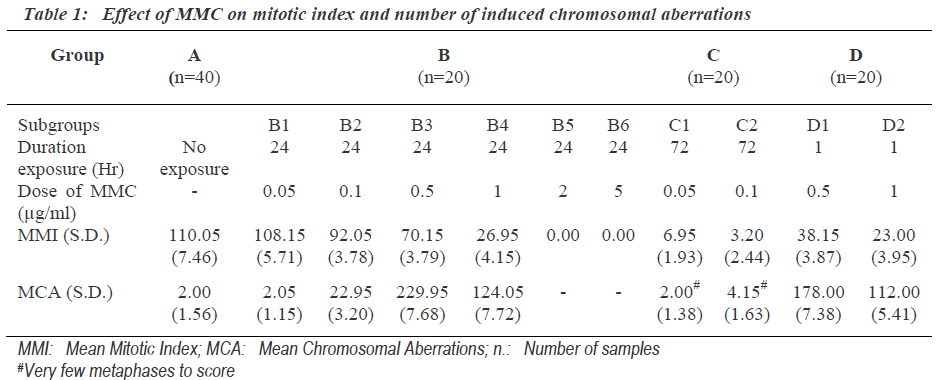
Mitotic index indicates proliferating activity of cultured cells. The mitotic index (Mitotic Index = Number of cells in mitosis per 1000 observed cells) was determined after exposure to different dosages of MMC for different duration (Table 1). The exposure of 0.05μg/ml MMC for 24 hours (subgroup B1) was not sufficient to induce mito-tic inhibition. The cells exposed to concentration 2.0μg/ ml and above for 24 hours (group- B5-6) showed complete inhibition of mitotic activity. Low concentration (0.05, 0.1μg/ml) MMC exposure for long duration of 72 (group C) hours inhibits mitosis rather than exposure to high concentration (0.5, 1.0μg/ml) for short duration of 1 hour (group D). Mitotic indexes for same duration of exposure were decreased significantly with the increase in the dose of MMC. The number of chromosomal aberra-tions induced after different doses of MMC for different time exposure is shown in Table 1. The frequencies of total chromosome aberrations in each group were express-ed per 100 metaphases examined.
The concentration 0.05μg/ml MMC was not sufficient to induce chromosomal aberration though the exposure was for the last 24 hours (subgroupB-1) (Table 1). Where as cultures treated with 0.5μg/ml of MMC for same duration i.e., last 24 hours showed the highest frequency of chromosomal aberrations. But cultures exposed to still higher concentration (1.0μg/ml) showed less number of chromosomal aberrations. This indicated that the frequen-cy of chromosomal aberration was not dose dependent. Cultures with MMC exposure to concentrations of (0.5μg/ ml) FOR 24 hours showed higher number of chromoso-mal aberrations than the cultures exposed to same concentration for 1 hour. This indicated that the frequency of chromosomal aberration was dependent on duration of exposure.
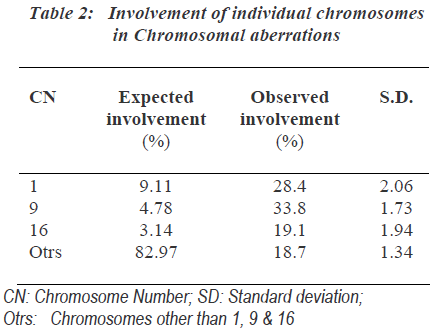
As the group B-3 exposed to 0.5μg/ml MMC for 24hours showed highest number of chromosomal aberrations, it was most suitable for further analysis of chromosomal aberrations. One hundred involved chromosomes in chro-mosomal aberrations from each sample of group-B-3 were identified and the involvement of individual chro-mosomes was counted (Table 2). The expected involve-ment of individual chromosome in chromosomal aberration induced by any drug was determined according to number of bases present in individual chromosome as per the available data of compiled statistics for the chro-mosomes as provided by the human genome information of Sanger Institute in the Vertebrate Genome Annotation database [14]. MMC did not induce chromosomal abe-rrations randomly and it more commonly affected chromosomes were 1, 9 as well as 16.
