- Biomedical Research (2015) Volume 26, Issue 4
Chemical constituents and biological activities of Stephania yunnanensis H. S. Lo
Xiaoli Shi, Xu Li and Ming Zou*Pharmacy Department of Affiliated Zhongshan Hospital of Dalian University, Dalian, China
- *Corresponding Author:
- Ming Zou
Pharmacy Department of Affiliated Zhongshan Hospital
of Dalian University Dalian 116000, China.
E-Mail: zouming2015@126.com
Accepted date: August 07 2015
Abstract
To study the chemical constituents of Stephania yunnanensis H. S. Lo and their in vitro antibiotic activities. Silica gel, alumina and Sephadex LH-20 chromatographic methods are used to isolate alkaloidal constituents. Structures of the compounds are identified based on physicochemical properties and spectral analysis. Kirby-Bauer test and porous plate method are used to study the inhibitory activities of isolated chemical constituents on methicillin-resistant S. aureus (MRSA). Five chemical constituents are isolated from the tuber extract of Stephania yunnanensis H. S. Lo, namely tetrahydropalmatine (1), palmatine (2), sinoacutine (3), dicentrine (4) and jatrorrhizine (5). Inhibition zone diameters of tetrahydropalmatine and palmatine against MRSA are larger at a concentration of 50 mg/mL, which are 13 mm and 16 mm, respectively; tetrahydropalmatine and palmatine both have inhibitory effects on MRSA, with MIC of 0.312 μg/ml and 0.156 μg/ml. In the porous plate method, IC50 of tetrahydropalmatine and palmatine are 0.436 μg/ml and 0.201 μg/ml. Alkaloidal constituents in Stephania yunnanensis H. S. Lo are mainly berberine type, morphinan type and protoberberine type alkaloids. Besides, tetrahydropalmatine and palmatine have rather significant anti-MRSA activities.
Keywords
Stephania yunnanensis H. S. Lo, Chemical constituent, Alkaloid, MRSA, Biological activity
Introduction
Stephania yunnanensis H. S. Lo is a famous traditional Chinese herbal medicine in Yunnan, China, which is often called Shanwugui, Jinbuhuan, Didan and Baididan in folk medicine. The herb is bitter, acrid, cold and slightly toxic, which has heat-clearing, detoxifying, sedative, analgesic and qi-regulating functions. In the folk medicine, it is used to treat snake bites, rheumatic pain, stomach ache, etc. Studies have shown that Stephania yunnanensis H. S. Lo contains a variety of chemical constituents, which are mainly alkaloids, and some polysaccharides [1-3]. Modern medical research has revealed that Stephania has insecticidal, antibiotic, and antiviral activities [4-7].
Snake bites are prone to cause infection; in folk medicine, Stephania is used to treat snake bites, suggesting that Stephania may contain some natural products with good antibiotic activities. To further discover natural products with antibiotic activities, this paper extracts and isolates monomer compounds in Stephania yunnanensis H. S. Lo, and conducts in vitro antibiotic activity tests on these monomer chemicals.
Materials
Melting point was measured with SGW X-4 micro melting point apparatus (Wuguang, Shanghai), and was uncorrected; MS was measured with Waters Synapt G2 mass spectrometer; and NMR was measured with Bruker- 400 SYS-600 NMR spectrometer using TMS as the internal standard. Miniature vortex mixer was manufactured by Huxi Analytical Instrument Factory, Shanghai; ZHJH-CH09B clean bench was manufactured by Zhicheng Analytical Instrument Manufacturing Co., Ltd., Shanghai; and ZD-85A dual-function gas bath thermostatic oscillator was manufactured by Jieruier Electrical Equipment Co., Ltd., Jintan.
