Research Article - Biomedical Research (2017) Volume 28, Issue 5
Comparing the incidence of heparin-induced thrombocytopenia (HIT) in patients receiving heparin (UHF) and enoxaparin (LMWH)
Mohammad Reza Motie1*, Gholamhossein Kazemzadeh2 and Sahar Gazeran11Department of Surgery, Surgical Oncology Research Center, Imam Reza Hospital, Faculty of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran
2Department of Vascular Surgery, Vascular and Endovascular Surgery Research Center, Imam Reza Hospital, Faculty of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran
- *Corresponding Author:
- Mohammad Reza Motie
Surgical Oncology Research Center
Imam Reza Hospital
Mashhad University of Medical Sciences, Iran
Accepted date: June 08, 2016
Abstract
The aim of this investigation is the evaluation of heparin-induced thrombocytopenia (HIT) abundance and relevant factors risks in the patients receiving unfractionated heparin and low molecular weight heparin. This cohort study was conducted on two groups of patients who were hospitalized in general and vascular surgery department: receiving fractioned heparin group and receiving low molecular weight heparin group (LMWH). Platelet count was measured before treatment and on days 4, 7, 14, and 30 post-treatment. If in each of these steps there HIT criteria including platelet count less than 100000 or loss of 50% of base numbers, SRA diagnostic test would conduct and if positive, definite HIT was diagnosed. In this research 486 patients participated (234 patients in each group). According to heparin induced thrombocytopenia criteria, in this study only 3 patients had such criteria. Using SRA diagnostic test, HIT was determined in two out of three patients. The three patients were in the group of receiving UFH, also all of them were male and they receive heparin prophylaxis. There was no significant difference between 2 UFH and LMWH receiving groups. Heparin-induced thrombocytopenia (HIT) is not a common side effect of consuming low molecular weight heparin, but a deadly side effect and it is a strong risk factor in thrombotic events such as VTE; so prevention, diagnosis and treatment is very vital. Furthermore, the main key to reduce the side effects of HIT is immediate diagnosis with measuring platelet and the drug is discontinued.
Keywords
Thrombocytopenia, Unfractionated heparin, Low molecular weight heparin, Heparin induced thrombocytopenia (HIT)
Introduction
Unfractionated heparin (UFH) and low molecular weight heparins (LMWHs) heparin are drugs consuming for thromboembolism prophylaxis after surgery of patients in the hospital [1]. It is evaluated that in America more than 12 million hospitalized patients consume heparin every year [2]. More than 50% of these patients are in the surgery department [3]. Bleeding events are the most common side effects of consuming heparin and its derivations [4]. Heparin induced thrombocytopenia (HIT) is an immunologic drug reaction [5-8]. There are 2 types of HIT: type I is benign, unsafe and transient thrombocytopenia that is found in 25% of patients receiving heparin in first 5 days due to increasing platelet aggregation and usually recover without any medical intervention. Contrary, type II or True HIT immune thrombocytopenia is found in the patients by antibodies between 4-14 days from receiving heparin and may lead to arterial venous thrombotic events [4,6,7,9].
Heparin joins with 4 platelet load factor and produced antibody bans with them. This complex joins with Fc receptor and leads to platelet aggregation and thromboembolism [4,8-10]. According to an ancient opinion, HIT occurs only due to consuming UFH, not consuming LMWHs. However, further studies shows that less common HIT occurs due to consuming LMWHs [8,11,12]. So far, there has been no research due to HIT abundance as the result of consuming UFH and LMWH in general and vascular surgery departments in Iran; so the aim of this study is to assessing the abundance and risks of relevant factors in creating HIT.
Materials and Methods
Study design
The participants of this cohort study were all hospitalized patients in general and vascular surgery department that receiving either therapy or as prophylaxis heparin or its derivations (LMWH). Totally, 468 patients participated in the study-234 patients in each group. All patients were aware of the study and a written consent was obtained from them. Drugs prescribed depending on patients' needs in two therapies or as prophylaxis doses in different periods. Platelet counts was measured before treatment and on days 4, 7, 14, and 30 posttreatment, then SRA diagnostic test was applied , if positive, definite HIT was diagnosed [13]. It should be mentioned that, if in any time of receiving drugs the patients showed the symptoms of HIT (fever, tachycardia, tachypnea, hypertension), taking drug would stop immediately and platelet was measured. If there was thrombocytopenia, final test was conducted and the clinical signs were recorded and finally compared. Furthermore, all patients filled a questionnaire including information such as sex, age, receiving heparin rate, the number of days of receiving drugs, type of receiving drug and diagnosis of patients.