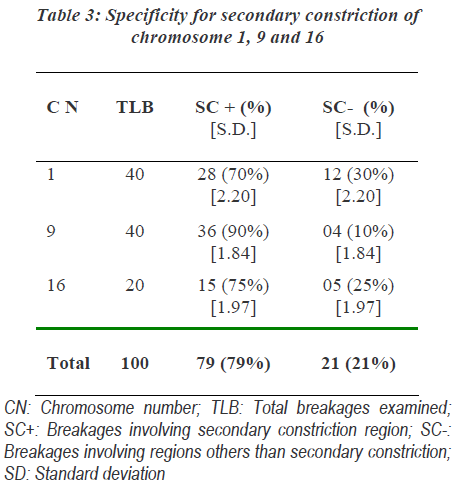
The involvement of these chromosomes was found to be significantly more than that expected as per the human genome information of Sanger Institutes. Out of chro-mosome 1, 9 and 16; chromosome 9 was more commonly affected followed by the involvement of chromosome 1 and chromosome 16 showed least involvements. Specifi-city of action of MMC to specific region of chromosome was found. The non random interchange breakages indu-ced in chromosome 1, 9 and 16 showed significant invol-vement of secondary constriction region. Secondary cons-triction region of chromosome 9 was most commonly involved (Table 3).
Discussion
Mitomycin C is an antitumor antibiotic. Due to its clinical applications, it is a centre of biomedical research. The primary action of MMC has been considered to inhibit the deoxyribonucleic acid (DNA) synthesis by cross-linking of the complimentary strands of DNA [15]. This would inhibit DNA replication and probably this might be the antitumor action of this drug. Very few workers have studied the effects of MMC on human chromosomes that were with many drawbacks. Cohen MM et al and Shaw MW et al used five samples and during that period, G-banding technique and individual chromosome identification criteria were not available [9-10]. Latt SA found in five individuals MMC-induced sister chromatid exchanges (SCE) in lymphocytes grown in medium containing 5-bromodeoxyuridine (5-BRDU) [11]. 5-BRDU itself was an agent which produces chromosomal damage. The observations in his study might be summative effects of two DNA –damaging agents i.e., 5-BRDU and MMC. Tchun TS et al studied MMC effects only in one individual [12]. Abdel-Halim et al used sample from one individual with 5-BRDU pretreatment. To overcome these short comings, in the present study, samples from 100 healthy individuals were studied [13]. The observations of the present study suggested that lower concentrations MMC exposure for long duration inhibited mitosis significantly more than exposure to higher concentrations for short duration. Thus, during chemotherapy, rather than giving high dosage of MMC at long intervals, it is better to give small doses of MMC repeatedly to cause maximum inhibition of mitotic activity of tumors so that toxicity will be minimal. Currently practiced standard dose of MMC is as intravenous bolous at 15-20 mg/m2 on day 1 of a 6 to 10 week cycle [3]. The usual dose-limiting toxicity of MMC is myelosuppression and this toxicity is dose related, increasing with doses >10 to 20 mg/m2 [8]. By using such methods of sustained release of MMC, MMC can be used at low concentration with sustained release which will be more beneficial for reduction of toxicity of MMC. The results of the present study suggested that MMC did not induce chromosomal aberrations randomly; t more commonly affected chromosome 1, 9 and 16.
The involvement of these chromosomes was significantly more than that of expected as per the human genome information of Sanger Institutes. It most commonly affected chromosome 9 followed by the involvement of chromosome 1 and least involvement of chromosome 16. In human chromosomes large segments of proximal heterochromatin in the secondary constriction regions appear most frequently in chromosome 1, 9 and 16 [16]. MMC induced chromatid breaks in the heterochromatic regions of chromosomes of plant Vicia faba root tip [17]. The observations of the present study suggested that MMC acts commonly on heterochromatin at secondary constrictions of chromosome 1, 9 and 16; most common was chrmosome 9.
Fauth E et al showed that MMC-induced micronuclei revealed the preferential occurrence of parts of chromo-some 9 by using interphase FISH technique [18]. This finding strongly correlated with preferential underconden-sation of heterochromatin of chromosome 9 [9]. Similar correlation had been noted in lymphocytes treated with 5-azacytidine [19] and idoxuridine [20]. Heterochromatin of chromosome 9 had unique DNA composition which had been shown to contain a mixture of various repeated sequences [21]. Molecular study by Kumar S et al indicated that MMC is selective for guanine nucleosides in the sequence 5’-CpG-3’ (CpG) [22]. Induction of DNA lesions by MMC is enhanced when the cytosines of these MMC-susceptible sequences are 5-methylated [23]. Para-centromeric heterochromatic bands have been reported to contain GC-rich repeats and harbor highly methylated DNA [24]. Thus, the findings of present study i.e., a non-uniform distribution of MMC induced interchanges among human chromosomes with a striking preference for heterochromatic region of chromosome1, 9 and 16 can be explained as per molecular basis. Secondary constric-tions correspond to late-replicating regions of DNA [25]. DNA damage to this secondary constriction regions with non-transcribed DNA was repaired less rapidly than in euchromatin [26]. This explains that MMC affected CpG sequences all over the DNA but the affected heterochro-matin regions did not get repaired and it was seen to be affected more than other regions. Thus the present study with the use of human cells may be useful model study for DNA damaging drugs.
