- Biomedical Research (2011) Volume 22, Issue 1
Anti-proliperative and antioxidant effects of Tinospora crispa (Batawali)
Ibahim MJ1*, Wan-Nor I’zzah WMZ1, Narimah AHH1, Nurul Asyikin Z2, Siti-Nur Shafinas SAR3, Froemming GA11Institute for Medical Molecular Biotechnology, Faculty of Medicine, Universiti Teknologi MARA, 40450 Shah Alam, Selangor, Malaysia
2Faculty of Biotechnology & Life Sciences, Universiti Industri Selangor, Malaysia
3Faculty of Health Science, Universiti Sains Malaysia, Malaysia
- Corresponding Author:
- Ibahim M.J.
Faculty of Medicine, Universiti Technologi MARA,
40450 Shah Alam, Selangor
Malaysia
Accepted Date: September 17 2010
Abstract
T. crispa is a well known traditional medicinal plant used in India, Indonesia, Philippines and Malaysia. To date, there are numerous studies on this plant emphasizing it’s antioxi-dant, antidiabetic, antimalarial and cosmetic effects. However, limited informations are available regarding T. crispa cytotoxicity potential. The objectives of this study were to de-termine the cytotoxicity potential and antioxidant activity of different T. crispa crude ex-tracts. Viability of cells was measured using the MTT assay on MCF-7, MDA-MB-231, HeLa and 3T3 normal fibroblast cells while antioxidant activity was determined by measur-ing total flavonoid content, total phenolic content and DPPH free radical scavenging activ-ity. Results obtained showed that the cell viability decreased in a dose-dependent manner in all cancer cells for each extract. The lowest IC50 (33.75 ± 4.65 μg/ml) was observed in MCF-7 using the methanol extract. Further more, there were significant differences for total pheno-lic content (p<0.01, df=2, X2=9.836) and flavonoid content (p<0.05, df=0.02, X2= 7.20) be-tween each extract with the methanol extract having the highest activity for both phenolic (255.33 ± 10.79 mg GAE/g sample) and flavonoid content (9.53 ± 0.50 mg QE/ g sample). The DPPH assay showed that the methanol extract had highest scavenging activity in a dose-dependent manner where the IC50 value was 12 μg/ml. T. crispa has a dose-dependent antiproliferative activity against many types of cancer cells where the lowest IC50 was pre-sent in the methanol extract on MCF-7. Further more, methanol crude extract of T. crispa had higher total phenolic and flavonoid content and free radical scavenging activity com-pared to water extract and chloroform extract.
Keywords
T. crispa, cytotoxicity, antioxidant
Introduction
Cancer is characterized by uncontrolled growth of cells that can cause by many factors such as exposure to UV light, certain chemicals [1] and imbalance between oxi-dant and antioxidant [2]. Novel anticancer agent is one of the important treatments to fight against cancer instead of the surgery and radiotherapy [1].
Malaysia is known for its biodiversity with many plants possessing medicinal value [3]. T. crispa is known as ba-tawali or seruntun in Malaysia, bratawali and andawali in Indonesia, makhamukay in Philippines and boraphet in Thailand. It had been used as traditional medicine in rural society to treat fever, cholera, snake bites, rheumatism and fever due to malaria [4]. T. crispa has shown to have an antihyperglycemia effect by augmenting the release of insulin [5]. Its antimalarial activity [3], antibacterial [6], anti-inflammatory [7] and anti-oxidant properties [4] are also recorded. Recent study by Chantong et al. [8] showed that T. crispa has cytotoxic effect on P19 embryonal car-cinoma cells. The cytotoxicity activity of plants might be due to the antioxidant activity or the presence of active compound such as alkaloid. The measurement of different extraction solvents produced valuable information about the antioxidant activity of different type of T. crispa crude extracts. This study was designed to measure the cytotox-icity and antioxidant properties in different type of T. crispa crude extracts
Materials and Methods
Chemicals.