Sample preparation and measurement of platelet
To measure the platelet counts, 2 cc citrated venous bloods was drawn and separation was prepared immediately in order to prevent clinging the platelet to one another; also prevent decreasing platelet due to clinging to the edge of the damaged vessel. After this stage, platelet count conducted by COBAS.
Thrombocytopenia and heparin- induced thrombocytopenia definition
In this study the criteria to determine heparin- induced thrombocytopenia was as follows:
1. Any reduction of platelet less than 1,00,000 per cubic millimeter of blood.
2. 50% reduction of platelet numbers in any stage of the assessment after treatment [1,5,11,13,14].
Test SRA
Serotonin release assay is a definitive diagnostic test for heparin-induced thrombocytopenia that platelet activation was determined after exposure to serum antibodies in the presence of heparin. SRA is a functional assessment that examines the activity relevant to heparin in the platelet. In this way, the patient’s serum is mixed with donor platelet including serotonin 14C and different concentrations of heparin. The present antibodies in the patient’s serum bind to the donor platelet and activate it that lead to secretion of marked serotonin from platelet granules. Positive SRA test is defined as secretion of more than 20% of serotonin 14C when the patient’s serum mixes with low-dose heparin. Moreover, highdose heparin should reduce the secretion caused by low-dose heparin at least 50% to show that platelet activity depends on heparin [15].
Statistical analysis
Frequency tables, and distribution and bar charts are used for descriptive data, also descriptive statistics of measures of central tendency and dispersion are calculated. Inferential statistics including Chi square test, T test or nonparametric equivalent and the logistic regression are used for analytical data to test hypothesis.
Results
486 patients in the general and vascular surgery departments participated in this study. 58.5% participants were male and 41.5% were female. In UFH group, 57.5% patients were male and the rest of patients were female. In LMWH group, 59.4% were male and the rest of patients were female. Moreover, by Fishers Exact test, there was no significant difference between two UFH and LMWH groups (P>0.05). The mean age of patients was 55.99 years old. Furthermore, the mean age of UFH group was 53.52 and LMWH was 58.45 years old; so there was significant difference between two UFH and LMWH groups (P<0.001).
Depending on what the patients need, drugs was prescribed in two therapy or prophylaxis doses that mostly (411 patients out of 468) prophylaxis dose was prescribed. Moreover, depending on the patients' needs and the length of stay in hospital, the period of receiving drugs are also different (between 2-30 days). The mean of the period of receiving UFH drugs was 6.57 days, and the mean of the period of receiving LMWH drugs was 11.57 days that there was a significant difference between 2 groups (P<0.001) (Table 1).
| Total | UFH Group | LMWH Group | P Value | |
|---|---|---|---|---|
| Sex, No. (%) | ||||
| Male | 274 (58.5) | 135 (57.7) | 139 (59.4) | 0.778* |
| Female | 194 (41.5) | 99 (42.3) | 95 (40.6) | < 0.001** |
| Age, y | ||||
| Range | 17-90 | 17-90 | 19-88 | |
| Mean ± SD | 55.99 ± 14.58 | 53.52 ± 14.76 | 58.45 ± 14 | |
| Drug dosage | ||||
| Prophylaxis | 411 | 207 | 204 | |
| Therapy | 57 | 27 | 30 | |
| Period of receiving drug, d | ||||
| Range | 10990 | 42420 | 10990 | < 0.001*** |
| Mean ± SD | 9.16 ± 7.12 | 6.57 ± 4.23 | 11.75 ± 8.38 | |
| HIT | ||||
| According to platelet counts | 3 | 3 | 0 | 0.248* |
| According to SRA test | 2 | 2 | 0 | |
| *Fishers Exact test, **Independent t-test, ***Mann-Whitney test | ||||
Table 1. Demographic data of participants.