Column chromatography and TLC silica gel plates were manufactured by Qingdao Haiyang Chemical Plant; column chromatography alumina was manufactured by Kaimei Chemical Reagent Factory; and Sephadex LH-20 was manufactured by Amersham Pharmacia, Sweden. Reagents like ethanol, acetone, chloroform and cyclohexane used were industrially or chemically pure solvents; and TLC reagents used were 10% sulfuric acid in ethanol and modified bismuth potassium iodide solution. Nutrient broth medium was provided by Aobox Biotechnology LLC, Beijing; and agar was provided by Solarbio Technology Co., Ltd., Beijing.
Stephania yunnanensis H. S. Lo medicinal herb was purchased from a medicinal material wholesale market in Gejiu, Yunnan, which was identified by Professor Zhang Dongfang of the China Medical University as the Stephania yunnanensis H. S. Lo in the genus Stephania of the family Menispermaceae. The specimens were preserved in the College of Pharmacy of Dalian Medical University. Methicillin-resistant S. aureus (MRSA) was isolated at the First Affiliated Hospital of Dalian Medical University, whose coincidence rate was 99% upon identification by automated microbial analyzer VITEKAMS.
Extraction and Isolation
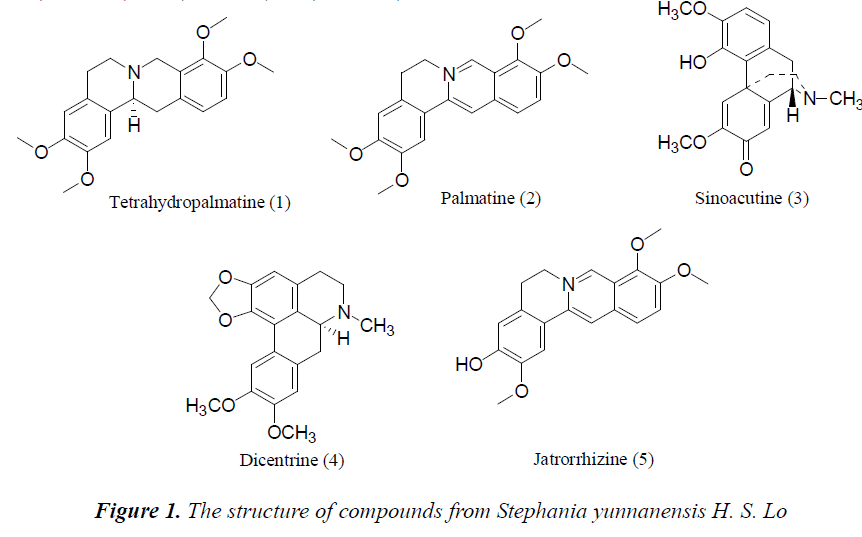
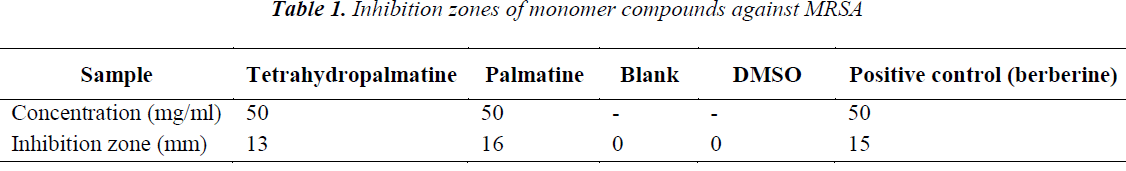
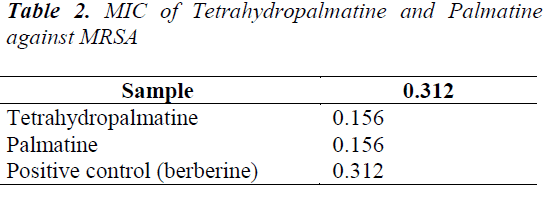
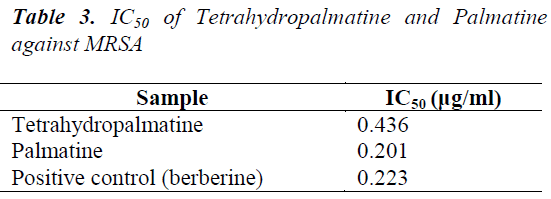
Stephania yunnanensis H. S. Lo was sliced, dried and crushed, then 5 Kg of the powder was taken and extracted for 2 h with 95% ethanol three times. The extracts were combined, and solvent was recovered under reduced pressure to give 700 g of extract. After suspending in water, the extract was extracted sequentially with petroleum ether, ethyl acetate and n-butanol to give respective fractions. 190 g of ethyl acetate fraction was isolated repeatedly by silica gel and alumina column chromatographies, and purified by Sephadex LH-20 column chromatography to obtain 5 compounds: tetrahydropalmatine (120 mg), palmatine (360 mg), sinoacutine (80 mg), dicentrine (56 mg) and jatrorrhizine (89 mg).
