Research Article - Asian Journal of Biomedical and Pharmaceutical Sciences (2017) Volume 7, Issue 63
Comparative Pharmacognostic and Phytochemical Studies of Flower, Leaf and Stem Extracts of Butea monosperma
Ashlesha Vaidya and Nancy Pandita*
Department of Chemical Sciences, Sunandan Divatia School of Science, SVKM’s NMIMS, Mumbai, India
- *Corresponding Author:
- Nancy Pandita
Department of Chemical Sciences
Sunandan Divatia School of Science, SVKM’s NMIMS
Mumbai, India
Tel: +919967471319
E-mail: nancy.pandita@nmims.edu
Accepted on September 13, 2017
Abstract
Background: Butea monosperma, known as Palas, is an Ayurvedic plant, and its various parts have been studied for their medicinal properties. Aim: To carry out a detailed pharmacognostic and phytochemical study of different extracts of flower, leaf and stem of Butea monosperma (BM). Materials and methods: Different plant parts (flowers, leaves, stem powder) were studied for their organoleptic, microscopic and physicochemical characteristics. Extraction of dried powder of different parts of BM, i.e. flowers, leaves and stem was carried out in succession with 3 solvents of increasing polarities, viz. alcohol, hydro-alcohol and water. Preliminary phytochemical screening was carried out on all the extracts using standard procedures. Thin layer chromatography was carried out to assess their qualitative nature. These were then tested quantitatively for their phenol, flavonoid and sterol contents. DPPH (2,2′- diphenyl- 1-picrylhydrazyl) assay was employed to assess their antioxidant capacities. Based on the results of these studies, the selected extracts were chosen for FRAP (Ferric reducing antioxidant potential) assay, their anti-glycation and anti-inflammatory activity, and the assays were performed using standard methods. Results: The hydroalcoholic and alcoholic extracts of the flower possessed the maximum phenol and flavonoid content, respectively, along with the highest anti-glycation and anti-inflammatory activities, while the alcoholic extract of the stem showed maximum anti-oxidant properties. Conclusion: A detailed pharmacognostic study was performed on the three parts of BM. The hydroalcoholic and alcoholic extracts of the flower and the alcoholic extract of the stem proved to be the most effective extracts among all the extracts of the three plant parts in terms of their phytochemical constituents and their antioxidant, anti-glycation and anti-inflammatory activities.
Keywords
Butea monosperma, Flower, Stem, Flavonoids, Phenols, Anti-oxidant, Anti-glycation.
Introduction
Medicinal plants contain numerous active ingredients that may be potentially useful for the development of therapeutic agents. The identification and isolation of phytochemical groups and/or single chemical entities from them are, hence, crucial for drug discovery as these entities often work as individual agents or as a collective group of phytocompounds (purified extracts) to achieve the desired therapeutic effect. However, to assess their quality, standardisation of these plant parts needs to be carried out, which includes a series of tests to determine the quality, quantity and the purity of the phytocompounds or the extracts, along with the measure of contaminants or foreign matter present in them [1]. In this study, the plant under consideration is Butea monosperma.
Butea monosperma (BM), also known as Palas in the traditional system of medicine is a medicinal plant. As reported by the Indian Ayurvedic texts, its leaves, stem, flowers, seeds, gum (stem) and roots have been widely used as traditional medicine [2,3]. Its classification is presented in Table 1.
| Kingdom | Plantae |
|---|---|
| Sub kingdom | Tracheobionta |
| Division | Magnoliophyta |
| Class | Magnoliopsida |
| Subclass | Rosidae |
| Order | Fabales |
| Family | Fabaceae |
| Genus | Butea |
| Species | Monosperma |
Table 1: Scientific classification of Butea monosperma.
The flowers have been reported to possess a range of therapeutic properties. Studies have proven their antiinflammatory and anti-oxidant potential [4-6]. The flavonoids contained in the flowers majorly contribute to these activities. Some of these flavonoids are butrin, isobutrin, and butein [7,8]. When the extracts of the flowers were tested in vivo, they exhibited hepatoprotective and anti-convulsive activities [9,10]. Besides in vivo studies, the effect of the alcoholic and aqueous floral extracts was tested in two separate in vitro studies on colon cancer and hepatoma cell lines. The cell lines, upon exposure to the extracts showed reduced proliferation [11,12].
Apart from flowers, the leaves and the stem portions of the plant have also been subjected to various studies to investigate their medicinal properties. The leaves have majorly exhibited anti-inflammatory and anti-diabetic properties [13,14], while the stem has been proven to possess strong hepatoprotective and anti-diarrhoeal activities [15,16].
Though Butea monosperma has been studied for its antidiabetic properties, the focus on the treatment of its complications is still poor. This study investigates the possible potential of BM as a treatment for the diabetic complications by assessing the in vitro anti-oxidant, anti-glycation and antiinflammatory activities of extracts of all the three plant parts, viz. flower, leaf and stem.
Materials and Methods
Reagents and instruments
All chemicals and reagents used during the experimentation were of analytical grade. Perkin Elmer Lambda 25 was the spectrophotometer used for all the assays. Motif, B1 Advanced Series photographic microscope was used to study the microscopic details.
