Research Article - Journal of Infectious Diseases and Medical Microbiology (2018) Volume 2, Issue 3
Assessment of ceftriaxone use in Eastern Ethiopian Referral Hospital: A retrospective study.
Hafte K1*, Tefera K2,3, Azeb W3, Yemsrach W4, Ayda R5
1College of Health Sciences, Discipline of Pharmacy, Defense University, Ethiopia
2Dire Dawa City Administration Health Bureau, Dire Dawa, Ethiopia
3Ethiopia Defense Minister, Health Department HIV/AIDS prevention and control office, Ethiopia
4Defense University College of Health Sciences, Addis Ababa, Ethiopia
5London School of Hygiene and Tropical Medicine, University of London, UK
- *Corresponding Author:
- Hafte Kahsay Kebede
Collage of Health Sciencess
Discipline of Pharmacy, Defense University
Bole Street
Addis Ababa,
Ethiopia
Tel: +251924955116
E-mail: maymegeltana@gmail.com
Accepted date: September 21, 2018
Citation: Hafte K, Tefera K, Azeb W, et al. Assessment of ceftriaxone use in Eastern Ethiopian Referral Hospital: A retrospective study. J Infectious Disease Med Microbiol. 2018;2(3):26-9.
Abstract
Introduction: Drug use evaluation (DUE) is a system of ongoing, systematic criteria based evaluation of drug use that will help ensure that medicines are used appropriately at the individual patient level. Ceftriaxone was the top and first frequently prescribing antibiotics in Dilchora Hospital. Therefore, this study was designed to evaluate the use of ceftriaxone by using performance improvement method that focuses on evaluation and improvement of drug use processes. Methodology: A cross sectional study design was used. Data collection format were developed according to WHO recommendation and validated to our context. The data was processed and analyzed using EX-Cell sheet as per WHO criteria for drug use evaluation as per standard treatment guideline of Ethiopia. Result: Out of the 174 patients on ceftriaxone, 43 (24.71%) were diagnosed CAP and 41 (23.56%) patients were used without indication. Majority of patients 71 (47.33%) were treated with ceftriaxone for 3-7 days followed by 49 (32.67%) for 8-14 days. ceftriaxone was found to be appropriate for the justification of use in 34 (19.56%) of patients. Conclusion: In Dilchora Hospital prescribers were not sticking to Ethiopian standard treatment guide line (STG). Majority of cases of indication for ceftriaxone was inappropriate and seen with duration of therapy on average 3-7 days. Therefore, we recommend DCH should adhere to national treatment guideline. In addition, DCH should promote the involvement of multidisciplinary team particularly the clinical pharmacists in all wards in monitoring of use of ceftriaxone and other antibiotics use.
Keywords
Ceftriaxone, Inappropriate, STG, Dire Dawa, Ethiopia.
Rationale of the Study
Drug use evaluation (DUE) is a system of ongoing, systematic criteria based evaluation of drug use that will help ensure that medicines are used appropriately at the individual patient level. DUE may be applied to a drug, therapeutic class, disease state or condition, a drug use process or outcomes [1,2]. DUE can assess the actual process of medication prescribing, administration, or dispensing. It involves a comprehensive review of patients’ prescription and medication data before, during and after dispensing in order to assure appropriate therapeutic decisionmaking and positive outcome [3,4]. Cephalosporins are the most widely used antibiotics for treating common infections [5]. Ceftriaxone is the most commonly prescribed drug due to its high anti-bacterial potency, wide spectrum of activity and low potential for toxicity. Despite its wide spectrum use, there are trends showing the misuse of ceftriaxone [6,7].
Studies conducted to assess appropriateness of ceftriaxone use in America [1], Port of Spain [7] Korea [6], black lion hospital, Ethiopia [8], Addis Ababa police hospital, Ethiopia showed 82%, 62%, 65%, 71%, 63% [9] of inappropriate use respectively. The appropriate use of ceftriaxone in Ayder Referral Hospital study was (35.8%) was close to the result obtained in Port of Spain [10]. In addition, a study performed by reviewing prescription from Dilchora OPD pharmacy at the end of 2009 E.C, to assess medicine use pattern in outpatient setting found that, 71.50% of the total prescription had antibiotics which is far from WHO recommendation (20-30%) [5].
To identify the specific antibiotic, we have done ABC-VEN analysis, and found that, ceftriaxone was the first among the top ten drugs; it utilized 7.8% of the total budget of pharmaceuticals. These findings indicating that ceftriaxone was the top and first frequently prescribing antibiotics in Dilchora Hospital. Therefore, this study was designed to evaluate the use of ceftriaxone by using performance improvement method that focuses on evaluation and improvement of drug use processes to achieve optimal patient outcomes in overall health care system at Eastern Ethiopian Hospital (Dilchora Hospital).
