Research Article - Biomedical Research (2017) Volume 28, Issue 8
Comparison of efficacy of linezolid and vancomycin for treatment of hospitalacquired pneumonia: A meta-analysis
Li Ma1, Xiaojuan Zhang1, Xingqin Zhao1, Lujing Zhao2 and Yuanyuan Qiao3*1Department of Emergency, Affiliated Hospital of Jining Medical College, Jining, PR China
2Department of Cardiology, Affiliated Hospital of Jining Medical College, Jining, PR China
3Intensive Care Unit, Affiliated Hospital of Jining Medical College, Jining, PR China
- *Corresponding Author:
- Yuanyuan Qiao
Intensive Care Unit
Affiliated Hospital of Jining Medical College, PR China
Accepted on November 29, 2016
Abstract
Objective: This study aims to systematically compare the efficacy and safety of linezolid andvancomycinfor treatment of hospital-acquired pneumonia (HAP).
Methods: PubMed, EMbase, MEDLINE, The Cochrane Library, CNKI, VIP, and Wan Fang databases were searched. Relevant conference proceedings in Chinese or English were manually searched. Randomized controlled trials (RCTs) were included in this study. RevMan 5.2 software was applied to do Meta-analysis.
Results: Totally 6088 patients with HAP in 7 RCTs were included. The meta-analysis results showed that the response rates between Linezolid and Vancomycin had no significant difference [OR=1.11, 95% CI (0.98, 1.17), P=0.10]. As to pathogen eradication, the efficacy of linezolid or vancomycin had no significant difference [OR=1.16, 95% CI (0.97, 1.40), P=0.10]. The exit event rate in trials induced by linezolid or vancomycin had no significant difference (P=0.40), and the incidence of gastrointestinal adverse events by linezolid or vancomycin was also not significantly different (P=0.18). The risk of renal dysfunction was significantly higher in vancomycin group than in linezolid group [OR=0.51, 95% CI (0.36, 0.73), P=0.0002], while the thrombocytopenia was significantly higher in linezolid group than in vancomycin group [OR=1.27, 95% CI (1.03, 1.57), P=0.02].
Conclusion: The efficacy of linezolid and vancomycin was similar for treatment of HAP. The risk dysfunction induced by linezolid was significantly lower than that byvancomycin, while the thrombocytopenia caused by linezolid was significantly higher than that by vancomycin.
Keywords
Hospital-acquired pneumonia, Linezolid, Vancomycin, Randomized controlled trial, Meta-analysis.
Introduction
Hospital acquired pneumonia (HAP) can extend the hospital stays of patients, which increases the medical cost for treatment. HAP mortality is next only to cancer and cerebrovascular diseases, which becomes the third cause of death in patients [1]. Staphylococcus aureus is the most common pathogen for HAP, accounting for 17% of isolated pathogens in hospital-acquired infection surveillance, and about 60% strains (such as methicillin-resistant Staphylococcus aureus, MRSA) were methicillin-resistant among all isolated Staphylococcus aureus [2].
In addition, increased mortality and morbidity is closely relevant to the infection caused by drug resistant bacteria, which increases the usage of medical resources and treatment costs [3]. Vancomycin is one representative drug for treatment of HAP caused by MRSA, but in recent years, some vancomycin-resistant Staphylococcus aureus strains were found in Japan and the United States [4], which presents new challenges for the treatment of HAP. Linezolid is a new generation fully synthetic antibiotics belonging to oxazolidinone class. Through binding to the 50S ribosomal subunit in bacteria and inhibiting the formation of 70S initiation complex, linezolid can play repression roles in the initiation stage of protein translation in bacteria.
Linezolid has better efficacy and tolerability for HPA caused by MRSA and other drug resistant bacteria [5]. However, it is still controversial to evaluate whether the efficacy and safety of linezolid is better than vancomycin. There are no specific studies to compare the efficacy and safety between linezolid and vancomycin for treatment of HAP. In this study, we applied Cochrane systematic reviews to collect studies about HAP treated by linezolid and vancomycin. Through objectively evaluate the clinical effects of the two drugs, the results in this study provides reliable evidence for clinical application.
