- Biomedical Research (2016) Volume 27, Issue 2
Antitumor activity of gossypol polyphenol is mediated through apoptosis induction and sub-G1 cell cycle arrest in SEG-1 human esophageal cancer cell line.
| Ping Chen1,2, Li Zhang3, Jing-Wen Li1, Lei Tan4, Hong-Wei Xu1* 1Department of Gastroenterology, Provincial Hospital Affiliated to Shandong University, Jinan 250021, PR. China 2Department of Gastroenterology, Taian Central Hospital, Taian 271000, PR. China 3Department of Respiratory Intensive Care Unit, Taian Central Hospital, Taian 271000, PR. China 4Department of Thoracic Surgery, Taian Central Hospital, Taian 271000, PR. China |
| Corresponding Author: Hong-Wei Xu Department of Gastroenterology Provincial Hospital Affiliated to Shandong University Jinan 250021 PR. China Email: xuhongweihw@hotmail.com |
| Accepted: January 30, 2016 |
Abstract
The main objective of the present study was to evaluate the antitumor effects of gossypol in human esophageal cancer cells (SEG-1) by studying its effects on apoptosis induction and cell cycle phase distribution. Gossypol is a naturally occurring polyphenolic aldehyde with numerous biological activities. MTT assay was used to demonstrate the cytotoxic effect of gossypol while as fluorescence and scanning electron microscopic techniques were employed to study the effect of gossypol on cellular morphology. Flow cytometry was used to investigate the effects on cell cycle phase distribution and apoptosis induced by gossypol. Results showed that gossypol exhibited significant and time dependent cytotoxicity against the cancer cells. With increase in gossypol dose there was an increase in number of cells that were stained orange and red which is an indication of apoptosis. Gossypol treatment at lower doses have shown surface projections and blebbing of the membrane while as, at higher doses apoptotic bodies were found. In addition it has shown to induce sub-G1 cell cycle arrest.
Keywords |
||||||||||||
| Esophageal cancer, Apoptosis, Gossypol, Anticancer activity. | ||||||||||||
Introduction |
||||||||||||
| Esophageal cancer is the sixth leading cause of cancer-related deaths and is the eighth most frequent cancer throughout the world. Around 450000 new cases and 400000 deaths from esophageal cancer are reported every year globally. In China, esophageal cancer accounts for about half of the total esophageal cancer incidences worldwide [1]. Esophageal cancer which affects more males than females has been recognized to have two main subtypes depending on the pathological characteristics. One type which is more dominant is the esophageal squamous cell carcinoma and the other which is less common is the adenocarcinoma. Because of the prospective features of invasion and metastasis in esophageal carcinoma cells, which are important prognostic factors, the overall 5-year survival rate is poor despite advanced treatment [2,3]. Despite many advances in treatment including radiotherapy, chemotherapy and surgery, the 5-year survival rate is low and the important reason for this treatment failure is the tumor recurrence and multidrug resistance [4,5]. Esophageal cancer usually remains undiagnosed till late stages, and advanced esophageal cancer is accompanied with poor outcome and is one of the most intractable of cancers. Thus, there is a strong demand for new curative approaches to advanced esophageal cancer [6]. The aim of the present study was to investigate the antitumor activity of gossypol in human esophageal cancer cell line SEG-1. Gossypol, which is a naturally occurring polyphenolic aldehyde, is isolated mostly from the genus Gossypium plants. It has shown diverse biological activities. The present study demonstrates the effects of gossypol on apoptosis induction, cell cycle arrest, cell cyclerelated proteins and mitochondrial membrane potential loss. | ||||||||||||
Materials and Methods |
||||||||||||
Materials |
||||||||||||