Structure Elucidation
(1) Tetrahydropalmatine: Colorless tabular crystals (methanol), Mp. 149.6~142.1°C, ESI-Ms (m/z) 356 [M+H]+, C21H25NO4. 1H-NMR (400 MHz, CD3OD) δ: 6.77 (1H, s, H-1), 6.65 (1H, s, H-4), 2.79 (2H, t, J=6.0 Hz, H-5), 3.13 (2H, t, J=6.0 Hz, H-6), 4.31 (1H, d, J=15.8Hz, H-8α), 3.54 (1H, d, J=15.8 Hz, H-8α), 6.89 (1H, d, J=8.4 Hz, H-11), 6.81 (1H, d, J=8.4 Hz, H-12), 2.90 (2H, m, H-13), 3.23 (1H, d, J=5.4 Hz, H-14), 3.83 (3H, s, 9- OCH3), 3.87 (3H, s, 10-OCH3), 3.89 (3H, s, 2-OCH3), 3.85 (3H, s, 3-OCH3); 13C-NMR (100 MHz, CD3OD) δ: 109.1 (C-1), 125.9 (C-1a), 147.9 (C-2), 147.1 (C-3), 110.6 (C-4), 129.0 (C-4a), 29.2 (C-5), 51.7 (C-6), 54.1 (C-8), 128.2 (C-8a), 151.0 (C-9), 145.5 (C-10), 110.8 (C-11), 121.6 (C-12), 126.9 (C-12a), 36.5 (C-13), 60.2 (C-14), 59.9 (9-OCH3), 56.1 (3-OCH3), 56.1 (10-OCH3), 56.1 (2- OCH3). 1H-NMR and 13C-NMR data were basically consistent with those reported in the literature [8], so the compound was identified as Tetrahydropalmatine.
(2) Palmatine: Pale yellow needle crystals (methanol), Mp. 205.2-207.9°C, ESI-Ms (m/z) 352 [M+H]+, C20H19NO4. 1H-NMR (400 MHz, DMSO) δ: 7.75 (1H, s, H-1), 7.12 (1H, s, H4), 3.22 (2H, t, J=6.5 Hz, H-5), 4.99 (2H, t, J=6.5 Hz, H-6), 9.85 (1H, s, H-8), 8.21 (1H, d, J=9.1 Hz, H-11), 8.11 (1H, d, J=9.1Hz, H-12), 9.12 (1H, s, H-13), 4.15 (3H, s, 9-OCH3), 4.09 (3H, s, 10-OCH3), 3.95 (3H, s, 2-OCH3), 3.81 (3H, s, 3-OCH3); 13C-NMR (100 MHz, DMSO) δ: 109.9 (C-1), 149.2 (C-2), 149.8 (C- 3), 112.1 (C-4), 132.8 (C-4a), 26.4 (C-5), 55.5 (C-6), 145.9 (C-8), 119.1 (C-8a), 152.0 (C-9), 143.5 (C-10), 123.6 (C-11), 126.9 (C-12), 129.1 (C-12a), 119.9 (C-13), 137.5 (C-14), 121.3 (C-14a), 61.5 (9-OCH3), 57.2 (3- OCH3), 56.1 (10-OCH3), 55.6 (2-OCH3). 1H-NMR and 13C-NMR data were basically consistent with those reported in the literature [9], so the compound was identified as Palmatine.