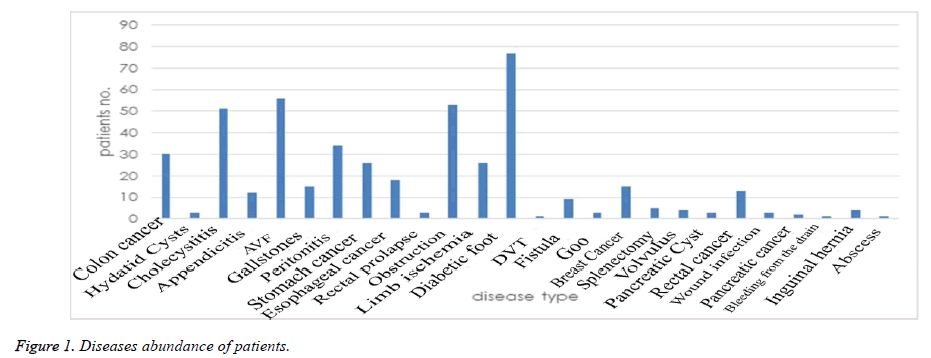
The platelet counts of the patients were measured before starting drug receiving, 4th, 7th day, 14th and 30th day. In this respect, there was a significant different between 2 UFH and LMWH groups only before starting the study (P<0.001). At this time, the mean of platelet counts in UFH group was 253.36 and in LMWH group was 240.73 (Table 2). A diabetic foot ulcer was the most common disease among the patients who need to receive heparin and enoxaparin (the reason why 77 patients (16.5%) receive such drugs). Furthermore, AVF (56 patients-12%) and obstruction of the gastrointestinal tract (53 patients-11.3%) were the most common diseases to receive such drugs (Figure 1).
| Day 0 | Day 4 | Day 7 | Day 14 | Day 30 | |
|---|---|---|---|---|---|
| UFH group | |||||
| Range | 126-822 | 126-604 | 126-635 | 108-781 | 156-675 |
| Mean± SD | 253.36 ± 94.6 | 245.87 ± 74.26 | 256.42 ± 74.77 | 266.75 ± 74.85 | 276.29 ± 65.5 |
| LMWH group | |||||
| Range | 123-753 | 117-684 | 126-673 | 147-689 | 163-661 |
| Mean± SD | 240.73 ± 104.63 | 248.7 ± 94.57 | 261.18 ± 92.21 | 275.04 ± 83.75 | 284.43 ± 81.18 |
| P value* | 0.004 | 0.455 | 0.702 | 0.359 | 0.726 |
| Mann-Whitney test* | |||||
Table 2. Platelet counts of patients on days 0, 4, 7, 14, 30.
In this research, according to heparin-included thrombocytopenia criteria only 3 patients had such criteria within 5-7 days. Using SRA diagnostic test, HIT was determined in two out of three patients. The three patients were in the group of receiving UFH, also all of them were male and they received heparin prophylaxis. One of the three patients had colon cancer, one of them had esophageal cancer and one of them had obstruction that diagnosis of thrombocytopenia was rejected in the last case. Comparing the sex distribution of the heparin-included thrombocytopenia patients with other patients, there was no significant difference between two groups (P=0.27). Moreover, the age average of the three patients was 57 years old that there was no significant difference between the age average of mentioned patient with age average of 55.98 of other patients (P=0.904). Although the three patients belonged to UFH group, there was no significant difference between two UFH and LMWH groups due to incidence of thrombocytopenia (P=0.248).
Discussion
HIT is an immunologic drug reaction that was found in 1980 [5,12]. At the present time, HIT is found in 3-5% of the patients who receive UFH heparin and 1% of those who receive LMWH heparin [4,5]. Thrombosis is the main side effect of HIT and in the patients who receive heparin due to DVT prophylaxis, venous thrombosis us more common than arterial thrombosis [9]. Although bleeding side effects was found only in 5% of HIT patients, it is the main cause of 90% mortality and the main factor to reduce the side effects is heparin discontinues [7].
"Battistelli" in his review study mentioned that some factors including age, cardiovascular risk factors, and prescribed form has been not influential on HIT. He also mentioned that risk factors of HIT are type of heparin, higher risk of receiving cow UFH than human UFH and lower risk when receiving LMWH. Other risk factors are as follow: the period of consuming the drug, consuming rate, history of recent drug consumption, sex, type of disease (internal, surgery) [4]. In this investigation three risk factors, type of heparin, type of disease and sex, were noticed between two groups. Three thrombocytopenia patients were in the group of receiving UFH. Although, in respect of the sex, there were no significant differences between two groups, all three patients were male. However, it is different from other HIT studies that it is more common among females [4,16]. In the current research all patients received prophylaxis heparin and the disease arose between days 4-8- similar to other study results [6,16]. Furthermore, the age average of three patients was 57 years old that there was no significant difference between the age average of mentioned patient with age average of 55.98 of other patients, but it was different with the results of "Brigitte" research that the age average was 66-69 years old [17]. HIT occurs at any age and old patients expose to higher risk because heparin is used to prevent and treat thromboembolism [17].