Folin-Ciocalteu reagent, sodium carbonate anhydrous (NaOH), gallic acid, 1,1-diphenyl-2-picrylhydrazyl (DPPH), aluminum chloride hexahydrate (AlCl3.6H20), quercetin, dimethyl sulfoxide (DMSO) and tamoxifen were purchased from Sigma Chemical Co. USA. Roswell Park Media Institute (RPMI) 1640, penicillin, streptomy-cin and foetal bovine serum (FBS) were obtained from PAA, GMBH, (Germany). Methanol and chloroform used were of the highest pure grade (Merck, Germany). 3T3, MCF-7, MDA-MB-231 and HeLa cell line cultures were obtained from the American Type Culture Collection (ATCC).
Plant materials
The raw plant materials (stems) were obtained from a village in Chenor, Pahang, cut into small pieces, dried and ground into powder. The powder was soaked in water, methanol or chloroform overnight at room temperature. The water, methanol and chloroform extractions were filtered and the filtrates were evaporated with a rotary evaporator. Then the dried residues were stored at -80°C until used for cytotoxicity testing and antioxidant deter-mination.
Preparation of stock solution
For cell culture, 5 mg of each dry residue extract was dis-solve in 5 ml DMSO to produce 1 mg/ml stock solution of each extract and further diluted with RPMI 1640 to pro-duce a working solution of 100 μg/ml. For determination of the total antioxidant activity, 5 mg of each dry residue extract was dissolve in 5 ml methanol to produce 1 mg/ml working solution of each extract.
Cell culture and cytotoxicity test using MTT
MCF-7, MDA-MB-231, HeLa and 3T3 cell lines were cultured in RPMI 1640 supplemented with 10% of FBS, 100 IU/ml of penicillin and 100 μg/ml of streptomycin using 75-cm2 flasks, in humidified incubator with 5% CO2 and 95% air at 37°C. Cells were harvested when it reached 70% confluent and 1 x 105/ml were plated onto a 96-well plate and incubated overnight to allow the cells to attach to the well. The medium was discarded and 100 μl of the working solution, which was diluted with the me-dium to yield the final concentrations of the test solutions ranging from 10 - 100 μg/ml were added into the well and incubated for 72 hours. As a control, cells were cultured in 100 μl of medium, while for positive control cells were cultured in the presence of tamoxifen which the concen-trations were similar to extracts. All treatments were done in replicates of six. Viability of the cells was determined using MTT assay according to manufacturer’s instruc-tions (Roche Diagnostic). The results were recorded as IC50 value which is the concentration of sample that in-hibits 50% of tumour cells growth.
Determination of total phenolic content
The total phenolic content of T. crispa extract was deter-mined by using Folin-Ciocalteu method [9] where 200 μl of samples (1 mg/ml) were added into the test tube fol-lowed by 1.5 ml of Folin-Ciocalteu’s reagent (diluted 10 times) and allowed to stand at 22oC for 5 minutes in the dark. Then 1.5 ml of NaOH (6% w/v) were added and were incubated for 90 minutes before absorbance were measuring at 725 nm. Total phenolic content was meas-ured as gallic acid equivalents (GAE) in 1 g material.
Determination of total flavonoid content
The total flavonoid content was determined using the Dowd method [10]. Briefly, 1 ml of 2 % AlCl3.6H20 in methanol was mixed with the same volume of the extract solution (1 mg/ml). Then, the solution was allowed to stand at the room temperature in the dark for 15 minutes before absorbance was measured at 430 nm using spec-trophotometer against blank sample consisting of metha-nol. The total flavonoid content was determined using standard curve with quercetin (mg/ml) as the standard. Total flavonoid content was expresses as mg of quercetin equivalents (CE) in 1 g of extract.
DPPH free radical scavenging assay
The DPPH method [11] was followed by adding 100 μl different concentrations of the T. crispa extracts ranging 10 - 100 μg/ml (in triplicate) to 5 μl of DPPH (5 mg/2 ml methanol). DPPH solution was then allowed to stand for 20 min before absorbance was measured at 517 nm. Spec-trophotometric measurements was made using methanol as blank. Antioxidant activity was expressed as IC50 (inhibitory concentration in mg/ml of plant material nec-essary to reduce the absorbance of DPPH by 50%). Vita-min C was used as positive control.