(3) Sinoacutine: Colorless blocky crystals (methanol), Mp. 197.9-199.3°C, ESI-Ms(m/z) 328 [M+H]+, C19H21O4N. 1H-NMR (400 MHz, CDCl3) δ: 6.74 (1H, d, J=8.3 Hz, H- 1), 6.66 (1H, d, J=8.3 Hz, H-2), 6.32 (1H, s, H-5), 7.61 (1H, s, H-8), 3.61 (1H, d, J=5.3 Hz, H-9), 2.93 (1H, dd, J=17.6 Hz, 5.3 Hz, H-10α), 3.32 (1H, d, J=17.6 Hz, H- 10β), 1.78 (1H, td, J=12.6 Hz, 4.5 Hz, H-15α), 2.41 (1H, d, J=12.6 Hz, H-15β), 2.52 (1H, dd, J=12.4 Hz, 3.0 Hz, H-16α), 2.62 (1H, dd, J=12.4 Hz,3.0 Hz, H-16β), 3.76 (3H, s, 3-OCH3), 3.89 (3H, s, 6-OCH3), 6.41 (1H, br.s, 4- OH), 2.46 (3H, s, N-CH3); 13C-NMR (100 MHz, CDCl3) δ: 121.2 (C-1), 109.0 (C-2), 144.9 (C-3), 144.1 (C-4), 118.6 (C-5), 161.5 (C-6), 182.1 (C-7), 123.4 (C-8), 61.2 (C-9), 37.9 (C-10), 130.8 (C-11), 124.1 (C-12), 44.2 (C- 13), 152.1 (C-14), 32.7 (C-15), 47.2 (C-16), 41.8 (NCH3), 56.4 (3-OCH3), 54.9 (6-OCH3). 1H-NMR and 13CNMR data were basically consistent with those reported in the literature [10], so it was identified as Sinoacutine.
(4) Dicentrine: Pale yellow blocky crystals (methanol), Mp. 168.1-170.3°C, ESI-MS (m/z) 340 [M+H]+. 1H-NMR (400 MHz, CDCl3) δ: 6.55 (1H, s, H-3), 2.67 (2H, m, H2- 4), 3.23 (4H, m, H2-5, H2-7), 3.98 (1H, m, H-6α), 5.95, 6.10 (each 1H, d, J=1.9 Hz, -OCH2O-), 6.82 (1H, s, H-8), 7.76 (1H, s, H-11), 2.53 (3H, s, N-CH3), 3.90 (6H, s, 9- OCH3, 10-OCH3); 13C-NMR (100 MHz, CDCl3) δ: 142.8 (C-1), 117.7 (C-1a), 127.2 (C-1b), 148.1 (C-2), 105.9 (C- 3), 123.9 (C-3a), 29.1 (C-4), 53.7 (C-5), 63.4 (C-6a), 35.9 (C-7), 128.1 (C-7a), 111.4 (C-8), 147.9 (C-9), 148.8 (C- 10), 110.6 (C-11), 126.9 (C-11a), 101.7 (-OCH2O-), 44.1 (N-CH3), 56.2 (9-OCH3), 56.9 (10-OCH3). The above data were consistent with those reported in the literature [11], so the compound was identified as Dicentrine.