Plant collection and authentication
The plant material, i.e. BM flowers (BMF), BM leaves (BML) and BM stem (BMS) was collected from a local vendor in Mumbai and was authenticated by Agharkar Research Institute, Pune. Each of the samples was deposited at the herbarium under the following voucher numbers: flower: I/F-044, leaf: L-073, and stem: S/B-141. The plant parts were then air dried and hand crushed and stored in air tight and dry containers until further use.
Organoleptic characteristics
The macroscopic observations of the powdered samples, i.e. colour, texture, odour, etc. were made in order to establish the identity of the plant.
Microscopic studies
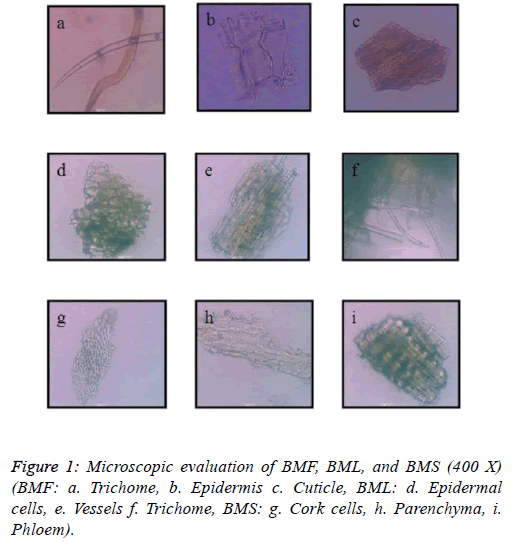
The microscopic evaluation of the powdered plant material (BMF, BML, and BMS) was carried out with the help of microscope. The plant material was soaked in a solution of 20% chloral hydrate, and then mounted on a glass slide with the help of glycerine. The mounted slides were then observed under a photographic microscope with a magnification of 400X. Figure 1 represents the microscopic images.
Physicochemical analysis
Physicochemical parameters like the determination of ash content (sulphated ash, acid insoluble ash, water soluble ash and total ash), extractive values and moisture content (or loss on drying) were checked for all the three plant materials [17]. The extractive values of the plant materials were measured with all the three solvents used for extraction.
Extraction of plant material
Two methods were employed for extraction of the three different types of plant samples. Powdered BMF and BMS were made to undergo successive maceration (500 g) with the help of solvents of increasing polarities, i.e. alcohol, hydro alcohol, and water. Successive Soxhlet extraction was employed for powdered BML (50 g) for the same solvents used for BMF and BMS (300 ml), after defatting it with petroleum ether. The extracts obtained were dried with the help of a rotary evaporator, and their yields (Table 5) were calculated. Table 2 denotes the nomenclature used for the extracts.
| BMF alcoholic extract | BMF AL |
| BMF hydroalcoholic extract | BMF HA |
| BMF aqueous extract | BMF AQ |
| BML alcoholic extract | BML AL |
| BML hydroalcoholic extract | BML HA |
| BML aqueous extract | BML AQ |
| BMS alcoholic extract | BMS AL |
| BMS hydroalcoholic extract | BMS HA |
| BMS aqueous extract | BMS AQ |
Table 2: The plant extracts and their abbreviations used in the study.
Qualitative phytochemical screening
The extracts were subjected to preliminary phytochemical screening as per the standard chemical methods for the identification of different phytocompounds present [18]. The results are displayed in Table 6.
| Plant part | Colour | Taste | Odour | Texture |
|---|---|---|---|---|
| BMF | Brown | Pungent | Pungent | Rough |
| BML | Green | Sour | Odourless | Coarse |
| BMS | Brown | Bitter | Odourless | Coarse |
Table 3: Macroscopic characteristics of the plant samples.
| Parameters (% w/w) | BMF | BML | BMS |
|---|---|---|---|
| Loss on Drying | 10.30 ± 0.7 | 7.16 ± 1.1 | 3.56 ± 0.98 |
| Total ash | 16.50 ± 0.5 | 11.50 ± 0.3 | 5.38 ± 0.5 |
| Sulphated ash | 12.05 ± 0.7 | 11.56 ± 0.5 | 7.76 ± 1.0 |
| Acid insoluble ash | 8.64 ± 0.44 | 3.00 ± 0.4 | 0.69 ± 0.05 |
| Water soluble ash | 1.89 ± 0.5 | 0.46 ± 0.05 | 0.63 ± 0.03 |
| Alcohol soluble extractive value | 6.41 ± 0.81 | 4.53 ± 1.09 | 2.38 ±1.1 |
| Hydroalcoholic extractive value | 38 ± 0.76 | 13.71 ± 0.59 | 7.34 ± 0.4 |
| Water soluble extractive value | 5.70 ± 0.56 | 7.92 ± 0.49 | 3.08 ± 0.91 |
Table 4: Physicochemical parameters of the plant material.
| Extract | Yield (% w/w) ± SD |
|---|---|
| BMF AL | 4.31 ± 0.81 |
| BMF HA | 2.31 ± 0.99 |
| BMF AQ | 3.13 ± 0.82 |
| BML AL | 7.22 ± 0.78 |
| BML HA | 2.67 ± 0.52 |
| BML AQ | 2.08 ± 0.66 |
| BMS AL | 6.84 ± 0.98 |
| BMS HA | 5.37 ± 0.74 |
| BMS AQ | 3.63 ± 0.95 |
Table 5: Percent yields of the plant extracts.