Statistical analysis
Data were expressed as mean ± standard deviation. Kruskal Wallis test was used to compare mean of each group. Probability levels of less than 0.05 were taken as statistical significance.
Results
Cell viability
MCF-7
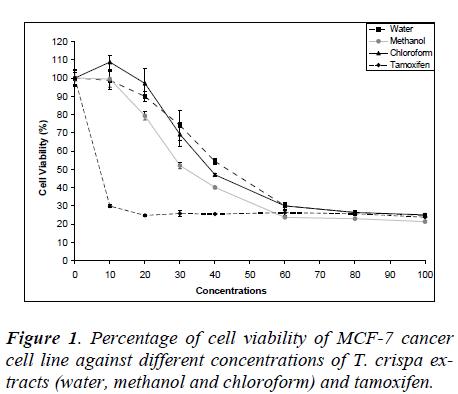
Fig. 1 shows the MCF-7 cell viability on various T. crispa extracts (water, methanol and chloroform) and tamoxifen as control. The cell viability was decreased in dose-dependent manner where the IC50 were 42.75 ± 4.61 μg/ml, 33.75 ± 4.65 μg/ml and 38.90 ± 3.21 μg/ml for water, methanol and chloroform extracts, respectively.
MDA-MB-231
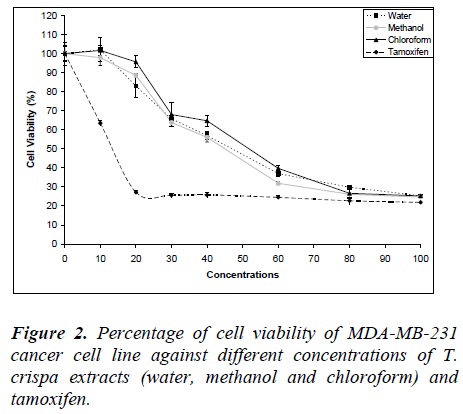
Fig. 2 shows the MDA-MB-231 cell viability on various T. crispa extracts (water, methanol and chloroform) and tamoxifen as control. The cell viability was decreased in dose-dependent manner where the IC50 were 46.88 ± 1.75 μg/ml, 44.83 ± 1.21 μg/ml and 51.25 ± 3.62 μg/ml for water, methanol and chloroform extracts, respectively.
HeLa
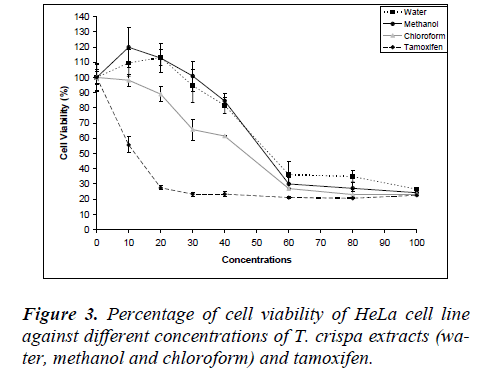
Fig. 3 shows the MDA-MB-231 cell viability on various T. crispa extracts (water, methanol and chloroform) and tamoxifen as control. The cell viability was decreased in dose-dependent manner where the IC50 were 53.83 ± 1.47 μg/ml, 52.5 ± 1.14 μg/ml and 46.13 ± 2.81μg/ml for water, methanol and chloroform extracts, respectively
3T3
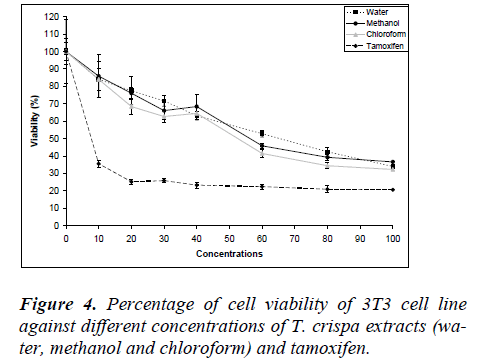
Fig. 4 shows the MDA-MB-231 cell viability on various T. crispa extracts (water, methanol and chloroform) and tamoxifen as control. The cell viability was decreased in dose-dependent manner where the IC50 were 65.50 ± 4.64 μg/ml, 57.50 ± 6.47 μg/ml and 52.58 ± 2.25 μg/ml for water, methanol and chloroform extracts, respectively. The IC50 values for 3T3 normal fibroblast cell were higher compared to all cancer cell line for each extract.