(5) Jatrorrhizine: Red prismatic crystals (methanol), Mp. 205.3-207.9°C, ESI-S (m/z) 338 [M]+. 1H-NMR (400 MHz, DMSO) δ: 7.72 (1H, s, H-1), 6.96 (1H, s, H-4), 3.13 (2H, t, J=6.5 Hz, H-5), 4.98 (2H, t, J=6.5 Hz, H-6), 9.82 (1H, s, H-8), 8.21 (1H, d, J=9.2 Hz, H-11), 8.10 (1H, d, J=9.2 Hz, H-12), 9.01 (1H, s, H-13), 4.13 (3H, s, 9-OCH3), 4.07 (3H, s, 10-OCH3), 3.94 (3H, s, 2-OCH3); 13C-NMR (100 MHz, DMSO) δ: 109.7 (C-1), 147.9 (C-2), 149.5 (C-3), 114.4 (C-4), 134.3 (C-4a), 26.7 (C-5), 56.3 (C-6), 145.8 (C-8), 117.9 (C-8a), 151.1 (C-9), 143.9 (C-10), 123.1 (C- 11), 126.0 (C-12), 129.6 (C-12a), 118.2 (C-13), 137.3 (C-14), 122.1 (C-14a), 61.9 (9-OCH3), 57.2 (2-OCH3), 56.7 (10-OCH3). The above data were consistent with those reported in the literature [12], so the compound was identified as Jatrorrhizine.
Determination of Antibiotic Activities
Preparation of samples
Tetrahydropalmatine and palmatine were dissolved separately in DMSO to prepare 50 mg/mL stock solutions for later use.
Activation of test strains
MRSA strains were seeded on slant medium, and cultured in a 37°C incubator for 24 h for later use.
Preparation of bacterial culture plate
The prepared agar medium was transferred into conical flask, autoclaved at 121°C for 15 min, and cooled to 50~60°C, then poured into plate on a clean bench, and cooled to give solid medium for late use.
Preparation of bacterial suspension
After activation, the strains were seeded on broth medium, then prepared into a bacterial suspension of a certain concentration, and stored in a 4°C refrigerator (not longer than 12 h).
Preparation of drug-containing paper discs
The samples were diluted, and sterilized for later use. Each paper disc (6 mm in diameter) was added with 5 μL of solutions, whereas blank control group was added with 5 μL of sterile distilled water. Negative control group was added with 5 μL of analytical grade DMSO.
K-B method
On a clean bench, sterilized agar media were poured into Petri dishes. After marking the dishes, 0.1 mL of prepared bacterial suspension was pipetted with a sterile pipette into the Petri dishes, and spread uniformly with a spreader. The above paper discs were placed equidistantly on the bacterium-containing plates to prepare bacterial plates. After setting aside at room temperature for 3~5 min, drug-containing paper discs were added, and the plates were incubated at a 37°C incubator for 24 h, then colony growths were observed, and inhibition zone diameters were determined accurately [7]. Each antibiotic test was repeated twice in parallel for each diluent.
Determination of minimum inhibitory concentration (MIC)
After samples were diluted proportionally with suitable amounts of solvent (DMSO), each paper disc was added with 5 μL of drug solutions, blank control group was added with 5 μL of sterile distilled water, and negative control group was added with 5 μL of analytical grade DMSO. The concentration at which there was no detectable inhibition zone diameter was the MIC. All operations were carried out under sterile conditions.
Determination of median inhibitory concentration (IC50) [8-16]
Clean and sterile 96-well culture plates were added separately with 90 μL of MRSA that was cultured in broth medium for 18 h, and then added with 10 μL of sample stock solutions with different concentration gradients; the final concentration of solvent (DMSO) was 1/10 the concentration of stock solutions. Meanwhile, negative, blank and solvent controls were set up, each with three replicates. After the 96-well plates were cultured at 37°C for 20 h, absorbance (A) was measured at 595 nm using a microplate reader. Supernatant A of each sample under the same conditions was taken as the blank control, after subtracting the blank control, A of bacterial amount was obtained, corresponding supernatant was prepared under 10000/6 min conditions, and drawn 100 μL correspondingly. Inhibition rate (%) was calculated according to the following formula:
Inhibition rate = [(Asolvent control - Acontrol supernatant) - (Adrug solution -Adrug supernatant)] / (A positive control - Anegative supernatant) × 100%
Curves were plotted with sample concentration versus inhibition rate, linear regression equation was solved, and concentration causing 50% inhibition, i.e. IC50 value, was calculated.
