Research Article - Biomedical Research (2017) Volume 28, Issue 1
Anti-allergic effects of probiotic Lactobacillus rhamnosus GG (LGG) on allergic rhinitis induced by ovalbumin in rats
Liu Wei1, Liu Fei1, Xiao Ning1, Tang Haiming1, Guo Lixin1* and Meng Xianqin2*1Department of Otolaryngology, the 169th Hospital of P.L.A. (Xiangnan Affiliated Hospital of Hunan Normal University) Hengyang, Hunan Province, PR China
2Department of Geriatrics, the 163th Hospital of P.L.A. (Second Affiliated Hospital of Hunan Normal University) Changsha, Hunan Province, PR China
- *Corresponding Author:
- Guo Lixin
Department of Otolaryngology
169th Hospital of P.L.A Hunan Normal University
PR China
Meng Xianqin
Department of Geriatrics
169th Hospital of P.L.A
Hunan Normal University
PR China
Accepted on May 2, 2016
Abstract
To explore the potential pharmaceutical effects of Lactobacillus rhamnosus GG (LGG) in treating allergic rhinitis in a rat model sensitized by ovalbumin (OVA). 8 week-old male Wistar rats sensitized with OVA were adopted as study objects. Allergic rhinitis animal model was prepared by OVA sensitization from day 1 to day 63. ELISA was performed to evaluate the serum level of histamine, total IgE and OVA-specific IgE in OVA sensitized rats. Flow cytometry (FCM) was performed to detect the CD4+CD25+ T-cells proportion alteration in groups treated with or without LGG. Passive cutaneous anaphylaxis (PCA) was performed to evaluate the inhibitory effect of LGG on allergic responses. qPCR was used to verify the secretion of CCL5, CCL24, CCL26 and expression of CCR3 and IL-3. WB was also used to confirm the stimulation of IL-3 and suppression of CCR3. Our results showed that LGG is protective in OVA-induced allergic rhinitis rat model. The serum level of histamine, total IgE and OVA specific IgE were significantly elevated in OVA sensitized allergic rhinitis rats assayed by ELISA indicating the effectiveness of our animal model. Further ELISA results showed that decrease of these cytokines treated with LGG. FCM results showed that the proportion of CD4+CD25+ and Foxp3+Treg cells in the mesenteric lymph nodes (MLNs) of OVA-sensitized allergic rhinitis rats was increased with LGG treatment. PCA results in vivo indicated the obvious anti-allergic effects of LGG treatment with a significant decrease of the amount of dye. Secretion and expression of CCL5, CCL24 and CCL26 were down regulated by LGG treatment; the expression of their receptor CCR3 was also decreased. IL-3 induction was detected by qPCR and WB after treatment of LGG. LGG treatment can significantly decrease the allergic responses of OVA-induced rhinitis rat models. LGG treatment was associated with a total decrease of allergic responses, secretion of CCL5, CCL24 and CCL26. LGG could also down regulate expression of CCR3 through IL-3 induction.
Keywords
Allergic rhinitis, Lactobacillus rhamnosus GG, Eotaxins, CCR3, IL-3.
Introduction
Allergic rhinitis (AR) is a very commonly seen immune disorder in daily lives. The high prevalence of AR made it a now serious public health problem [1,2]. It was estimated that about 20% of the total population were suffering from AR [3]. According to the allergic responses, AR can be classified to seasonal or perennial AR.
Patients have onset of AR have symptoms such as rhinorrhoea, nasal congestion, sneezing, itching, etc. The characterization of AR is nasal mucosa inflammation and hypersensitivity with high secretion level of IgE and eosinophil’s infiltration. Mast cells play a crucial role in the hypersensitivity reactions and allergic reactions in AR. IgE secreted from B cells activates mast cells to produce inflammatory cytokines such as IL-6, IL-1-β, tumour necrosis factor (TNF)-α. These chemotactic cytokines attract and stimulate Th2 cells and also eosinophil’s [4]. Reports have been made that Th1 cells also take part in this allergic action with an inhibitory function during sensitization [5,6]. AR is also often associated with other airway diseases such as asthma, which is also resulted from immune disorder of hypersensitivity resulting in severer regional inflammation [7].
Probiotic is term referring to microbial supplements in food or beverage for its beneficial effects on health. Probiotics nowadays are so widely spread over various kinds of food and drinks as supplements. The beneficial aspects of probiotics rely on the potential alterations of macrobiotic metabolic activities and modulation of immune system [8]. The application of probiotics in treating allergic diseases emerged decades ago [9]. Treatments of different allergies by probiotics have been carried out in infants to manage ailments like milk allergy and eczema. But the study is still insufficient as the evidence on each age group is not consistent [10,11].