Material and Methods
Inclusion and exclusion criteria
Patients who were diagnosed with HAP based on American Thoracic Society diagnostic criteria and hospital standards for HAP [1] were included. The randomized controlled trials (RCTs) were included whether they were blinded or not. For the intervention measures, the treatment group received linezolid while vancomycin was used in the control group, and conventional internal medicine treatment was used at the same time. The main endpoints include: (1) clinical cured rate: defined as at least two items were improved in clinical baseline symptoms and signs; (2) pathogen eradication rate; (3) hospital mortality; (4) adverse events.
Search strategy
Computer-based retrieval method was applied to search PubMed, EMbase, The Cochrane Library, CNKI, VIP, and WanFang database. Relevant studies were included using the following key words: “randomized controlled trial”, “Staphylococcus”, “Gram-positive”, “infections”, “lungs”, “respiratory”, “hospital-acquired”, “ventilator-associated and nosocomial pneumonia”, “linezolid* or oxazolone*”, “vancomycin”, and “glycopeptide”. And relevant conference proceedings were manually searched. The time for all searched publications was limited from date of database construction to August 10, 2015, and no language limitation.
Data extraction and quality evaluation
Two researchers independently screened literature, extracted trail associated data, and evaluate the quality of included studies based on inclusion and exclusion criteria. Any encountered discrepancies were resolved by discussion with a third party. The main content for extraction from literatures includes: clinical characteristic of patients (case number, gender proportion, and average age), intervention characteristic (intervention method, dosage, treatment course, and follow-up time), trail endpoints (clinical cured rate, bacteria eradication rate), mortality, and adverse events. Then the improved Jadad rating scale was used to evaluate the risk of publication bias.
Statistical analysis
Meta-analysis was performed using the Cochrane Collaboration Rev Man 5.2 software [6]. For enumeration data, the Odds Ratio (OR) was used, while the weighted mean difference (WMD) was applied for measurement data, and each effect variable was expressed in 95% CI. A χ2-test statistic was performed to assess the heterogeneity among studies. When I2<50% and P>0.1, a fixed effects model was used to do combined analysis. If heterogeneity was significant (I2>50% and P<0.1), subgroup analysis and sensitivity analysis were used to detect the causes that may induce clinical or statistical heterogeneity. If heterogeneity was still significant after excluding the interference by above factor, random effect model was applied to do meta-analysis.
Results
Literature searching and screening
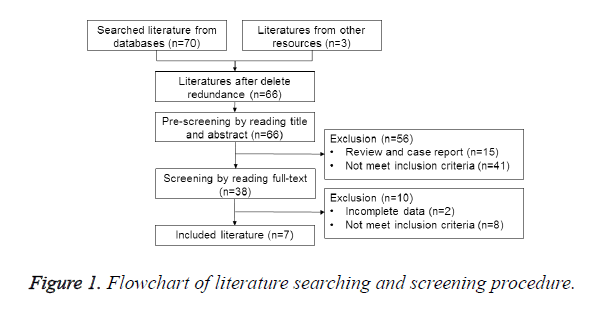
According to the searching strategy, 73 relevant literatures were selected. After exclusion of 66 literatures whose therapeutic effect index did not meet the criteria, 7 RCTs were selected [6-13]. Totally 6088 patients were included, and the procedure for searching and screening literatures was shown in Figure 1.
Basic characteristic of included studies
Patients in 7 included RCTs were diagnosed with HAP, and 5 studies focused specifically on population with HAP [6,9-11,14]. In the left 2 studies, we extracted associated materials about HAP through subgroup analysis [7,8]. In Jaksic et al. study [7], they mainly focused on patients with neutropenia with fever, while patients with Gram-positive infections were subjects in Lin et al. study [8]. The ages of patients were more than 56 years old except Jaksic et al. study [7] whose average age was less than 48 years old. The number of clinical cure and the number of adverse events were reported in all 7 studies. All studies reported there were no significant differences between two groups through comparing the baseline data. All 7 studies were multi-center, randomized controlled studies, and no details about randomization method and allocation concealment. The numbers of exit and lost population were reported, and intention to treat (ITT) analysis was used in all 7 studies. Double-blind method was used in all 7 studies, and the characteristics and JADAD score were shown in Table 1.