Results
Determination of inhibition zone
Tests were carried out as per the procedure under "K-B method", respectively. Inhibition zone data of each sample are shown in Table 1.
Determination of MIC
Each sample was tested as per the method under "Determination of minimum inhibitory concentration (MIC)" for antibiotic activity against MRS; the data are shown in Table 2.
Determination of IC50
Each sample was tested for IC50 in accordance with the "Determination of median inhibitory concentration (IC50); the results are shown in Table 3.
Stephania may achieve treatment of snake bites in the folk medicine by two ways: 1. it can detoxify snake venom, and avoid the venom from damaging other organs; 2 it can heal wounds, and kill bacteria around the wounds. The aim of this study is to find active constituents in Stephania yunnanensis H. S. Lo by studying its chemical constituents and antibiotic activities, so as to lay the foundation for development and utilization of Stephania yunnanensis H. S. Lo resources.
Five compounds isolated from Stephania yunnanensis H. S. Lo are all alkaloids, of which tetrahydropalmatine and palmatine are berberine alkaloids, sinoacutine and dicentrine are morphinane alkaloids, and jatrorrhizine protoberberine alkaloid. This suggests that alkaloids are the main chemical constituents of Stephania yunnanensis H. S. Lo. Alkaloids have a high biological activity, and berberine alkaloids have a good antibiotic activity [17,18]. To study in depth the chemical constituents in Stephania yunnanensis H. S. Lo, we choose tetrahydropalmatine and palmatine as the study objects. Main reasons are as follows: 1. tetrahydropalmatine and palmatine are alkaloids, which have good biological activities. 2 Tetrahydropalmatine and palmatine have similar structures, so differences in antibiotic activities can be explored from the structural perspective.
Experimental results show that the inhibition zone diameters of tetrahydropalmatine and palmatine against MRSA are larger at a concentration of 50 mg/mL, which are 13 mm and 16 mm, respectively. Tetrahydropalmatine and palmatine both have inhibitory effects on MRSA, with MIC of 0.312 μg/ml and 0.156 μg/ml. In the porous plate method, IC50 of tetrahydropalmatine and palmatine are 0.436 μg/ml and 0.201 μg/ml. All anti-MRSA activity indicators of palmatine are higher compared with the positive control berberine, whereas tetrahydropalmatine has anti-MRSA activity indicators all slightly lower than berberine. Main reasons may be: 1. compared with tetrahydropalmatine, palmatine has conjugated structure, and uniform electron cloud distribution, its solubility is better than tetrahydropalmatine, which can easily penetrate bacterial membrane and fully act with bacteria. 2. Palmatine is smaller in terms of spatial volume, which is substantially in the same plane, while tetrahydropalmatine occupies larger space structurally. Spatial difference may result in deviation in targets, leading to difference in antibiotic activities.
There is still space for in-depth study of this topic. To be able to further explore its antibiotic mechanism, the following experiments can be conducted: 1. TEM observation of microscopic damage of bacteria by drug. 2. Flow cytometric detection of changes in bacterial metabolic cycle. 3. Establishment of wound infection model to clarify the healing effect of drug on infected wounds, so as to lay a solid foundation for development and utilization of Stephania yunnanensis H. S. Lo.
Acknowledgements
This work was supported by the Department of Education of Liaoning Province (No. L2014500).
References
- Dai X, Hu R, Sun C, Pan Y. Comprehensive separationand analysis of alkaloids from Stephaniayunnanensisby counter-current chromatography coupled with liquid chromatography tandem mass spectrometry analysis. Journal Of Chromatography A 2012; 1226:18-23.
- Hu RL, Dai XJ, Lu YB, Pan YJ.Comprehensive separation and analysis of alkaloids from Stephania Preparative separation of isoquinoline alkaloids from Stephaniayunnanensis by pH-zone-refining countercurrent chromatography. Journal of Chromatography B: Analytical Technologies in the Biomedical and Life Sciences 2010; 878: 1881-1884.