In this research, thrombocytopenia and HIT did not occur due to consuming LMWH low molecular weight heparin and thrombocytopenia (1.5%) and HIT (0.85%) occurs due to consuming UFH heparin. According the high risk of HIT due to consuming UFH rather than LMWH, the results were similar to other researches [18-22] (Table 3). However, in the current study the percentage of HIT occurrence (0.85%) was less than other studies (2-3%) [4,5], and it could be compared with the study results of "Smythe" (0.2%) [3]. This research is similar to "Nurmohamed" studies due to the occurrence of thrombocytopenia [23].
| No. | Study | Mean Duration, d | Patients, No. | HIT Outcome | Thrombocytopenia Outcome |
|---|---|---|---|---|---|
| Warkentin et al. [18] | UFH | 10 | 332 | 8 | 100 |
| Enoxaparin | 10 | 332 | 0 | 96 | |
| Leyvraz et al. [19] | UFH | 42624 | 175 | 2 | 2 |
| Enoxaparin | 42624 | 174 | 0 | 0 | |
| Mahlfeld et al. [20] | UFH | 9 | 252 | 5 | NA |
| Enoxaparin | 9 | 252 | 1 | NA | |
| Ganzer et al. [21] | UFH | NA | 307 | 10 | 89 |
| Enoxaparin | NA | 325 | 0 | 26 | |
| Pouplard et al. [22] | UFH | 10 | 157 | 6 | 0 |
| Dalteparin | 30 | 171 | 0 | 2 | |
| Our Study | |||||
| UFH | 6 | 234 | 2 | 1 | |
| Enoxaparin | 11 | 234 | 0 | 0 | |
| Nurmohamed et al. [23] | UFH | 10 | 709 | NA | 1 |
| Enoxaparin | 10 | 718 | NA | 0 |
Table 3. Comparison of the result of this study with other studies.
Conclusion
It is concluded that thrombocytopenia and heparin-induced thrombocytopenia (HIT) is not a common side effect of consuming low molecular weight heparin, but a deadly side effect and it is a strong risk factor in thrombotic events such as VTE; so screening, diagnosis, management and prevention are very important factors. It is recommended that consuming LMWH heparin is a better drug for therapy and prophylactic purposes and with the aim of early diagnosis, the platelet was measured at intervals of 3 to 4 days in the first week. In the case of occurrence of thrombocytopenia, stop taking the drug is the main factor to reduce it. Limitation of the study: during the study some patients didn’t continued participating in the study.
Acknowledgment
The results described in this paper constituted as a part of a MD thesis submitted by the third author to Mashhad University of Medical Sciences. The study was supported by the vice chancellor for research at Mashhad University of Medical Sciences.
References
- Martel N, Lee J, Wells PS. Risk for heparin-induced thrombocytopenia with unfractionated and low-molecular-weight heparin thromboprophylaxis: a meta-analysis. Blood 2005; 106: 2710-2715.
- Swanson JM. Heparin-induced thrombocytopenia: a general review. J Infusion Nursing 2007; 30: 232-240.
- Smythe MA, Koerber JM, Mattson JC. The incidence of recognized heparin-induced thrombocytopenia in a large, tertiary care teaching hospital. Chest 2007; 131: 1644-1649.
- Battistelli S, Genovese A, Gori T. Heparin-induced thrombocytopenia in surgical patients. Am J Surgery 2010; 199: 43-51.
- Davoren A, Aster RH. Heparin-induced thrombocytopenia and thrombosis. Am J Hematol 2006; 81: 36-44.
- Fabris F, Luzzatto G, Stefani PM, Girolami B, Cella G, Girolami A. Heparin-induced thrombocytopenia. Haematologica 2000; 85: 72-81.