| Included literature | Study design | Group | Cases | Age (Year) | Course of the disease (Day) | Intervention method | JADAD scoring |
|---|---|---|---|---|---|---|---|
| [14] | Randomize double-blind | T | 203 | 63 | 9.6 | Linezolid+aztreonam | 4 |
| C | 193 | 61 | 8.9 | Vancomycin+aztreonam | |||
| [6] | Randomize double-blind | T | 321 | 63 | 9.5 | Linezolid+aztreonam | 3 |
| C | 302 | 62 | 9.4 | Vancomycin+aztreonam | |||
| [7] | Randomize double-blind | T | 304 | 48 | 11.4 | Linezolid+Gram-negative coverage | 4 |
| C | 301 | 47 | 11.5 | Vancomycin +Gram-negative coverage | |||
| [8] | Randomize double-blind | T | 71 | 56.3 | 12.2 | Linezolid +aztreonam | 3 |
| C | 71 | 59.6 | 10.7 | Vancomycin+aztreonam | |||
| [9] | Randomize double-blind | T | 1496 | 60.7 | 10 | Linezolid +Gram-negative Coverage | 4 |
| C | 1454 | 61.6 | 10 | Vancomycin +Gram-negative Coverage | |||
| [10] | Randomize double-blind | T | 101 | 59 | NR | Linezolid+aztreonam | 3 |
| C | 87 | 56 | NR | Vancomycin+aztreonam | |||
| [11] | Randomize double-blind | T | 597 | 60.5 | NR | Linezolid | 4 |
| C | 587 | 60.5 | NR | Vancomycin |
Table 1. Characteristics of included studies and methodological quality assessment.
Clinical cured rate analysis
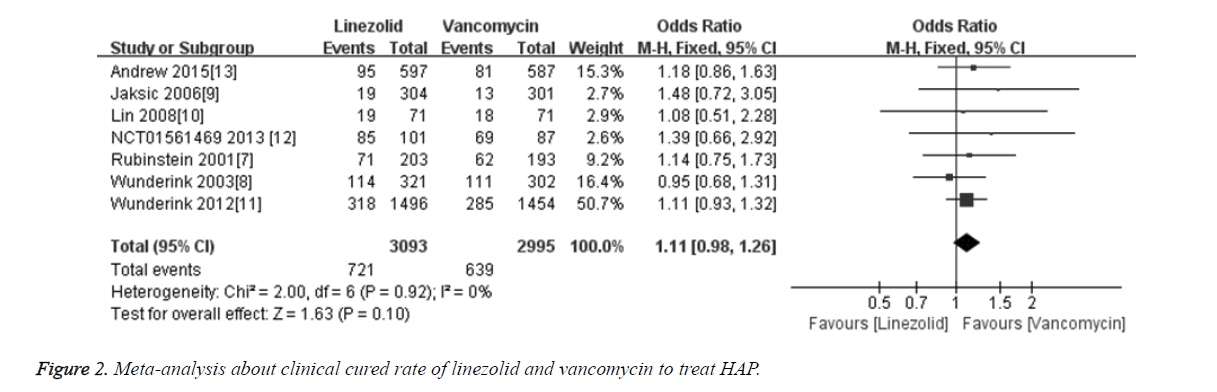
The cure rate was compared in all 7 included studies [6-11,14] between linezolid and vancomycin groups. Totally 6088 patients were included, among which 3093 cases were treated with linezolid and 2995 cases were treated with vancomycin. Meta-analysis results showed that the merged OR of clinical cured rate between linezolid and vancomycin groups was 1.11 [95% CI (0.98, 1.17), P=0.10], and the difference was not statistically significant (Figure 2). The results indicate that the treatment efficacy of linezolid for treatment HAP is not superior to vancomycin.