| Main group | Test | BMF AL | BMF HA | BMF AQ | BML AL | BML HA | BML AQ | BMS AL | BML HA | BML AQ |
|---|---|---|---|---|---|---|---|---|---|---|
| Carbohydrates | Benedicte’s Test | + | + | + | - | + | + | + | + | + |
| CoCl3 test for hexoses | + | + | + | + | + | + | + | + | + | |
| Fehling’s test | - | - | - | - | - | - | + | + | + | |
| Alkaloids | Wagner’s test | - | - | - | - | - | - | - | - | - |
| Hager’s test | - | - | - | - | - | + | - | - | - | |
| Proteins | Ninhydrin test | + | + | - | - | + | - | - | + | - |
| Biuret test | - | - | - | - | - | - | + | + | - | |
| Steroids | Salkowski’s reaction | + | + | + | + | + | + | + | + | + |
| Liebermann- Burchard reaction | + | + | - | - | - | - | - | - | - | |
| Flavonoids | Shinoda test | + | - | - | - | - | - | - | - | - |
| Sulphuric acid test | + | + | + | - | - | - | - | - | - | |
| Lead acetate test | + | + | - | - | - | - | - | - | - | |
| Tannins/phenolic compounds | 5% FeCl3 test | - | - | - | - | - | + | + | - | - |
| Lead acetate test | + | + | + | - | - | - | - | - | + | |
| KMnO4 test | + | + | + | - | - | - | - | - | - | |
| Cardiac glycosides | Keller –Killiani’s test | - | - | - | + | - | - | + | - | - |
Table 6: Qualitative phytochemical screening of the plant extracts .
Thin layer chromatography (TLC)
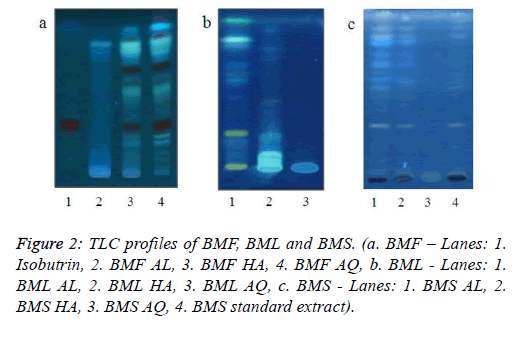
TLC fingerprinting has the potential to authenticate the presence of chemical constituents of herbal drug or formulations. The thin layer chromatography of all the extracts of the three plant parts was carried out, and their Rf values were recorded. Aluminium plates pre-coated with silica gel 60G F254 were used for the technique. The plates were cut carefully according to the sizes required, and application of the samples was done, each of 20 μl having a lane length of 0.5 cm. The plates were then run in the respective solvent systems and were observed under UV light [19]. Figure 2 represents the TLC profiles of the different extracts of BMF, BML and BMS, respectively.
Solvent systems
a. BMF: Ethyl acetate: methanol: formic acid (8:2:0.4), Scanning at 348 nm
b. BML: Butanol: Acetic acid: Water (5:3:2), Scanning at 366 nm
c. BMS: Butanol: Acetic acid: Water (5:3:2), Scanning at 366 nm
Total phenol content
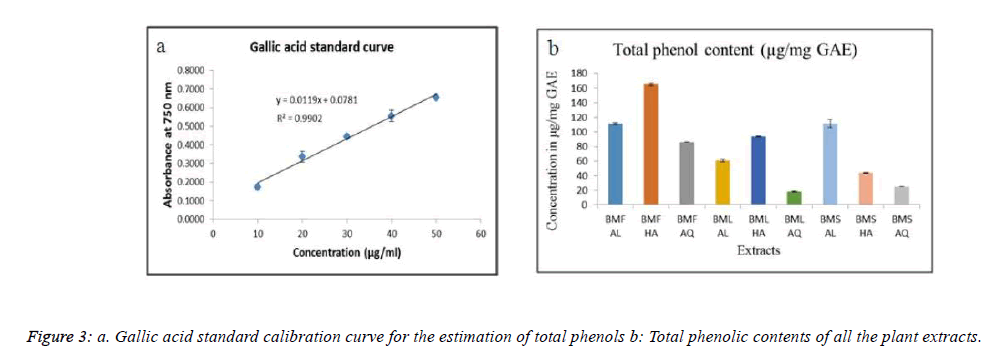
The Folin Ciocalteau’s method was employed for the quantitative estimation of the total phenolic content [20]. Gallic acid was used as the reference standard to prepare a calibration curve. Different concentrations of gallic acid were prepared in methanol (mention the range). A volume of 0.5 ml of each concentration of gallic acid was mixed with 2.5 ml of (a 10 fold diluted) Folin Ciocalteu reagent and 2 ml of 7.5 % sodium carbonate solution. The tubes were allowed to stand for 30 minutes at room temperature and the absorbance at 765 nm was measured using the spectrophotometer. Similarly, 0.5 ml of all the extracts (250 μg/ml) were treated and the absorbance was measured.