Total phenolic content
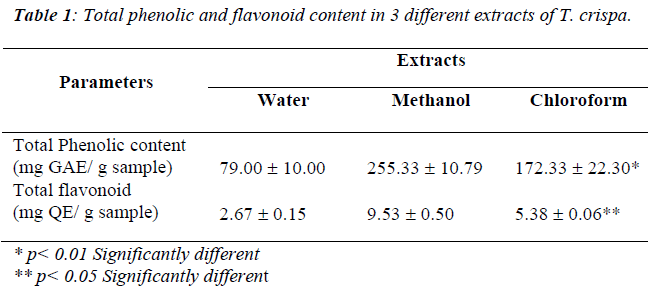
Table 1 showed the total phenolic contents of water, methanol and chloroform extract of T. crispa expressed in milligram gallic acid per gram samples. A significant dif-ference was evident of the total phenolic content for wa-ter, methanol and chloroform extracts (p<0.01, df=2, X2=9.836) where methanol extract poses highest activity (255.33 ± 10.79 mg GAE/ g sample) compared to water and chloroform extracts.
Total flavonoid content
Table 1 shows the total flavonoid content of water, methanol and chloroform extracts of T. crispa expressed in milligram quercetin per gram samples. A significant difference of the total phenolic content was evident for water, methanol and chloroform extracts (p<0.05, df=2, X2=7.20) where methanol extract poses highest activity (9.53 ± 0.50 mg QE/ g sample) compared to water and chloroform extracts.
DPPH free radical scavenging assay
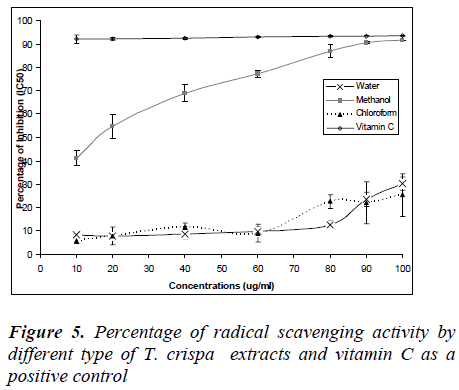
Figure 5 shows the percentage of radical scavenging ac-tivity of T. crispa extracts and vitamin C as a positive control. The percentage radical scavenging activities were increased with concentrations except for vitamin C which was constant from 10 – 100 ug/ml where the percentage of radical activity nearly 100%. Furthermore, percentage of radical activity for water and methanol extracts in-creased with concentration, however no IC50 values were obtained. Only methanol extract showed IC50 values (12 μg/ml) and percentage of radical activity increased to 100% which was similar to vitamin C.
Discussion
In this study, the purpose of testing different types of T. crispa extracts was to determine the best extraction method of T. crispa that provides the strongest antiprolif-erative effect against cancer cell lines and antioxidant activity. The antiproliferative activity was measured us-ing MTT assay where it is based on the reduction of a soluble tetrazolium salt by mitochondrial dehydrogenase activity into soluble colored product which could be measured by spectrophotometer. The IC50 values obtained from the viability graph were used as parameter for cyto-toxicity where it refers to 50% of cells inhibited by the plant extract [13].
Different type of extraction methods contributes different active compounds of T. crispa due to polarity of the sol-vent. Regarding in vitro study on anti-malarial activity, chloroform extract of T. crispa provided best result com-pared to methanol extract [3]. Similarly in this study, methanol extract gave the best IC50 value compared to water and chloroform extracts in MCF-7 and MDA-MB-231 but not for HeLa cell. Cisplatin and tamoxifen are well established human anticancer drugs and have been used to treat cancer [14]. In this study, we used tamoxifen as a positive control and each T. crispa extract showed similar pattern of cell viability curve compared to that of tamoxifen.