Pathogen eradication rate comparison
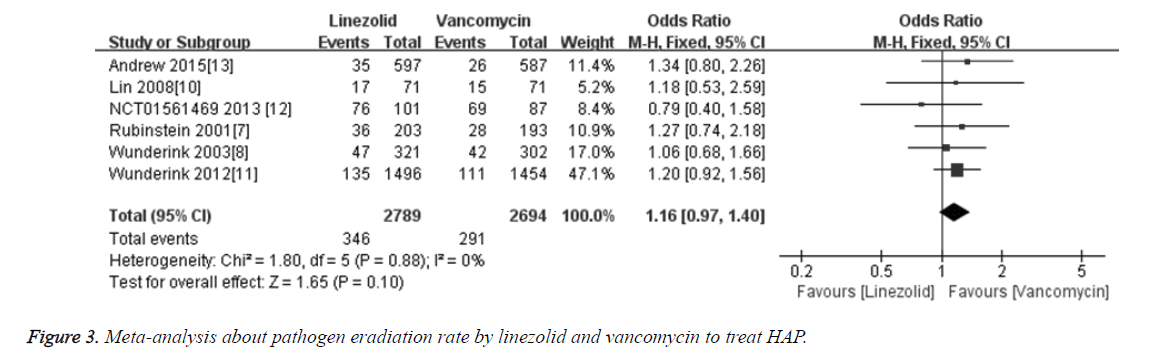
The pathogen eradication rate was compared in 6 included studies [6,9-11,14] between linezolid and vancomycin groups. Totally 5483 patients were included, among which 2789 cases were treated with linezolid and 2694 cases were treated with vancomycin. Meta-analysis results showed that the merged OR of pathogen eradication rate between linezolid and vancomycin groups was 1.16 [95% CI (0.97, 1.40), P=0.10], and the difference was not statistically significant (Figure 3). The results indicate that the pathogen eradication rate of linezolid for treatment HAP is not superior to vancomycin.
Mortality in hospital
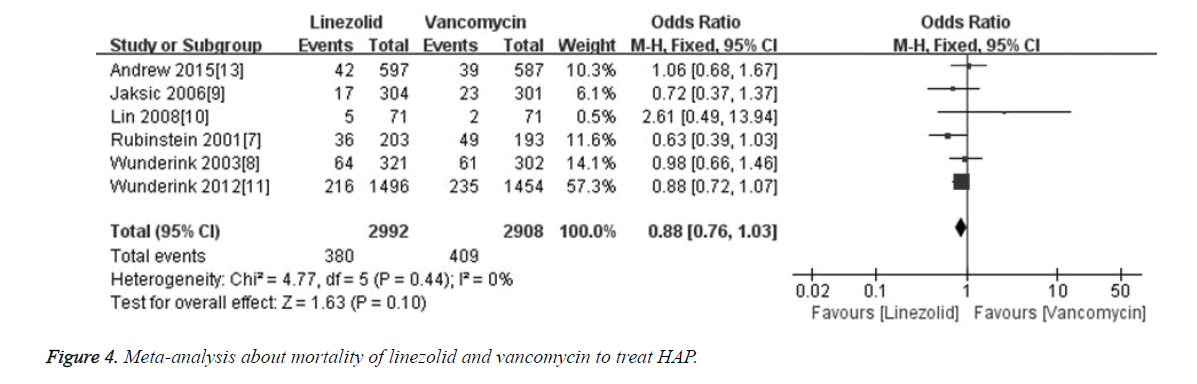
The mortality rate was compared in 6 included studies [6-9,11,14] between linezolid and vancomycin groups. Totally 5900 patients were included, among which 2992 cases were treated with linezolid and 2908 cases were treated with vancomycin. Meta-analysis results showed that the merged OR of mortality between linezolid and vancomycin groups was 0.88 [95% CI (0.76, 1.03), P=0.10], and the difference was not statistically significant (Figure 4). The results indicate that linezolid is not superior to vancomycin in decreasing mortality rate for treatment HAP.