- Slocum MM, Adams JG, Teel R, Spadone DP, Silver D. Use of enoxaparin in patients with heparin-induced thrombocytopenia syndrome. J Vasc Surgery 1996; 23: 839-843.
- Walenga JM, Prechel M, Jeske WP, Bakhos M. Unfractionated heparin compared with low-molecular-weight heparin as related to heparin-induced thrombocytopenia. Current Opin Pulmonary Med 2005; 11: 385-391.
- Shah MR, Spencer JP. Heparin-induced thrombocytopenia occurring after discontinuation of heparin. J Am Board Family Pract 2003; 16: 148-150.
- Lubenow N, Hinz P, Ekkernkamp A, Greinacher A. Should patients be informed about the risk of heparin-induced thrombocytopenia before prolonged low-molecular-weight heparin thromboprophylaxis post-trauma/orthopedic surgery? Euro J Haematol 2007; 79: 187-190.
- Warkentin TE, Sheppard J-AI, Heels-Ansdell D, Marshall JC, McIntyre L, Rocha MG, Mehta S, Davies AR, Bersten AD, Crozier TM. Heparin-induced thrombocytopenia in medical surgical critical illness. CHEST 2013; 144: 848-858.
- Dailiana ZH, Malizos KN, Varitimidis S, Hantes M, Basdekis G, Rigopoulos N. Low-molecular-weight heparin for prevention of thrombosis: inverted role. J Trauma 2007; 63: E111-115.
- Junqueira DR, Perini E, Penholati RR, Carvalho MG. Unfractionated heparin versus low molecular weight heparin for avoiding heparin-induced thrombocytopenia in postoperative patients. Cochrane Database Syst Rev 2012; 9: CD007557.
- Warkentin TE, Greinacher A. Heparin-induced thrombocytopenia: recognition, treatment, and prevention: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest 2004; 126: 31S-37S.
- Leo A, Winteroll S. Laboratory diagnosis of heparin-induced thrombocytopenia and monitoring of alternative anticoagulants. Clin Diagn Lab Immunol 2003; 10: 731-740.
- Lindhoff-Last E, Nakov R, Misselwitz F, Breddin HK, Bauersachs R. Incidence and clinical relevance of heparin-induced antibodies in patients with deep vein thrombosis treated with unfractionated or low-molecular-weight heparin. Br J Haematol 2002; 118: 1137-1142.
- Tardy-Poncet B, Tardy B. Heparin-Induced thrombocytopenia: minimising the risks in the elderly patient. Drugs Aging 2000; 16: 351-364.
- Warkentin TE, Levine MN, Hirsh J, Horsewood P, Roberts RS, Gent M, Kelton JG. Heparin-induced thrombocytopenia in patients treated with low-molecular-weight heparin or unfractionated heparin. England Journal Medicine 1995; 332: 1330-1335.
- Leyvraz PF, Bachmann F, Hoek J, Buller HR, Postel M, Samama M, Vandenbroek MD. Prevention of deep vein thrombosis after hip replacement: randomised comparison between unfractionated heparin and low molecular weight heparin. Br J Med 1991; 303: 543-548.
- Mahlfeld K, Franke J, Schaeper O, Kayser R, Grasshoff H. Heparin-induced thrombocytopenia as a complication of postoperative prevention of thromboembolism with unfractionated heparin/low molecular weight heparin after hip and knee prosthesis implantation. Der Unfallchirurg 2002; 105: 327-331.
- Ganzer D, Gutezeit A, Mayer G. Potentials risks in drug prevention of thrombosis-low-molecular-weight heparin versus standard heparin. Zeitschrift fur Orthopadie und ihre Grenzgebiete 1999; 137: 457-461.
- Pouplard C, May MA, Iochmann S, Amiral J, Vissac AM, Marchand M, Gruel Y. Antibodies to platelet factor 4-heparin after cardiopulmonary bypass in patients anticoagulated with unfractionated heparin or a low-molecular-weight heparin : clinical implications for heparin-induced thrombocytopenia. Circulation 1999; 99: 2530-2536.
- Nurmohamed MT, Verhaeghe R, Haas S, Iriatte JA, Vogel G, van Rij AM, Prentice CR, Jan W, Group SS. A comparative trial of a low molecular weight heparin (enoxaparin) versus standard heparin for the prophylaxis of postoperative deep vein thrombosis in general surgery. Am J Surgery 1995; 169: 567-571.