| Gossypol (98% purity), Penicillin, Streptomycin, Rhodamine-123, Triton X-100 and 5% paraformaldehyde were purchased from Jinchan Biochemistry Company Ltd. (Anhui, China). All the reagents used in the experiments were of analytical grade. Fetal Bovine Serum (FBS), 2.5% trypsin, Dulbecco's modified Eagle's medium, 3-(4,5- dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)and DMSO were purchased from Gibco (Carlsbad, CA, United States). Annexin V-FITC and Acridine Orange (AO) was purchased from (Beyotime Institute of Biotechnology, Shanghai, China). Cell cycle phase determination kit was procured from Keygen Biotechnology (Nanjing, China). | ||||||||||||
Cell lines and cell culture conditions |
||||||||||||
| SEG-1 human esophageal cancer cell line was procured from the Cell Institute, Chinese Academy of Sciences (Shanghai, China) and cultured in Dulbecco's modified Eagle's medium containing 10% FBS, 100 U/ml Penicillin and 100 μg/ml Streptomycin in humified atmosphere with 5% CO2 at 37°C. Further the cells were sub cultured after attaining 95-100% confluency. | ||||||||||||
MTT cell proliferation assay |
||||||||||||
| The cytotoxicity of gossypol on human esophageal cancer cells was evaluated by MTT cell viability assay which is a colorimetric assay for assessing cell metabolic activity.In brief, SEG-1 cells were seeded in 96-well plates (1 × 105 cells/well). The cells were treated with various doses (0, 5, 10, 25, 75, 100 and 125 μM) of gossypol molecule for 12, 24 and 48 hours. After incubation, MTT dye (10 mg/ml) was added to each well and incubated for another 4 hours, further the formazan precipitate formed was dissolved in 400 μL DMSO, and then the absorbance was measured in Automated Microplated Reader (Bio-Tek, VT, USA) at 570 nm. The percentage of viable cells was calculated by the following formula: | ||||||||||||
| Inhibitory ratio (%) = (OD control – OD treated) / OD control × 100%. | ||||||||||||
| Cytotoxicity was expressed as the concentration of gossypol inhibiting cell growth by 50% (IC50 value). | ||||||||||||
Apoptotic assessment using fluorescence microscopy and acridine orange staining |
||||||||||||
| Cells were seeded at a density of 2 × 105 cell/ml in a volume of 4 ml/well on sterile cover slips placed in 6-well tissue culture plates. After incubation, the medium was removed and replaced with fresh medium with 5% FBS. The cells were treated with gossypol (0, 10, 25 and 100 μM). Following drug treatment, the cover slips with monolayer of cells were inverted on the glass slide with 10 μL of AO stain (20 μg/ml). Images were captured using a UV fluorescence microscope (Olympus Optical Co., LTD, Tokyo, Japan) using UV filter at 400 X magnification to detect morphological changes due to apoptosis. | ||||||||||||
Scanning Electron Microscopy (SEM) studies of ultrastructural cellular morphology |
||||||||||||
| SEG-1 cells at a density of 2 × 105 cells were seeded in sixwell microtitre plates and were treated with (0, 10, 25 and 100 μM) concentrations of gossypol for 12 h. The cells were then centrifuged at 12,000 rpm for 20 minutes followed by Phosphate Buffer Saline (PBS) washing. The supernatant was removed and resuspended in 20 ml of 0.15 M cacodylate buffer. The sample was again centrifuged at 12,000 rpm for 10 min. The cacodylate buffer was removed and 1.2% of Osmium Tetroxide (OsO4) in 0.2 M cacodylate buffer was added. The cover slips were washed three times with 0.2 M cacodylate buffer. After that 30% ethanol was added for 5 minutes. The coverslips were glued onto stubs with silver print and dried for about 20 minutes at room temperature. Then the samples were covered with gold using sputter coater. The images were captured by a scanning Electron Microscope (JOEL 64000, Japan) at accelerating voltage of 15-25 KV). | ||||||||||||
Annexin V-FITC binding assay/quantification of apoptotic cell death |
||||||||||||
| In order to quantify and confirm whether gossypol induced apoptosis, Annexin V-FITC binding assay using flow cytometry was performed. Briefly, SEG-1 cells were treated with gossypol (0, 10, 25 and 100 μM) for 48 h, and then harvested by trypsinization. Harvested cells were then incubated in annexin V-FITC (25 μg/ml) and propidium iodide (25 μg/ml), at room temperature for 20 min in the dark, and analysed using BD FACSCalibur™ flow cytometer (BD Bioscience) taking a minimum of 15000 cells per sample. | ||||||||||||