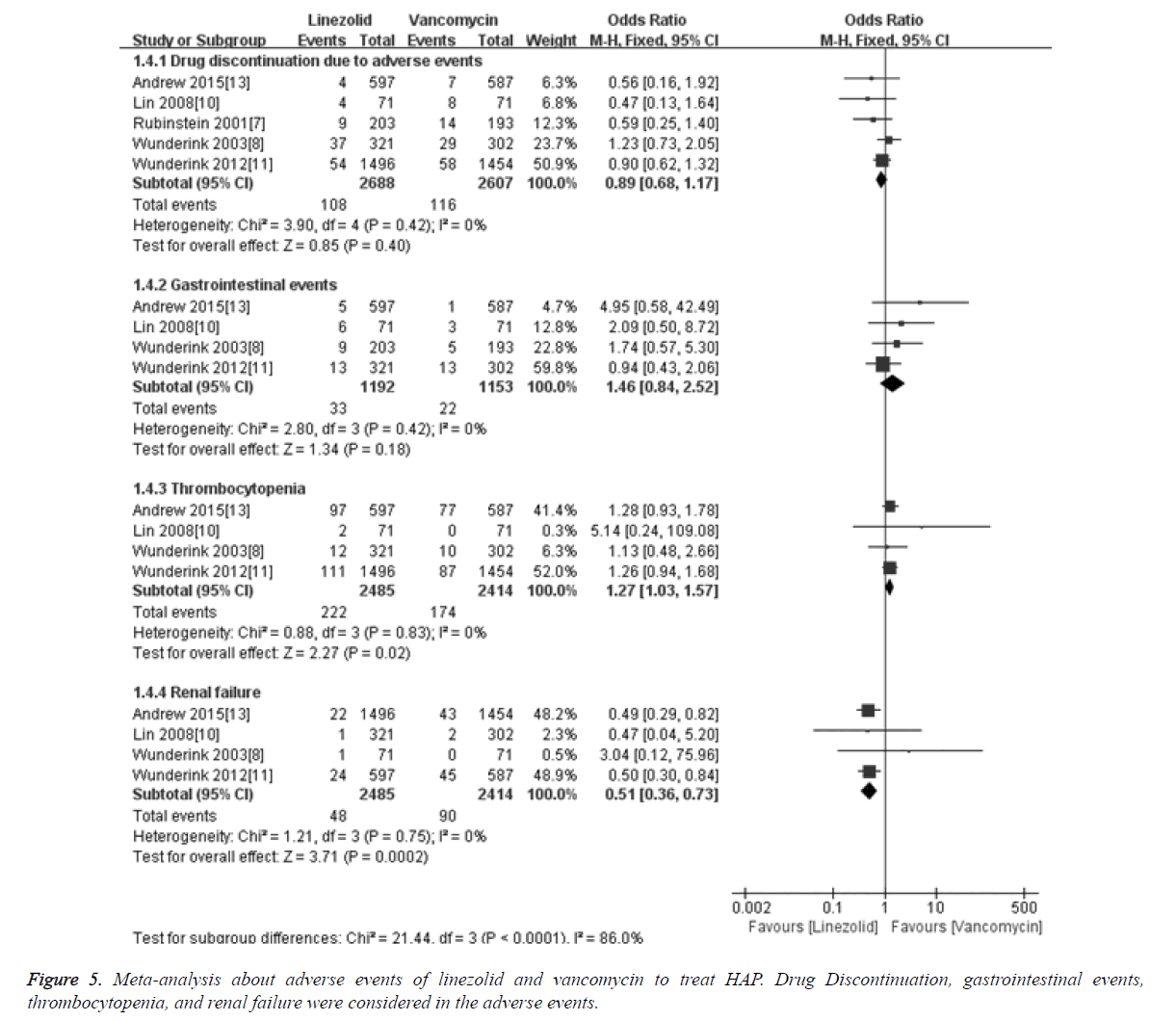
Adverse events
Meta-analysis results about adverse events showed that the merged OR of drug discontinuation due to adverse events between linezolid and vancomycin groups was 0.89 [95%CI (0.68, 1.17), P=0.40], and the difference was not statistically significant. For gastrointestinal events, the merged OR between linezolid and vancomycin was 1.46 [95%CI (0.84, 2.52), P=0.18], and there was no significant difference. The renal failure in linezolid group was 1.93, which was significantly lower than in vancomycin group (3.72%). And the merged OR for renal failure was 0.51 [95% CI (0.36, 0.73), P=0.0002]. The thrombocytopenia rate in linezolid group was 8.93, which was significantly higher than in vancomycin group (7.21%). And the merged OR for thrombocytopenia was 1.27 [95% CI (1.03, 1.57), P=0.02], details was shown in Figure 5.