Materials and Methods
Study design, settings and participants
A retrospective cross sectional study design was used to evaluate the use and appropriateness of ceftriaxone in Dilchora Hospital, Dire Dawa Ethiopia from December 2017-January 2018. The Hospital provides comprehensive curative, preventive and rehabilitative service for around 1,000,000 people living in Dire Dawa and its surrounding community. Data extraction tool was developed using WHO tools.
Sampling procedure
A total of 207 hospitalized patients received ceftriaxone between August 2016-July 2017, and 170 patient’s files were complete and available. The rest 37 patient’s files were lost and incomplete. They used systematic random sampling method. The data was extracted from patients' medical records during hospitalization in Dilchora Hospital.
Data collection procedures
Data collection format were developed according to WHO recommendation and validated to our context. The tool was tested by taking ten patient medical files and the pre-tested files were excluded from the study. Each case from the patient medication records were evaluated against the STG of Ethiopia for indication, dosage, frequency and duration of ceftriaxone therapy. Supervised by the principal investigators, five diploma nurses collected the data. We gave a half day training for the data collectors. To ensure the quality of data, the principal investigator checked the questionnaire for completeness and consistency daily.
Statistical analysis
The data was processed and analyzed using EX-Cell sheet as per WHO criteria for drug use evaluation as per standard treatment guideline of Ethiopia. Four WHO criteria namely indication for use, dosage, frequency and duration were used to evaluate ceftriaxone use.
Ethical considerations
The ethical clearance of this thesis was approved by Ethical clearance committee of Dilchora Hospital to the start of data collection. Permission was taken from Dilchora Hospital Administration.
Results
Socio-demographic characteristics
Among the 174 patients, 122(70.11%) were female and 52(29.89%) male. Nearly ninety percent (90%) of patients were in the age group of 15-65 years (Table 1).
Table 1.Distribution of patients on ceftriaxone as disaggregated by sex and age inpatient ward at Dilchora Hospital, East Hararge, Ethiopia from August 2016 to July 2017 (n=174 encounters).
| Variables | Frequency | Percentage (%) |
|---|---|---|
| Sex | ||
| Male | 52 | 29.89 |
| Female | 122 | 70.11 |
| Age (year) | ||
| <15 | 15 | 8.62 |
| 15-65 | 155 | 89.08 |
| >65 | 4 | 2.30 |
| Weight | ||
| Measured | 9 | 5.17 |
| Not measured | 165 | 94.83 |
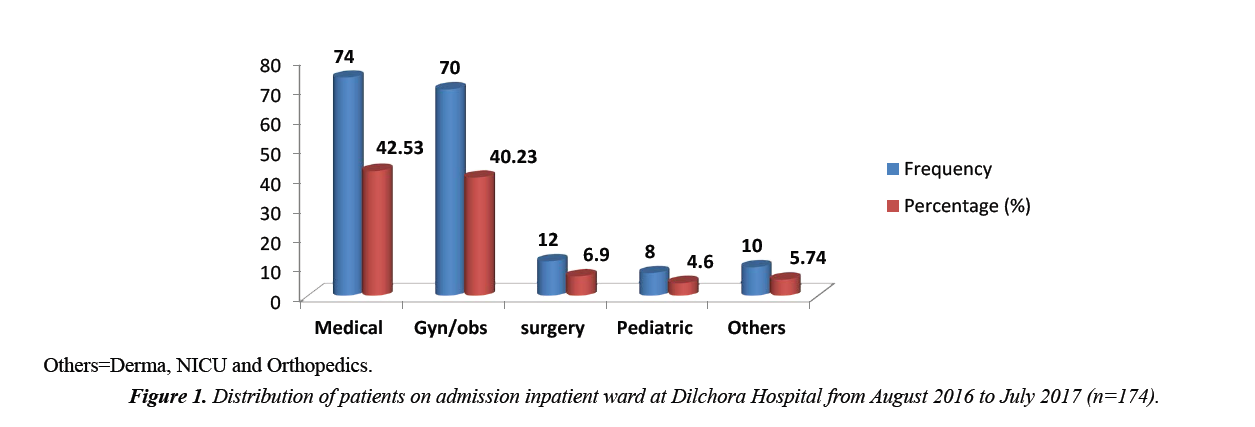
Most cases of ceftriaxone utilization in Medical ward 74(42.53%); the remaining were gynecology and obstetrics ward 70(40.25%), surgical ward 12(6.90%), pediatric ward 8(4.60%) and others 10(5.74%) (Figure 1).
Distribution of diagnosis and its indication of ceftriaxone
Majority of patients 43(24.71%) were diagnosed CAP and indicated for ceftriaxone and 41(23.56%) patients were used ceftriaxone without indication (Table 2). Majority of patients 71(47.33%) were treated with ceftriaxone for 3-7 days followed by 49(32.67%) for 8-14 days.