Total flavonoid content
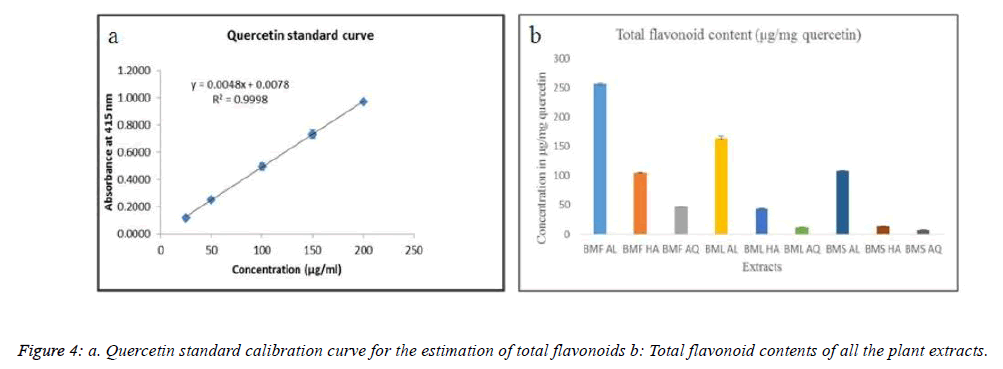
The total flavonoid content in extracts was determined with the help of a spectrophotometric method using aluminum chloride [21]. A calibration curve was prepared using quercetin as the standard and its various concentrations were prepared. A volume of 0.5 ml of each of these concentrations were mixed with 1.5 ml of ethanol, 0.1 ml of 10% aluminum chloride, 0.1 ml of 1 M potassium acetate and 2.8 ml of distilled water. These reaction mixtures were then incubated at room temperature for 30 min, and their absorbance was measured at 415 nm with a spectrophotometer. The amount of 10% aluminum chloride was substituted by the same amount of distilled water in the blank. Similarly, 0.5 ml of all the plant extracts (250 μg/ml) were used for determination of flavonoid content.
DPPH radical scavenging activity assay
The free radical scavenging activity of the extracts was measured in vitro by 2,2′- diphenyl- 1-picrylhydrazyl (DPPH) assay, according to the method by Kasangana et al., with minor modifications [22]. A 0.36 mg/ml stock solution of the same was prepared with methanol and stored in an amber coloured bottle at 20°C. Three ml of this solution was mixed with 100 μl of each of the plant extracts, with a range of concentrations. The reaction mixture was shaken and incubated in the dark for 15 min at room temperature. Absorbance was measured at 517 nm. The control was prepared as above with methanol. The scavenging activity of the extracts was calculated using the following equation:
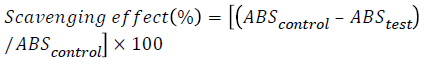
FRAP assay
The reducing power of a sample tests its ability to transform Fe (III) to Fe (II). The procedure described by authors Kumar and Jain was used, with minor modifications [23]. Formation of a blue coloured complex confirms the transformation to Fe (II) and it can be measured spectrophotometrically at 700 nm. Each of the plant extracts (2 ml) were mixed with 2 ml of phosphate buffer (0.2 M, pH 6.6) and 2 ml of potassium ferricyanide (10 mg/ml). The mixture was incubated at 50°C for 20 min and 2 ml of trichloroacetic acid (100 mg/l) was later added to it. The mixture was centrifuged at 3000 rpm for 10 min, and the upper layer of the solution was collected. Two ml of this mixture was mixed with 2 ml of distilled water and 0.4 ml of 0.1% (w/v) fresh ferric chloride. After 10 min, the absorbance was measured at 700 nm. Higher absorbance of the reaction mixture indicates a higher reducing power.
Antiglycation assay
Bovine serum albumin (BSA, 20 mg/ml, 10 ml) was mixed with glucose (500 mM, 5 ml) and 0.02% sodium azide in phosphate buffer (200 mM, pH 7.4). The sample with different concentration dissolved in phosphate buffer (200 mM, pH 7.4, 5 ml) was added to the reaction mixture, and then the mixture was incubated for 30 days at 37°C to obtain glycated materials. Aminoguanidine was used as a positive control in two concentrations, 300 μg/ml (Pos 300) and 400 μg/ml (Pos 400). Aliquots of the glycated materials were taken from the system for the following experiments in 0, 3rd, 6th, 12th, 18th, and 21st day after incubation. The glycated material (0.5 ml) and NBT reagent (0.3 mM, 2.0 ml) in sodium carbonate buffer (100 mM, pH 10.35) were incubated at room temperature for 15 min, and the absorbance was read at 530 nm against a blank [24].
Anti-inflammatory assay
The reaction mixture consisted of 0.2 mL of 1% BSA, 2.8 mL of phosphate buffered saline (PBS, pH 6.4) and 2 ml of varying concentrations of extract so that final concentrations become 100, 200, 300, 400, 500 μg/ml. Similar volume of doubledistilled water served as control. Then the mixtures were incubated at 15°C in an incubator for 10 min and then heated at 70°C for 10 min in water bath. After cooling, their absorbance was measured at 660 nm by using vehicle (only buffer) as blank. Diclofenac at the final concentration of (100, 200, 300, 400, 500 μg/ml) was used as reference drug and treated similarly for determination of absorbance [25]. The percentage inhibition of protein denaturation was calculated by using the following formula:
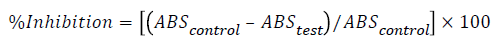
Statistical evaluation
Values reported are expressed as Mean ± S.E.M. Standard calibration curves were prepared for the quantitative estimation of phenol and flavonoid content in the extracts, and graphical representations of the results were made with the help of MS Excel. The results of the anti-oxidant and anti-inflammatory assays were derived by employing the equations of percentage of scavenging effect and percent inhibition, and the average values calculated with their standard deviations, were plotted on graphs using MS Excel. The values obtained for the antiglycation assay were observed as a trend for a period of 21 days, and compared with those of the negative and positive controls.