Cell cycle analysis |
||||||||||||
| Cell cycle analysis was performed by using FACSCalibur™ instrument (BD Biosciences, San Jose, CA). ModFit LT cell cycle analysis software was used to determine the percentage of cells in the various phases of the cell cycle. Briefly, SEG-1 cells (2 × 105) cells/well were treated with various doses of gossypol (0, 10, 25 and 100 μM) for 48 hours. Subsequently, the cells were collected, washed with ice cold PBS buffer, fixed with 70% alcohol at 4°C for 12 hours, and stained with propidium iodide in presence of 1% RNAase A at 37°C for 30 minutes prior to flow cytometric analysis. | ||||||||||||
Statistical analysis |
||||||||||||
| All experiments were done in triplicate and the results are conveyed as the mean ± standard deviation. Graphpad Prism (Graphpad Software, Inc., CA, USA) was used for performing statistical analyses and P<0.05 was taken to designate a statistically significant difference. | ||||||||||||
Results |
||||||||||||
Antitumor activity of gossypol against SEG-1 cells |
||||||||||||
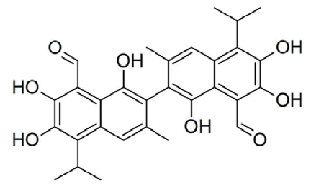
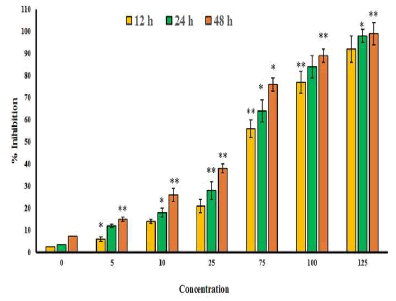
| The chemical structure of gossypolis shown in Figure 1. MTT assay was used to assess the antitumor activity of gossypol in SEG-1 cells. Results revealed that gossypol exhibited a potent, concentration dependent as well as time-dependent growth inhibitory effect on the proliferation of SEG-1 cancer cells with IC50 values 69.5, 49.2 and 31.3 μM at 12, 24 and 48 h time intervals respectively (Figure 2). IC50 value gives an indication about the potency of the compound, higher its value lower will be the potency of the given drug. | ||||||||||||
Apoptotic detection of SEG-1 cancer cells by fluorescence microscopy using AO staining |
||||||||||||
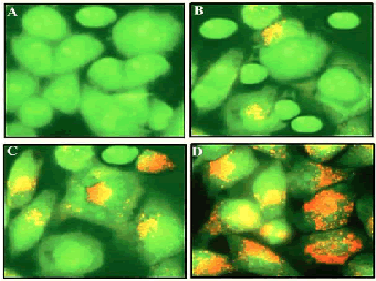
| The results from this assay are shown in Figure 3 and indicate that viable cells with undamaged DNA show green fluorescence indicating normal cellular morphology while as apoptotic cells have damaged or fragmented DNA and stain as orange and red. Gossypol treatment in SEG-1 cells showed reduction in the number of viable cells significantly and in a dose-dependent manner. With an increase in the dose of Gossypol, an increasing number of cells were stained orange and red indicating apoptosis process happening within the cells. Gossypol-treated cells displayed characteristic features of apoptosis including membrane blebbing, chromatin condensation and apoptotic body formation which were not detected in untreated cells (Figure 3A). | ||||||||||||
SEG-1 esophageal cancer cell surface analysis by SEM |
||||||||||||
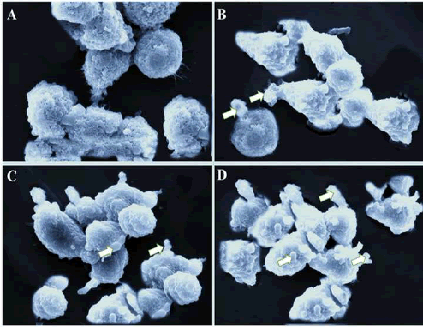
| Scanning electron microscopy is used to reveal the morphological features of the cell surface. The apoptosis process in a cell usually leads to cell surface changes including shrinkage, membrane blebbing, loss of microvilli structures and smoothing. As can be seen from Figure 4A, untreated SEG-1 cells were rounded with normal surface. However, gossypol treatment at lower dose led to various surface projections and blebbing of the membrane. At higher gossypol concentration, apoptotic body formation was witnessed (Figure 4 B-D). Overall, scanning electron microscopy study clearly showed that gossypol resulted in the formation of apoptotic body formation in human esophageal cancer cells. | ||||||||||||
Gossypol-induced Apoptosis quantification using annexin V-FITC assay |
||||||||||||
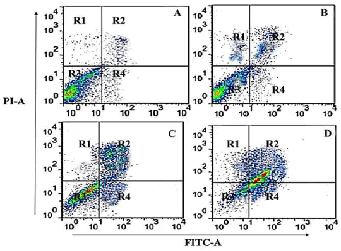