Sensitivity analysis
To ensure the stability of the conclusions, we applied sensitivity analysis for evaluating the clinical cured rate for linezolid and vancomycin to treat HAP. After excluding study with small sample size [8], the pooled OR value 1.11 [95% CI (0.98, 1.27), P=0.16]. So the conclusions did not change after merging.
Discussion
In this study, we systematically compare the treatment efficacy between linezolid and vancomycin to treat HAP based on 7 included studies. The results showed that linezolid was not superior to vancomycin in clinical cured rate, pathogen eradiation rate, and other aspects. However, Logman [12], Dodds [13], and Bounthavong [15] found that linezolid was better than vancomycin for treatment of skin and soft tissue infections, which may be caused by the different penetrability of the two drugs. The penetration is good for linezolid in skin and soft tissues with high tissue concentration, while vancomycin has poor penetration in skin and soft tissue [16,17]. Falagas et al. [18] have performed a meta-analysis including 12 randomized control studies of linezolid, and compared the therapeutic efficacies of linezolid and glycopeptide/β-lactam antibiotics in the treatment of 6093 patients with Gram-positive cocci infection. They have found that, linezolid is superior to glycopeptide/β-lactam antibiotics, and linezolid is more efficient in the treatment of skin and soft tissue infection and sepsis. However, no significant differences have been observed between linezolid and glycopeptide/β- lactam antibiotics in treating pneumonia. Moreover, another meta-analysis has compared the therapeutic efficacies of linezolid and vancomycin in the treatment of sepsis, and no significant differences have been observed [19]. In another 3 Meta-analysis [20-22], treatment efficacy for HAP induced by Gram-positive cocci bacteria between linezolid and vancomycin. The results showed that there was no significant difference between 2 drugs, which was consistent with results in our meta-analysis.
Beibei et al. [21] have compared the efficacies of linezolid and vancomycin in the treatment of Gram-positive cocci infection-caused pneumonia, bacteremia, and skin and soft tissue infection, which is similar to this study regarding the perspective and focus. However, based on their findings, extensive reviewing and detailed retrieving of the literature were performed in our study, finally including an enlarged sample size of 6088 subjects, which might better reflect the differences in efficacy between linezolid and vancomycin in the treatment of hospital-acquired pneumonia. Another randomized control study has shown that, linezolid has significant advantages over vancomycin regarding the cure rate, microbiologic clearance, and adverse effects in the clinical treatment of MRSA pneumonia, which has been, however, questioned in the methodology and statistical analysis [23]. Although the adverse events of two drugs had no significant difference, we also found that renal failure rate in linezolid group was significantly lower than that in vancomycin group, which indicated that nephrotoxicity of linezolid was lower than vancomycin. The reason may be that linezolid had renal clearance and non-renal clearance ways [24], so it had lower renal toxicity and good tolerability. And the bioavailability of oral absorption for linezolid is 100% compared to intravenous administration, and this oral absorption feature can significantly shorten the hospital stay for HAP patients [25]. In addition, thrombocytopenia rate in linezolid was significantly higher than in vancomycin group. It was reported that the thrombocytopenia may be associated with treatment course (usually more than 2 weeks) [26]. Platelet number in majority of patients can return to normal or baseline level. So it is necessary to pay more attention to blood routine examination when applies linezolid in long treatment course.
The sensitivity analysis showed that the meta-analysis results did not changed when we excluded the literature [8] with small sample size, although it was not indicated whether this literature has influence on final conclusion or not. For the publication bias, it was difficult to generate funnel plot to analyse bias as small number of literatures were included. But as all included literatures were in English, and no more studies in other languages were included, which may cause some bias for searching results. And the low quality of literature results may also influence the reliability of the conclusions. Meanwhile it should be careful to apply the conclusion due to the following methodological deficiencies: (1) We found most studies did not descript the details about randomized method, random allocation, and allocation concealment; (2) Only 1 study [9] mentioned the details, although all selected literatures used double-blinding method, which may induce some bias about implementation and measurement.