Each extract showed that cell viability decreased in a dose-dependent manner in all types of cancer cells and normal cell. Most plant extracts show that their effects depend on time and the dose [15]. However, the IC50 ob-tained for 3T3 normal fibroblast cell was relatively higher than that of MCF-7, MDA-MB-231 and HeLa cells. The IC50 of 3T3 appears different from that in some animal cells where T. crispa extracts failed to produce any toxic effects even up to a concentration of 1 g/ml [16]. The IC50 values in this study were also found to be lower than those reported recently in HepG2, Caov-3, MCF-7 and HeLa cancer cells but not normal HUVEC cell using aqueous extracts [17]. Methanol extract of T. crispa againt MDA-MB-231 and MCF-7 had lower IC50 which showed the potential antiproliferative effect. The antipro-liferative effect of T. crispa might be due to its chemical substances especially alkaloids, which are known to have anticancer properties [4].
Total phenolic content of T. crispa extracts were meas-ured according to the Folin–Ciocalteu method. This method was widely acceptable for determination of phe-nolic content. However, this method was not entirely spe-cific for phenolic compound but not all phenolic com-pounds display the same level of activity in the assay [10]. The total phenolic and flavonoid content of T. crispa were higher in methanol compared to water and chloro-form extracts. In this study, total amount of phenolic compound for methanol extract was 3.2 folds compared to water extract and 1.5 folds compared to chloroform ex-tract. The difference of total phenolic content may be due to the specificity of Folin-Ciocalteu to the compound in methanol extract compared to water or chloroform ex-tracts.
Flavonoid is plant pigment which has antioxidant activity through scavenging or chelating process and their effects on human nutrition and health are substantial [18]. AlCl3 was used in this measurement because it is specific for total flavonoid which consists of flavanols and flavones compared to the other method for example by using 2,4-dinitrophenlhydrazine which is specific for flavanol only [12]. For total flavonoid content, the value for methanol extract was found to be 3.6 folds compared to water and and 1.8 fold to chloroform extract, respectively. Results showed that methanol extract had higher phenolic and flavonoid contents compared to other study which had reported low association between phenolic and flavonoid content [12].
Antioxidant activity measurement was done using DPPH radical scavenging where principle of the method was based on the ability of the compounds to act as free radi-cal scavengers or hydrogen ion donors [18]. In this study, free radical scavenging activity of the methanol extract was found to be higher compared to water and chloroform extracts. Free radical scavenging activity of the methanol extract was increased from 50% up to 90% on dose-dependent manner and equivalent to vitamin C free radi-cal scavenging activity. It however, showed that T. crispa extract has a strong antioxidant activity. In most cases, total phenolic content as determined by Folin-Ciocalteu method was correlated with the antioxidant capacities [19]. Similarly, in this study the methanol extract had shown high level of total phenolic and flavonoid that may contribute to the high level of free radical scavenging ac-tivity.
Our results clearly showed that the solvent used during extraction did affect the total phenolic content, flavonoid and free radical scavenging activity. Methanol extract of
buckwheat showed highest antioxidant activity when se-quentially extracted with hexane, diethyl ether, ethyl ace-tate and acetone [20]. Determination of antioxidant activ-ity using different methods and extraction media lead to different observations. A combination of several tests could give a more consistent judgment of the antioxidant activity [20]
Conclusion
In conclusion, T. crispa has a dose-dependent antiprolif-erative activity against many types of cancer cells where the lowest IC50 is found to be present in the methanol ex-tract on MDA-MB-231 breast cancer cells. Further more, methanol crude extract of T. crispa showed higher total phenolic and flavonoid content and free radical scaveng-ing activity compared to water extract and chloroform extract. Further studies are needed to elucidate the me-chanism of cell death caused by T. crispa extract.