Table 2: Reasons for ceftriaxone use during admission at Dilchora Hospital from August 2016 to July 2017 (n=174).
| Diagnosis at Admission | Frequency | Percentage (%) |
|---|---|---|
| Community acquired pneumonia | 43 | 24.71 |
| Caesarian section | 33 | 18.97 |
| CPD/obstructed labor | 9 | 5.17 |
| DVT + CHF + severe anemia + IUFD + APH+ term prom+ pancytopenia + RVI + burn + severe head injury + AFSOL primegravida + (Jaundice + pre-term-1) + (LONS + NEC +EONS-5) (disease without indication) |
41 | 23.56 |
| SBP | 12 | 6.90 |
| UTI | 8 | 4.60 |
| Mastitis + cellulitis + pyomyoscitis | 3 | 1.72 |
| Septic arthritis | 3 | 1.72 |
| p/o BPH/TVP | 4 | 2.30 |
| Upper GI bleeding-prophylaxis | 3 | 1.72 |
| Foot ulcer | 3 | 1.72 |
| AGE | 3 | 1.72 |
| Sepsis | 3 | 1.72 |
| Others | 7 | 5.14 |
Other=Brain abscess, Necrotizing fasciitis, Inguinal hernia p/o-prophylaxis, Acute appendicitis, Soft tissue injury, Cholilitiasis and brain abscess.
Nearly 95% of patients took 2 g ceftriaxone in daily based doses. From the total patients only two of them were took ceftriaxone despite contraindication (Table 3).
Table 3: Distribution of indication, duration and dose of ceftriaxone for different cases at Dilchora Hospital from August 2016 to July 2017 (n = 174).
| Category | Frequency | Percentage (%) |
|---|---|---|
| Indication of ceftriaxone | ||
| Treatment | 95 | 54.60 |
| Prophylaxis | 38 | 21.84 |
| Not indicated | 41 | 23.56 |
| Duration of therapy | ||
| Stat | 2 | 1.33 |
| 1 -2 days | 21 | 14.0 |
| 3-7 days | 71 | 47.33 |
| 8-14 days | 49 | 32.67 |
| 15-21 days | 6 | 4.0 |
| >21 days | 1 | 0.67 |
| Dose of drug | ||
| < 1 gm/day | 9 | 5.17 |
| 2 gm/day | 164 | 94.25 |
| 4 gm/day | 1 | 0.57 |
| Missed doses | ||
| 1-2 dose | 34 | 50.0 |
| 3-5 dose | 20 | 29.41 |
| 6-8 dose | 12 | 17.65 |
| >8 dose | 2 | 2.94 |
| Contra indication | ||
| Yes | 2 | 1.15 |
| No | 172 | 98.85 |
Outcome monitoring and documentation
From the total study participants (174), patient clinical vital signs were not recorded in 135(87.59%) patients on daily bases. Only 29(16.67%) and 15(8.62%) of patient charts had CBC within 48 hours and CBC within 2-7 days respectively. Nearly three fourth (3/4) of patient charts had no documented patient outcome (Table 4).
Table 4: Distribution of outcome monitoring at Dilchora Hospital from August 2016 to July 2017 (n=174).
| Clinical characteristics | Frequency | Percentage (%) |
|---|---|---|
| Clinical monitoring | ||
| V/S at least 3x/day | ||
| Yes | 39 | 22.41 |
| No | 135 | 87.59 |
| Laboratory monitoring | ||
| CBC within 48 hrs. | ||
| Yes | 29 | 16.67 |
| No | 145 | 83.33 |
| CBC within 2-7 days | ||
| Yes | 15 | 8.62 |
| No | 159 | 91.38 |
| Out come | ||
| Documented | 47 | 27.01 |
| Not-document | 127 | 72.99 |
Co-administered drugs
Among the total drugs co-administered with ceftriaxone, metronidazole was the most commonly used medication (Table 5). Among the 174 cases, 140(80.46%) were found to be inappropriate (not according to the Ethiopian STG). While the rest 34(19.54%) were appropriate (Table 6).