| In order to quantify the extent of apoptosis induced by gossypol in human SEG-1 cells, annexin V-FITC assay was carried out using flow cytometry. The results revealed that gossypol induced both early and late apoptosis in a concentration-dependent manner. As compared to the untreated control cells (Figure 5A), gossypol treated cells at 10, 50 and 100 μM led to potent apoptosis induction (Figure 5 B-D). The four quadrants R1, R2, R3 and R4 represent necrotic cells, late apoptotic cells, viable cells and early apoptotic cell populations respectively. Percentage of apoptotic cells increases from 3.3% in control cells (A), to 24.5%, 39.9% and 56.3% in 10 μM (B), 50 μM (C) and 100 μM(D) respectively. | ||||||||||||
Gossypol induced sub-G1 cell cycle arrest in SEG-1 cells |
||||||||||||
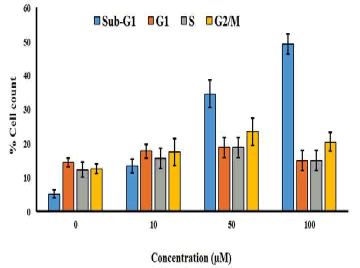
| The effect of gossypol on cell cycle phase distribution was evaluated by using flow cytometry using propidium iodide staining. The cells were treated with various doses of gossypol (0, 10, 50 and 100 μM) for 48 h and then subjected to flow cytometric detection. The results indicated that gossypol induced potent and dose-dependent increase in the percentage of cells in sub-G1 phase of the cell cycle. This was also accompanied by a small increase in the percentage of G2/M cells also (Figure 6). | ||||||||||||
Discussion |
||||||||||||
| The results of the current study demonstrates that gossypol exerts potent and dose-dependent anti-proliferative effects in SEG-1 cells. The cytotoxic effects induced by gossypol were found to be time-dependent, as the antiproliferative effect was shown to increase with increase in time intervals after gossypol treatment. Acridine orange staining of the SEG-1 cells followed by fluorescence microscopic examination revealed that gossypol treatment led to DNA fragmentation and the cells were stained orange and red. Gossypol-treated cells displayed characteristic features of apoptosis including membrane blebbing, chromatin condensation and apoptotic body formation which was not detected in untreated cells. Scanning electron microscopy revealed that gossypol treatment induced alterations in cell surface morphology. On treating cells with increasing doses of gossypol, several cellular surface projections and loss of microvilli structures and surface smoothing was observed. Apoptotic body formation in gossypol-treated cells can be easily seen in SEM analysis. Further, annexin V-FITC assay quantified the percentage of apoptotic cells after gossypol treatment indicating the increase in percentage of apoptotic cells from 3.3% in control cells, to 24.5%, 39.9% and 56.3% in 10 μM (B), 50 μM (C) and 100 μM(D) respectively. Gossypol also induced potent and dosedependent increase in the percentage of cells in sub-G1 phase of the cell cycle which was further accompanied by a small increase in the percentage of G2/M cells also. | ||||||||||||
| Gossypol has been reported to have several biological properties including antimalarial and proapoptotic properties due to the regulation of Bax and Bcl-2 genes [7]. It has also been reported to inhibit replication of HIV-1 virus [8]. Some authors report that gossypol is an effective protein kinase C inhibitor. This compound has also been reported to induce apoptosis in ovarian cancer cells through the mediation of oxidative stress. It has also shown to induce apoptosis in human prostate cancer cells via the downregulation of Bcl-2and upregulation of Bax at the mRNA and protein levels. Gossypol also activated caspases-3, 8 and 9 and increased PARP [poly (ADP-ribose) polymerase] cleavage. Gossypol has been identified as a BH3-mimetic inhibitor of pro-apoptotic Bcl-2 family members, including Bcl-2, Bcl-xL, and Mcl-1, and induces apoptosis in various types of cancer [9-13]. To the best of our knowledge, anticancer activity of gossypol against human esophageal cancer (SEG-1) cells has not been reported till date. As such, the present report constitutes the first such report on this type of human cancer. | ||||||||||||
Conclusion |
||||||||||||
| The findings of the present study reveal that gossypol inhibited cancer cell growth in human esophageal tumor cell lines via the induction of apoptosis and sub-G1 cell cycle arrest. | ||||||||||||
Figures at a glance |
||||||||||||
|
||||||||||||
References |
||||||||||||
|