This meta-analysis may have some limitations: (1) Only 4 studies were specifically for HAP among 7 included studies, and the data from the left 3 studies were extracted by subgroup analysis, which induce small sample size, then may induce some bias. (2) The clinical cured rate and other objective indicators were lack for intention-to-treat patients after ending of follow-up, which may weaken the proof intensity. Therefore, large-scale RCTs specifically for HAP are necessary if we want to improve the quality of the meta-analysis. And more long-term follow-up and objective endpoints to evaluate efficacy and safety should be considered when design the randomized controlled trials.
In summary, current studies indicate that the treatment efficacy of linezolid for HPA is similar to vancomycin. Considering the better tolerance and smaller nephrotoxicity, linezolid may be a better choice to replace vancomycin when treating vancomycin-resistantpatients, severe patients, especially renal insufficiency patients.
Acknowledgements
We thank Dr. Zhihong Li, Xiangjie Guo and Haiwei Yang from Affiliated Hospital of Jining Medical College for their valuable help during the preparation of this work.
References
- American Thoracic Society and Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J RespirCrit Care Med 2005; 171: 388-416.
- Klevens RM, Morrison MA, Nadle J, Petit S, Gershman K, Ray S, Harrison LH, Lynfield R, Dumyati G, Townes JM, Craig AS, Zell ER, Fosheim GE, McDougal LK, Carey RB, Fridkin SK, Active Bacterial Core surveillance MI. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA 2007; 298: 1763-1771.
- Engemann JJ, Carmeli Y, Cosgrove SE, Fowler VG, Bronstein MZ, Trivette SL, Briggs JP, Sexton DJ, Kaye KS. Adverse clinical and economic outcomes attributable to methicillin resistance among patients with Staphylococcus aureus surgical site infection. Clin Infect Dis 2003; 36: 592-598.
- Stevens DL. The role of vancomycin in the treatment paradigm. Clin Infect Dis 2006; 42 Suppl 1: S51-57.
- Sanchez Garcia M, De la Torre MA, Morales G, Pelaez B, Tolon MJ, Domingo S, Candel FJ, Andrade R, Arribi A, Garcia N, Martinez Sagasti F, Fereres J, Picazo J. Clinical outbreak of linezolid-resistant Staphylococcus aureus in an intensive care unit. JAMA 2010; 303: 2260-2264.
- Wunderink RG, Cammarata SK, Oliphant TH, Kollef MH. Continuation of a randomized, double-blind, multicenter study of linezolid versus vancomycin in the treatment of patients with nosocomial pneumonia. ClinTher 2003; 25: 980-992.
- Jaksic B, Martinelli G, Perez-Oteyza J, Hartman CS, Leonard LB, Tack KJ. Efficacy and safety of linezolid compared with vancomycin in a randomized, double-blind study of febrile neutropenic patients with cancer. Clin Infect Dis 2006; 42: 597-607.
- Lin DF, Zhang YY, Wu JF, Wang F, Zheng JC, Miao JZ, Zheng LY, Sheng RY, Zhou X, Shen HH, Ijzerman MM, Croos-Dabrera RV, Sheng W. Linezolid for the treatment of infections caused by Gram-positive pathogens in China. Int J Antimicrob Agents 2008; 32: 241-249.
- Wunderink RG, Niederman MS, Kollef MH, Shorr AF, Kunkel MJ, Baruch A, McGee WT, Reisman A,Chastre J. Linezolid in methicillin-resistant Staphylococcus aureus nosocomial pneumonia: a randomized, controlled study. Clin Infect Dis 2012; 54: 621-629.
- Pfizer. Evaluation of Patients With Methicillin-Resistant Staphylococcus Aureus Hospital-Acquired Pneumonia Treated With Linezolid or Vancomycin.