Results
Organoleptic characteristics
Organoleptic characteristics of a particular plant sample give a measure of its outward appearance and employ the sense of touch, smell and taste to determine the nature of the sample and its differentiating characteristics. The results of the organoleptic analysis are given in Table 3.
Microscopic studies
The powder microscopy of all the three plant parts revealed peculiar characteristics. The flower powder of BM showed the presence of trichomes, which were unbranched and unicellular in nature having a narrow lumen. Single layer epidermal cells and parts of cuticle were also observed. Microscopy of the leaf powder showed cells of the upper epidermis, unicellular trichomes, which tapered towards the ends, and annular vessels. The stem powder under the microscope shows traces of parenchymatous cells, phloem fibres and outer cork cells. The results of microscopic analysis are shown in Figure 1.
Physicochemical analysis
Various physicochemical parameters like the ash content and extractive values were checked for the three plant materials and their results are summarized in Table 4. The extractive values, the moisture content (indicated by loss of drying) and the total ash of BMF were higher than those of BML and BMS.
Extraction of plant material
The use of solvents is made in plant studies to extract the phytochemical compounds from the plant material into the solvent. Here, after extraction of all the three plant materials with solvents alcohol (AL), hydro alcohol (HA) and water (AQ), the yield of each extract was calculated, and the results are displayed in Table 5. The average yield of BMS was comparatively higher than that of BMF or BML. The results also indicated that alcohol was the preferred solvent for extraction of all the three plant materials as the yield of the alcoholic extracts were higher than those of the hydro alcoholic and aqueous ones.
Qualitative phytochemical screening
Phytochemical screening was done with the help of standard chemical tests to test for the available phytocompounds present in the extracts. Proteins and alkaloids were found in all the extracts but carbohydrates and steroids were found to be present in all the extracts. Flavonoids and phenolic compounds were primarily found in the all extracts of BMF. Table 6 lists the results of the phytochemical screening.
Thin layer chromatography
The thin layer chromatographic profiles of the three extracts of the three different plant parts are shown in Figure 2. The alcoholic and hydroalcoholic extracts of BMF (lanes 2 and 3) show a band whose Rf corresponds to the standard isobutrin, Rf 0.28 (Lane 1, Figure 2a). In case of BML, the alcoholic extract (Lane 1) exhibited an accurate separation, compared to its other two extracts. There were bands of Rf 0.25, 0.57, and 0.82 in the BML AL sample, while bands of Rf 0.21, 0.42 and 0.78 were seen in BML HA (Lane 2, Figure 2b). The BMS AL and BMS HA lanes (1 and 2) showed a profile very similar to that of the standardised extract of BMS (lane 4), with corresponding Rf of 0.33 and 0.53. The aqueous extracts of all the three plant parts failed to run and exhibit any separation (Figure 2c).
Total phenol content
Total phenolic contents of the extracts were expressed as milligrams of gallic acid equivalents. Figure 3a represents the standard curve for the total phenol content using gallic acid as the standard. As seen in Fig 3b, BMF HA exhibited the highest total phenolic content, with 165.3 μg/mg of gallic acid equivalents (GAE), while BMS AQ showed only 25.2 μg/mg of GAE.
Total flavonoid content
A quercetin standard curve was plotted and is represented in Figure 4a. The alcoholic extract of the flower, BMF AL showed flavonoid content in μg/mg equivalents of quercetin (256.5 μg/mg), followed by that of the leaf, BML AL (164.4 μg/mg), as shown in Figure 4b.
DPPH radical scavenging activity assay
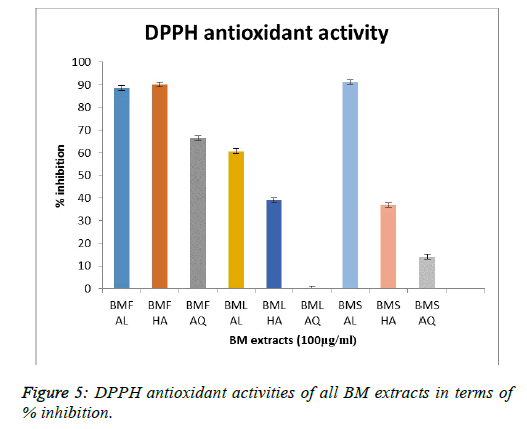
The DPPH radical scavenging activity of the extracts was recorded in terms of percent inhibition, with BMS AL exhibiting an inhibition of 91.27 %, followed closely by BMF HA (90.11%) and BMF AL (88.58%). Figure 5 represents the results of all the other extracts. With respect to the above results so far, it was found that BMF AL, BMF HA and BMS AL were proven to be effective compared to the rest of the extracts, in terms of the amount of flavonoid and phenol contents and their anti-oxidant activities. Hence, these three extracts were studied for their reducing power, their antiglycation and their anti-inflammatory activity.