Acknowledgements
This study was supported by Datta Meghe Institute of Medical Sciences University, Nagpur, India. Thanks are due to Dr. Vedprakash Mishra, Vice-chancellor, DMIMS University and Dr. S.R. Joharapurkar, Director, DMDPG-MER for keen interest and encouragement; Dr. S.S. Patel, Dean, JN Medical College, Wardha, for coopera-tion; Dr. S. Z. Quazi, Asso. Prof., Community Medicine, JNMC, for help in statistical analysis.
References
- Hata T, Sano Y, Sugawara R, Matsumac A, Kanamori K, Shima T, Hoshi T. Mitomycin, a new antibiotic from streptomyces. I J Antibiot 1956; 9: 141-146.
- Crawford ED. Diagnosis and treatment of superficial bladder cancer: an update. Semin Urol Oncol 1996; 14: 1-9.
- Schnall S, Macdonald JS. MMC therapy in gastric cancer. Oncol 1993; 50: 70-77.
- Kelsen D. The use of chemotherapy in the treatment of advanced gastric cancer and pancreas cancer. Semin Oncol 1994; 21: 58-66.
- Coia L. The use of MMC in esophageal cancer Oncol 1993; 50(1): 53-62.
- Miller TP, Mc Mahon LJ, Livingston RB. Extensive adenocarcinoma and large cell undifferentiated carci- noma of the lung treated with 5-FU, vincristine and MMC. Cancer Treat Rep 1980; 64: 1241-1245.
- Workman P, Stratford IJ. The experimental develop- ment of bioreductive drugs and their role in cancer therapy. Cancer Metastasis Rev 1993; 12: 73-82.
- Doll DC, Weiss RB, Issell BF. Mitomycin: ten years after approval for marketing. J Clin Oncol 1985; 3: 276-286.
- Cohen MM, Shaw MW. Effects of MMC on human chromosomes. J Cell Biol 1964; 23: 386-395.
- Shaw MW, Cohen MM. Chromosome exchange in human leucocytes induced by MMC. Genet 1965; 181- 190.
- Latt SA. Sister chromatid excanges, indices of human chromosome damage and repair: Detection by fluores- cence and induction by MMC. Proc Nat Acad Sci USA. 1974; 71: 3162-3166.
- Abdel-Halim HI, Natarajan AT, Mullenders LHF, Boei WA. MMC-induced pairing of heterochromatin reflects initiation of DNA repair and chromatid exchange formation. J Cell Science 2005; 118: 1757-1767.
- Tchun TS, Hahn, Kim DS. The effect of MMC on human chromosomes with G banding patterns. Yonsei Med J 1976; 17(2): 115-130.
- http: //vega.sanger.ac.uk/Homo_sapiens/index.html
- Iyer VN, Szybalski W. Mitomycin and Porfiromycin : Chemical mechanism of activation and cross-linking of DNA . Science 1964; 145(3627): 55-58.
- Patau K. The identification of individul chromosomes especially in man. Am J Hum Genet 1960; 12: 250- 276.
- Merz T. Effect of MMC on lateral root tip chromo- somes of Vicia faba. Science 1961; 133: 329.
- Fauth E, Scherthan H, Zankl H. Chromosome painting reveals specific patterns of chromosome occurrence in MMC and diethyl stilbostrol-induced micronuclei. Mutagen 2000; 15(6): 459-467.
- Fauth E, Zankl H. Comparison of spontaneous and ido- xuridine- induced micronuclei by chromosome painting. Mutat Res 1999; 440: 147-156.
- Lee C, Wevrick R, Ferguson-Smith MA, Lin CC. Human centromeric DNAs. Human Genet 1997; 100: 291-304.
- Kumar S, Lipman R, Tomasz M. Recognition of speci- fic DNA sequence by mitomycin C for alkylation. Biochem 1992; 31: 1399-1407.
- Li VS, Tang MS, Kohn H. The effect of C(5) cytosine methylation at CpG sequences on mitomycin-DNA bonding profiles. Bioorg Med Chem 2001; 9: 863-873.
- Meneveri R, Agresti A, Marozzi A, Saccone S, Rocchi M, Archidiacono N, Corneo G, Valle GD, Ginelli E. Molecular organization and chromosomal location of human GC-rich heterochromatic blocks. Gene 1993; 123: 227-234.
- Schimid W. DNA replication patterns of human chromosomes. Cytogent 1963; 2: 4-5.
- Bohr VA, Wassermann K. DNA repair at the level of the gene. Trends Biocem Sci 1988; 13: 429-433.