Acknowledgements
Present research was funded by UiTM Excellence Re-search Grant Fund 600-IRDC/ST/DANA/5/3/DST (4/2008). Critical review and valuable suggestion by Pro-fessor Dr. Amar Chatterjee, Faculty of Medicine, UiTM during preparation of this manuscript is highly appreci-ated.
References
- Larsen HR. Cancer: Causes, Prevention and Treatment. The International Journal of Alternative and Comple-mentary Medicine 1996; 14: 9-11
- Kikuzaki H, Hisamoto M, Hirose K, et al. Antioxidant properties of ferulic acid and its related compounds. J Agric Food Chem 2002; 50: 2161-2168
- Nik Rahman NN, Furuta T, Kojima S, et al. Antimalar-ial activity of extracts of Malaysian medicinal plants. J Ethnopharmacol 1999; 64: 249-254
- Dweck CA, Andawali CJ. (Tinospora crispa): A review. 2006. http://www.dweckdata.co.uk/Published_papers/A_List_of_published_papers.htm
- Noor H, Ashcroft SJ. Pharmacological characterization of the antihyperglycaemic properties of Tinospora crispa extract. J Ethnopharmacol 1998; 62: 7-13
- Zakaria ZA, Mat Jais AM, Somchit MN, et al. The in vitro antibacterial activity of Tinospora crispa extracts. J. Biol. Sci. 2006; 6: 398-401
- Sulaiman MR, Zakaria ZA, Lihan R. Antinoceptive and anti-inflammatory activities of Tinospora crispa in various animal models. Int J Tropic Sci. 2008; 3: 66- 69
- Chantong B, Kampeera T, Sirimanapong W, et al. An-tioxidant activity and cytotoxicity of plants commonly used in veterinary medicine. Acta Hort (ISHS) 2008; 786: 91-98
- Kähkönen MP, Hopia AI, Vuorela HJ, et al. Antioxi-dant activity of plant extracts containing phenolic com-pounds. J Agric Food Chem 1999; 47: 3954-3962
- Meda A, Lamien CE, Romito M, et al. Determination of total phenolic, flavonoid and proline contents in bukina fasan honey, as well as their radical scavenging activity. Food Chemistry 2005; 91: 571-577
- Chan, EWC, Lim YY, Chew, YL. Antioxidant activity of Camellia sinensis leaves and tea from a lowland plantation in Malaysia. Food Chemistry 2007; 102: 1214-1222
- Edrini S, Rahmat A, Ismail P, Hin, TYY. 2002. Anti-carcinogenic properties and antioxidant activityof henna (Lawsenia Inermis). J Med Sci. 2002; 2: 194-197
- Smit HF, Waedenbag HJ, Singh RH, et al. Ayurvedic herbals drugs with cytotoxicity activity. J Ethnophar-macol 1995; 47: 75-84
- Behrens D, Gill JH, Fichtner I. Loss of tumourigenicity of stably ER transfected MCF-7 breast cancer cells. Mollec Cell Endocrinol 2007; 274: 19-29.
- Shahin SA, Naresh K, Abhinav L, et al. Indian Medi-cainal Herb. Food Res Intl 2008; 41: 1-15.
- Hartl M, Humpf HU. Toxicity assessment of fumonis-ins using the brine shrimp (Artemia salina) bioassay. J Food Chem Toxicol 2000; 38: 1097-1102.
- Zulkhairi A, Abdah MA, Kamal NH, et al. Biological Properties of Tinospora crispa (Akar Patawali) and Its antiproliferative activities on selected human cancer cell lines. Mal J Nutr 2008; 14: 173-187
- Cook NC, Samman S. Flavonoids-chemistry, metabo-lism, cardioprotective effects, and dietary sources. . J Nutr Biochem 1996; 7: 66-76
- Roginsky V, Lissi EA. Review of methods to determine chain breaking antioxidant activity in food. Food Chem. 2005; 92: 235-254
- Sun T, Chi-Tang H. Antioxidant activities of buck-wheat extracts. Food Chemistry 2007; 90: 743-749