FRAP assay
The reducing power of the three extracts, i.e. BMF AL, BMF HA, and BMS AL, was measured with the help of FRAP assay. Ascorbic acid, the standard, showed a significantly high reducing power at 700 nm, but among the extracts, BMS AL exhibited the highest reducing power of 0.43 as against the second highest of 0.28 recorded by BMF AL (Table 7).
| Sample | Reducing power at 700 nm |
|---|---|
| Ascorbic acid | 1.65 ± 0.96 |
| BMF AL | 0.28 ± 0.54 |
| BMF HA | 0.23 ± 1.02 |
| BMS AL | 0.43 ± 1.10 |
Table 7: Reducing powers of the standard and extract at 700 nm.
Anti-glycation assay
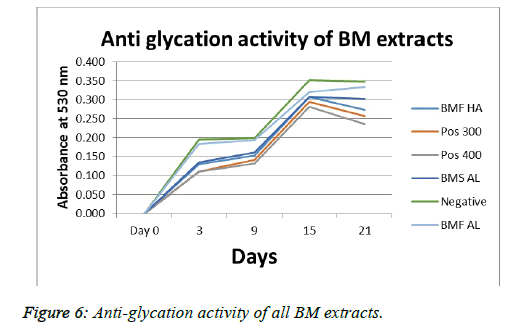
The measure of an extract to inhibit or restrict the glycation of a standard protein in conditions mimicking in vivo ones is given by the anti-glycation assay. Here, a negative was introduced so as to check the amount of glycation occurring in the absence of either a standard inhibitor, or any extract. Hence, the negative showed maximum glycation over a period of 21 days. As seen in Figure 6, the extent of inhibition of glycation was in the order: Pos 400> Pos 300> BMF HA> BMS AL> BMF AL> Negative.
Anti-inflammatory assay
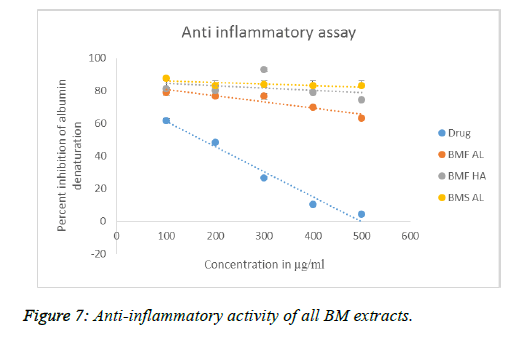
The anti-inflammatory assay employs the principle of albumin denaturation at high temperatures. Here, the drug diclofenac was used as the standard. The results are shown in Figure 7. BMF AL, BMS AL, and BMF HA worked better at inhibiting albumin denaturation, compared to the standard drug. Though the drug showed a progressive decrease in albumin denaturation as its concentration increased, none of the extracts displayed such a trend. BMF HA proved to be an effective anti-inflammatory extract, with the other two extracts are coming a close second.
Discussion
Butea monosperma is found in the tropical and subtropical regions of the Indian subcontinent, and has been used not only as a medicinal plant, but is also used in the dyes and pesticide industry [26]. Though BM has been studied as an antidiabetic and all the parts of the plant, i.e. the seeds, flowers, roots, leaves and stem have been used for medicinal purposes, the plant is yet to be studied extensively as a possible treatment for complications of diabetes. Hence this study was aimed at comparing the pharmacognostic and phytochemical profiles of the different extracts of the flowers, leaves and stem of the plant and choosing the extracts most endowed with phytochemicals to further assess their in vitro anti-oxidant, anti-glycation and anti-inflammatory activities, as oxidation, glycation and inflammation are the most common etiology of diabetic complications.
Preliminary studies like those of the organoleptic characteristics of a particular plant sample, their microscopic details, their chromatographic profiles and their physicochemical parameters give a measure of their purity and authenticity.
Alcohol was the ideal solvent for extraction of the phytoconstituents as the yield was the highest in all the alcoholic extracts. Most of the in vivo studies have been carried out with the alcoholic flower extracts of BM [16,27,28]. Phytochemical screening served as a preliminary test to identify the phytochemical groups present in the extracts, and phenols and flavonoids were found to be present in abundance, compared to alkaloids or steroids. These results as echoed in other studies which have found that flavonoids are present in abundance in the flower of BM [8,29]. This was confirmed by the fact that the quantitative sterol content estimation revealed almost no sterols, but the quantitative phenol and flavonoid content was high enough to be the possible reason for the medicinal properties of the extracts.
Total phenolic content of the extracts was estimated by using Folin- Ciocalteu reagent and BMF HA exhibited the highest total phenolic content. The total flavonoid content of all the extracts was carried out by the AlCl3 method and the alcoholic extract of the flower, BMF AL showed the maximum amount of flavonoid content. This finding serves as an indication to the fact that alcohol extracts a large amount of phytoconstituents (it gives the higher yield too) thus increasing the therapeutic pharmacological activity.
Phenols and flavonoids have been known to possess antioxidant activities, hence these results validated the purpose of studying the extracts for their anti-oxidant, anti-glycation and anti-inflammatory activities. The DPPH radical scavenging assay is a gold standard to assess the anti-oxidant nature of extracts. Recorded in terms of percent inhibition, BMS AL exhibited the highest inhibition. Keeping these observations in mind, BMF AL, BMF HA and BMS AL were carried forward to study their reducing power, their anti-glycation and their anti-inflammatory activity.