- Shorr AF, Puzniak LA, Biswas P, Niederman MS. Predictors of Clinical Success in the Treatment of Patients with Methicillin-Resistant Staphylococcus aureus (MRSA) Nosocomial Pneumonia (NP). PLoS One 2015; 10: e0131932.
- Logman JF, Stephens J, Heeg B, Haider S, Cappelleri J. Comparative effectiveness of antibiotics for the treatment of MRSA complicated skin and soft tissue infections. Curr Med Res Opin 2010; 26: 1565-1578.
- Dodds TJ, Hawke CI. Linezolid versus vancomycin for MRSA skin and soft tissue infections (systematic review and meta-analysis). ANZ J Surg 2009; 79: 629-635.
- Rubinstein E, Cammarata S, Oliphant T,Wunderink R. Linezolid (PNU-100766) versus vancomycin in the treatment of hospitalized patients with nosocomial pneumonia: a randomized, double-blind, multicenter study. Clin Infect Dis 2001; 32: 402-412.
- Bounthavong M, Hsu DI. Efficacy and safety of linezolid in methicillin-resistant Staphylococcus aureus (MRSA) complicated skin and soft tissue infection (cSSTI): a meta-analysis. Curr Med Res Opin 2010; 26: 407-421.
- Stein GE1, Schooley SL, Peloquin CA, Kak V, Havlichek DH. Pharmacokinetics and pharmacodynamics of linezolid in obese patients with cellulitis. Ann Pharmacother 2005; 39: 427-432.
- MacGowan AP. Pharmacokinetic and pharmacodynamic profile of linezolid in healthy volunteers and patients with Gram-positive infections. J Antimicrob Chemother 2003; 51 Suppl 2: ii17-25.
- Falagas ME, Siempos II, Vardakas KZ. Linezolid versus glycopeptide or beta-lactam for treatment of Gram-positive bacterial infections: meta-analysis of randomised controlled trials. Lancet Infect Dis 2008; 8: 53-66.
- Shorr AF, Kunkel MJ, Kollef M. Linezolid versus vancomycin for Staphylococcus aureus bacteraemia: pooled analysis of randomized studies. J Antimicrob Chemother 2005; 56: 923-929.20.
- Xiao L, Zhu J. A meta-analysis about therapeutic efficacy of linezolid and vancomycin for treatment infections by methicillin-resistant Staphylococcus aureus. Chinese J Antibiotics 2008; 33: 178-181.
- Beibei L, Yun C, Mengli C, Nan B, Xuhong Y, Rui W. Linezolid versus vancomycin for the treatment of gram-positive bacterial infections: meta-analysis of randomised controlled trials. Int J Antimicrob Agents 2010; 35: 3-12.
- Kalil AC, Murthy MH, Hermsen ED, Neto FK, Sun J. Linezolid versus vancomycin or teicoplanin for nosocomial pneumonia: a systematic review and meta-analysis. Crit Care Med 2010; 38: 1802-1808.
- Docobo-Pérez F, López-Rojas R, Domínguez-Herrera J, Jiménez-Mejias ME, Pichardo C, Ibáñez-Martínez J, Pachón J. Efficacy of linezolid versus a pharmacodynamically optimized vancomycin therapy in an experimental pneumonia model caused by methicillin-resistant Staphylococcus aureus. J Antimicrob Chemother 2012; 67: 1961-1967.
- Beringer P, Nguyen M, Hoem N, Louie S, Gill M, Gurevitch M, Wong-Beringer A. Absolute bioavailability and pharmacokinetics of linezolid in hospitalized patients given enteral feedings. Antimicrob Agents Chemother 2005; 49: 3676-3681.
- Wiederhold NP, Coyle EA, Raad II, Prince RA, Lewis RE. Antibacterial activity of linezolid and vancomycin in an in vitro pharmacodynamic model of gram-positive catheter-related bacteraemia. J Antimicrob Chemother 2005; 55: 792-795.
- Falagas ME, Siempos II, Vardakas KZ. Linezolid versus glycopeptide or beta-lactam for treatment of Gram-positive bacterial infections: meta-analysis of randomised controlled trials. Lancet Infect Dis 2008; 8: 53-66.