- Zhang LB, Rao GX. Aporphine, protoberberine and morphine alkaloids from the tubers of Stephaniayunnanensis.Biochemical Systematics and Ecology 2009; 37: 622-625.
- Deng YC, Xu HH. Studies on Insecticidal Activities and Active Ingredients of Stephaniakwangsiensis Lo.ScientiaAgriculturaSinica 2005; 3: 523-527.
- Deng YC, Li JR, Gao CW, Yang LL. Inhibitory activity of the extract from the root tubers of Stephaniakwangsiensis and its compounds against pathogenic fungi and bacteria. Plant Protection 2007; 4: 43-46.
- Hao J, Wang YF, Zhang MY, Yang K, Luo Y. Experimental study on the anti-Coxsackie virus B3 activity of Stephania crude extract. Jiangsu Journal of Traditional Chinese Medicine 2008; 9: 125-128.
- Deng YC, Yu YZ,Luo HY, Zhang M, Qin X, Li LF. Antimicrobial activity of extract and two alkaloids from traditional Chinese medicinal plant Stephaniadielsiana. Food Chemistry 2011; 124: 1556-1560.
- Wu Y, He Y, He WY, Zhang YM, Lu J, Dai Z, Ma SC, Lin RC. Application of quantitative 1H-NMR for the calibration of protoberberine alkaloid reference standards. Journal of Pharmaceutical and Biomedical Analysis 2014; 90: 92-97.
- Lv ZM, Zhao SH, Liang JQ, Tu PF. Synthesis and characterization of 9-O-alkyl substituted palmatinederivatives. ZhongguoZhong Yao ZaZhi 2014; 39: 699-703.
- Kashiwaba N, Momoka S, Kimura M, Minoru O, Jun T, Hideki S, Takehiro S. New morphinane and hasubanane alkaloids from stephaniacepharantha. J Nat Pmd 1996; 59(5): 476-480.
- Garcez WS, Garcez FR, Silva LM, Hamerski L. Larvicidal activity against Aedesaegypti of some plants native to the West-Central region of Brazil. Bioresource Technology 2009; 100: 6647-6650.
- Ding PL, Chen LQ, Lu Y, Li YG. Determination of protoberberine alkaloids in RhizomaCoptidis by ERETIC 1H-NMR method. Journal Of Pharmaceutical And Biomedical Analysis 2012; 60: 44-50.
- Jiang WU, Zhang GL, Liu J, Gao HB, Song CX, Du HR, Zhang L, Gong ZP, Lv YG. Synthesis, characteristics, and antibacterial activity of a rare-earth samarium/silver/titanium dioxide inorganic nanomaterials. Journal of Rare Earths 2014; 32: 727-732.
- Kovárová M, Komers K, Stepánková S, Parík P, Cegan A. New method for the determination of the half inhibition concentration (IC50) of cholinesterase inhibitors. Z Naturforsch C 2013; 68: 133-138.
- Caldwell WG, Yan ZY, Lang WS, Masucci AJ. The IC50 Concept Revisited. Current Topics in Medicinal Chemistry 2012; 12: 1282-1290.
- Carrillo W; García-Ruiz A, Recio I, Moreno-Arribas MV. Antibacterial Activity of Hen Egg White Lysozyme Modified by Heat and Enzymatic Treatments against Oenological Lactic Acid Bacteria and Acetic Acid Bacteria. J Food Prot 2014; 77: 1732-1739.
- Zhang DF, Li AH, Xie J, Cheng Ji. In vitro antibacterial effect of berberine hydrochloride and enrofloxacin to fish pathogenic bacteria.Aquaculture Research 2010; 41: 1095-1100.
- Wojtyczka RD, Dziedzic A, Kępa M, Kubina R, Kabała-Dzik A, Mularz T, Idzik D. BerberineEnhances the Antibacterial Activity of Selected Antibiotics against Coagulase-Negative Staphylococcus Strains in Vitro. Molecules 2014; 19: 6583-6596.