The reducing power of a sample tests its ability to transform Fe (III) to Fe (II), confirmed by the formation of a blue coloured complex and can be measured spectrophotometrically at 700 nm. Among the extracts, BMS AL exhibited the highest reducing power. Hence it can be said that as far as anti-oxidant properties are concerned, the alcoholic extract of the stem (BMS AL) was the most effective [30]. This also indicates that though flavonoids are known to be anti-oxidant in nature, other phytoconstituents complement their anti-oxidant activity. The stem of BM is known to contain flavonoids and other constituents, which may contribute to its efficacy [31-33].
In the anti-glycation assay, NBT was used as a mediator. Glycated products are formed when the extracts are unable to inhibit the glycation of proteins. Such glycated products possess the ability to reduce NBT. The higher the reduction of NBT by the reaction mixture, the higher is the amount of glycated products present, and the higher is the absorbance. Here, this indicated that BMF HA, possessed the highest antiglycation activity, followed by BMS AL and BMF AL. The negative control which had no extract showed highest absorbance till day 21. The standard anti-glycating agent aminoguanidine showed higher powers of anti-glycation as was expected, compared to the extracts. Such a study of glycation activity BM flower extracts has not been reported.
The anti-inflammatory assay employs the principle of deliberate albumin denaturation at high temperatures and checking for agents that inhibit this denaturation. BSA expresses antigens associated with type III hypersensitive reactions and which are related to inflammatory diseases. Hence, agents that inhibit denaturation of serum albumin (or BSA) are likely to have high anti-inflammatory properties. The assay results showed that all the three extracts, i.e. BMF AL, BMS AL, and BMF HA worked better at inhibiting albumin denaturation, compared to the standard drug. Other studies, too, have focussed on the in vitro anti-inflammatory activity of BM, albeit in a different manner, but nevertheless, they found that both flowers and stem possess good anti-inflammatory activity, which is reflected in this study [33,34].
From the above studies, it can be postulated that the extracts of BMS and BMF could be used in unison, or individually to develop an agent possessing anti-oxidant, anti-glycation and anti-inflammatory activities. Moreover, flavonoids and phenols could be exclusively extracted and tested for these pharmacological activities to further strengthen their use as agents against diabetic complications. The leaf extracts in this study proved to be weaker, compared to the stem and flower extracts.
Conclusion
A detailed pharmacognostic and phytochemical study was performed on the three plant parts of BM. BMF HA and BMF AL possessed the maximum phenol and flavonoid content, respectively, along with the highest anti-glycation and antiinflammatory activities, while BMS AL showed maximum anti-oxidant properties.
Conflict of Interest
There are no conflicts of interest between authors.
References
- Pradhan N, Gavali J, Waghmare N. WHO (World Health Organization) guidelines for standardization of herbal drugs. International Ayurvedic Medical Journal 2015; 3: 2238-43.
- Nadkarni AK. Indian Materia Medica. Popular Prakashan, Mumbai, 2007.
- Kirtikar KR, Basu BD. Indian Medicinal Plants 2nd ed. Dehradun, India: International Book Distributors; II, 1999.
- Shahavi VM, Desai SK. Anti-inflammatory activity of Butea monosperma flowers. Fitoterapia. 2008; 79: 82–85.
- Krolikiewicz-Renimel I, Michel T, Destandau E, Reddy M, André P, Elfakir C, Pichon C. Protective effect of a Butea monosperma (Lam.) Taub. flowers extract against skin inflammation: Antioxidant, anti-inflammatory and matrix metalloproteinases inhibitory activities. J Ethnopharmacology. 2013; 148: 537-43.
- Jarald EE, Nalwaya N, Manish M, Jain A, Edwin S. Determination of Rutin Content and Antioxidant Activity of Extracts of Butea monosperma Flowers Extracted Using Various Extraction Methods. Phcog J. 2009; 1: 126-129.
- Rasheed Z, Akhtar N, Khan A, Khan KA, Haqqi TM. Butrin, Isobutrin, and Butein from medicinal plant Butea monosperma selectively inhibit Nuclear Factor-_B in activated human mast cells: Suppression of Tumor Necrosis Factor, Interleukin (IL)-6, and IL-8. J Pharmacol Exp Ther. 2010; 333: 354-363.
- Jassbi AR, Singh P, Vivek K, Gupta PK, Tahara S. Antioxidant study and assignments of NMR spectral data for 3’,4’,7-trihydroxyflavanone 3’,7-Di-O-β-D-glucopyranoside (butrin) and its hydrolyzed product. Chem Nat Compd. 2004; 40: 250-253.
- Sharma N, Shukla S. Hepatoprotective potential of aqueous extract of Butea monosperma against CCl4 induced damage in rats. Exp Toxicol Pathol. 2011; 63: 671– 676.
- Kasture VS, Kasture SB, Chopde CT. Anticonvulsive activity of Butea monosperma flowers in laboratory animals. Pharmacol Biochem Behav. 2002; 72: 965–972.