Table 5: Frequently co-administered drugs with ceftriaxone, at Dilchora Hospital from August 2016 to July 2017 (n=174).
| Drugs | Frequency | Percent (%) |
|---|---|---|
| Metronidazole | 45 | 13.39 |
| Furosemide | 33 | 9.82 |
| Spironlactone | 29 | 8.63 |
| Azithromycin | 21 | 6.25 |
| Fluconazole | 17 | 5.06 |
| Diclofenac | 36 | 10.71 |
| Tramadol | 38 | 11.31 |
| Propranolol | 8 | 2.38 |
| Feso4 | 15 | 4.46 |
| Metoclopramide | 14 | 4.17 |
| Cimetidine | 20 | 5.95 |
| Cotrimoxazole | 15 | 4.46 |
| Anti TB | 9 | 2.68 |
| Other | 36 | 10.74 |
Table 6: Distribution of inappropriate use of ceftriaxone based on DUE criteria.
| Clinical parameters | # of inappropriate | Percentage (%) |
|---|---|---|
| Indication | 41 | 29.29 |
| Dose | 0 | 0.00 |
| Frequency | 29 | 20.71 |
| Duration | 70 | 50.00 |
Discussion
In this study, the use of ceftriaxone was found to be appropriate for the justification of use in 34(19.56%) which is much lower than the value obtained in a retrospective study conducted in Black Lion Hospital, Addis Ababa (71.43%) [8], police Hospital, Addis Ababa (73.03%) [9], and Ayder Referral Hospital, Mekelle Ethiopia (35.8%) [10]. This difference might be due to Black Lion Hospital and Police Hospital have adequate man power, well experience staff and well equipped.
According to our study finding, more than 90% of the indicated drugs were taken 2 g/day. Among the total daily dose ordered to be taken by the patients, 68 dosses were missed or not taken by the patient. Most of them (50%) were missed the range of 1-2 dosses. These results showed that there was a poor patient adherence and these types of practice makes poor patient outcome and have a great role in drug resistance.
Concerning to the clinical and laboratory patient monitoring parameter, our study found that; more than 87.59% of patients were not taken their vital sign at least three times per day despite a lot of internship students were available in each wards. The other laboratory parameter, CBC which is important for diagnosis and response monitoring for utilization for ceftriaxone, was ordered and documented the results only for 16.67% with in the first 48 hours of visit, and only 8.62% patient cases in the next 2-7 days of admission. This indicated that; there was a poor patient care and monitoring system in the hospital.
Conclusion and Recommendations
In Dilchora Hospital prescribers were not sticking to Ethiopian standard treatment guide line (STG). Majority of cases of indication for ceftriaxone was inappropriate and seen with duration of therapy on average 3-7 days. Most of the patient clinical and laboratory results and treatment outcome were not documented. Therefore, we recommend DCH should adhere to national treatment guideline. In addition, DCH should promote the involvement of multidisciplinary team particularly the clinical pharmacists in all wards in monitoring of use of ceftriaxone and other antibiotics use. It is also important to provide audit feedbacks to prescribers and other hospital staff on judicious use of antimicrobial agents and ceftriaxone in particular.
Acknowledgments
We are grateful to the data collectors who extracted the data. We acknowledge Mrs. Ayda Redie (MSc) from London School of Hygiene and Tropical Medicine for editing the paper. This research was funded by the investigators.
Authors' Information
sTefera K is from Dilchora Hospital, HKK is a lecturer and clinical pharmacist at Defense University, College of Health Sciences Ethiopia.
Authors’ Contributions
All authors were involved in designing of the study, data collection, data analysis, drafting and critically reviewing the manuscript. All authors read and approved the final manuscript.
Availability of Data and Materials
The dataset supporting the conclusions of this article is included within the article.
Funding
This research was granted by investigators. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Competing Interests
The authors have declared that no competing interests exist.
References
- Phillips MS, Gayman JE, Todd MW. ASHP guidelines on medication use evaluation. American Society of Health-system Pharmacists. Am J Health Syst Pharm. 1996;53(16):1953-5.
- SHPA Committee of Specialty Practice in Drug Use Evaluation. SHPA Standard of practice for drug use evaluation in Australian Hospital. J Pharm Pract Res. 2004;34(3):220-3.
- http://www.amcp.org
- World Health Organisation. Drug and therapeutic committee, A practical guide to drug use evaluation; Drug use evaluation (Drug utilization review). WHO, Geneva. 2003.
- Marshall WF, Blair JE. The cephalosporins. Mayo Clin Proc. 1999;74(2):187-95.
- Hyuck L, Dongsik J, Joon S, et al. Evaluation of ceftriaxone utilization at multicenter study. Korean J Intern Med. 2009;24(4):374-80.
- Pereira LMP, Phillips M, Ramlal H, et al. Third generation cephalosporin use in a tertiary hospital in Port of Spain, Trinidad: Need for an antibiotic policy. BMC Infect Dis. 2004;4(1):59.
- World Health Organisation. Antimicrobial use, resistance and containment baseline survey, syntheses of findings, Addis Ababa, Ethiopia. WHO, Geneva. 2009.
- Michael M, Mulugeta T. Comparative retrospective drug use evaluation of ceftriaxone injection in Police and Black Lion Hospitals, EPA, 2009.
- Abebe F. Retrospective drug use evaluation of ceftriaxone in Ayder referral hospital, Ethiopia.