- Polachi N, Nagaraja P, Subramaniyan B, Mathan G. Antiproliferative activity of n-butanol floral extract from Butea monosperma against HCT 116 colon cancer cells; drug likeness properties and in silico evaluation of their active compounds toward glycogen synthase kinase-3β/axin and β-catenin/t-cell factor-4 protein complex. Asian J Pharm Clin Res. 2015; 8: 134-141.
- Choedon T, Shukla SK, Kumar V. Chemopreventive and anti-cancer properties of the aqueous extract of flowers of Butea monosperma. J Ethnopharmacol. 2010; 129: 208–213.
- Mengi SA, Deshpande SG. Anti-inflammatory activity of Butea frondosa leaves. Fitoterapia. 1999; 70: 521-22.
- Sharma N, Garg V. Anti diabetic and antioxidant potential of ethanolic extract of Butea monosperma leaves in alloxan induced diabetic mice. Indian J Biochem and Biophys. 2009; 46: 99-105.
- Tiwari P, Kumar K, Panik R, Pandey A, Pandey A, Sahu PK. Hepatoprotective potentials of Butea monosperma stem bark extract against carbon tetrachloride induced hepatotoxicity in albino rats. Int J Med Med Sci. 2011; 3: 252-255.
- Gunakkunru A, Padmanaban K, Thirumal P, Pritila J, Parimala G, Vengatesan N, Gnanasekar N, Perianayagam JB, Sharma SK, Pillai KK. Anti-diarrhoeal activity of Butea monosperma in experimental animals. J Ethnopharmacol. 2005; 98: 241-244.
- Quality Control Methods for Medicinal Plant Materials, World Health Organization Geneva, 1998.
- Harbourne AJ. Phytochemical Methods: A Guide to Modern Techniques of Plant Analysis, Springer, 1998.
- Wagner, Bladt. Plant Drug analysis: A Thin Layer Chromatography Atlas. 2nd Edition, Springer, 2004.
- Saeed N, Khan MR, Shabbir M. Antioxidant activity, total phenolic and total flavonoid contents of whole plant extracts Torilis leptophylla L. BMC Complement Altern Med. 2012; 12: 221.
- Malla MY, Sharma M, Saxena RC, Mir MI, Mir AH, Bhat SH. Phytochemical screening and spectroscopic determination of total phenolic and flavonoid contents of Eclipta Alba Linn. J Nat Prod Plant Resour. 2013; 3: 86-91.
- Kasangana PB, Haddad PS, Stevanovic T. Study of polyphenol content and anti-oxidant capacity of Myrianthus arboreus (Cecropiaceae) root bark extracts. Antioxidants. 2015; 4: 410-426.
- Kumar T, Jain V. Appraisal of total phenol, flavonoid contents, and antioxidant potential of folkloric Lannea coromandelica using in vitro and in vivo assays. Scientifica (Cairo). 2015; 203679.
- Wu JW, Hsieh CL, Wang HY. Inhibitory effects of guava (Psidium guajava L.) leaf extracts and its active compounds on the glycation process of protein. Food Chem. 2009; 113: 78-84.
- Arif Ullah HM, Zaman S, Juhara F, Akter L, Tareq SM, Masum EH et al. Evaluation of antinociceptive, in-vivo & in-vitro anti-inflammatory activity of ethanolic extract of Curcuma zedoaria rhizome. BMC Complement Altern Med. 2014; 14: 346.
- Sinha K, Saha PD, Datta S. Extraction of natural dye from petals of Flame of forest (Butea monosperma) flower: Process of optimization using response surface methodology (RSM). Dyes Pigm. 2012; 94: 212-216.
- Fageria D, Rao DV. A review on Butea monosperma (Lam.) kuntze: A great therapeutic valuable leguminous plant. Int J Sci Res. 2015; 5: 1-8.
- Subramaniyan B, Polachi N, Mathan G. Isocoreopsin: An active constituent of n-butanol extract of Butea monosperma flowers against colorectal cancer (CRC). J Pharm Biomed Anal. 2016; 6: 318-325.
- Gupta A, Sheth NR, Pandey S, Yadav JS, Joshi SV. Screening of flavonoids rich fractions of three Indian medicinal plants used for the management of liver diseases. Rev bras farmacogn. 2015; 25: 485-490.
- Salar RK, Seasotiya L. Free radical scavenging activity, phenolic contents and phytochemical evaluation of different extracts of stem bark of Butea monosperma (Lam.) Kuntze. Front Life Sci. 2011; 5: 107-116.
- Dutta NK, Mazumdar K, Mishra US, Dastidar SG, Park JH. Isolation and identification of a flavone (quercetin) from Butea frondosa bark. Pharm Chem J. 2007; 41: 269-271.
- Wound healing activity of flavonoid fraction isolated from the stem bark of Butea monosperma (Lam) in albino Wistar rats. Eur J Exp Biol. 2013; 3: 1-6.
- Muralidhar A, Sudhakar Babu K, Ravi Shankar T, Reddanna P, Reddy GV, Latha J. In vitro and in vivo anti-inflammatory activity of Butea monosperma stem bark extract. Int J Pharm Sci Res. 2010;1:44-51.
- Sapkale AP, Thorat MS, Dighe DA, Munot NM, Singh MC. Investigation of anti-inflammatory activity of whole flower extract of Butea monosperma by ex-vivo and in-vitro techniques and their correlation. Int J Pharm Pharm Sci. 2013; 5: 84-86.