Lactobacillus rhamnosus GG (LGG) is one of the Lactobacillus species most commonly used as probiotics. LGG showed efficient therapeutic and preventative potency in treating gastroenteritis induced by rotavirus [12]. Recent studies also showed that LGG can alleviate neonatal chronic visceral hypersensitivity caused by inflammation in rats [13]. LGG has also been shown to have immune system modulatory function on the inflammation of airways in mice asthma model [14]. Other species like Lactobacillus crispatus KT-11 was shown have anti-allergic effects on ovalbumin (OVA)- sensitized mice model. However, to our knowledge, no study has been performed investigating the relationship between LGG and ovalbumin induced allergic rhinitis.
In this study, we found that LGG reduced the allergic symptoms on the ovalbumin induced allergic rhinitis rat models. This attenuation was due to the alterations of CD4+CD25+ T-cells proportions. Moreover, LGG induction of IL-3 causes suppression of CCR3, which in turn inhibit the function of exotoxins leading to, decreased eosinophil’s infiltration.
Materials and Methods
Animals
8 week-old male Wistar rats were purchased from Shanghai Experimental Animal Centre (Shanghai, China). Animals were kept under thermostat conditions at about 24ºC with 55% humidity with free access to food and clean water. Light was provided 12 h per day.
Bacterial growth
LGG was raised in Difco medium 37ºC under anaerobic conditions. Bacteria were diluted in a rate of 1:10 in fresh medium with overnight incubation and grow daily to mid log phase. The concentration of bacteria was calculated by densitometry and viability tested by CFU counts after agar plating. Cells were harvested by centrifugation at 3000 g for 20 min. Then, bacteria were washed with sterile water, and resuspended in PBS. Cell suspension was then boiled for 10 min at temperature of 100ºC followed by lyophilisation.
OVA-induced allergic rhinitis rat model preparation
The detailed method has been reported by Jeong et al. [15]. Rats were sensitized on day 1, 7 and 14 by intraperitoneal injection of 40 mg aluminium hydroxide (Sigma-Aldrich) containing 20 mg OVA. Control groups were set receiving only aluminium hydroxide. From day 21, rats were daily administrated with 20 mg of OVA intranasally from day 21 to day 63 to induce AR. To study the effects of LGG on the OVA induced AR, LGG cells were orally administered to mice. The oral treatments of LGG started at day 35 and continued daily until day 63. LGG strain cells were lyophilized with proper amount and suspended in distilled H2O2 before the treatment. Two groups were set according to the different dosages of LGG intake (50 mg/kg and 500 mg/kg). Tranilast was used as positive control at dosage of 500 mg/kg.
Detection of IL-4, CCL5, eotaxin-2 and eotaxin-3 in serum
Enzyme-linked immunosorbent assay (ELISA) was adopted to detect the serum levels of IL-4, CCL5, Eotaxin-2 and Eotaxin-3 in experimental rat models according to the manufacturer’s recommendations (R&D Systems, MN, USA). The concentrations of IL-4, CCL5, Eotaxin-2 and Eotaxin-3were measured in an ELISA plate reader at a wavelength of 450 nm.
Flow cytometry
Flow cytometry was used for analysis of proportion of Foxp3+ (CD4+, CD25+) cells. Cells were isolated and incubated from rats with or without treatments of LGG (50 mg/ml, 500 mg/ ml). Cells were then washed in PBS for 2 times and fixed by 70% ethanol. After fixation, cells were re-suspended in 500 μl of binding buffer (10 mM HEPES/NaOH (pH 7.4), 140 mM NaCl, 2.5 mM CaCl2) washed with PBS for another time and incubated with CD4 and CD25 antibodies for 120 min (37ºC). A FACStar flow cytometer (BD, CA, USA) was adopted to measure the percentages of cells with different expression of CD4 and CD25.
Passive cutaneous anaphylaxis (PCA)
PCA was generated through intradermal injection of monoclonal IgE anti-DNP in PBS into dorsal skin followed twenty-four hours later with a tail vein injection of 200 μl of DNP-HSA (in PBS) with 2% Evans blue (10 mg/ml). After 30 min, rats were sacrificed, dorsal skin was removed and Evans blue dye spots and area were measured.
qPCR
Total RNA was isolated using Trizol reagent (Life Technologies). Reverse transcriptase andoligo’dT primers were used to prepare cDNA from 1 μg of RNA according the manufacturer’s instructions (Takara, Japan). Two microliters of each cDNA was then used for PCR amplification using primers for IL-4, Eotaxin-2, Eotaxin-3, IL-3, CCL5, CCR3, CD4 and CD25.
WB
Cells were lysed in prepared buffer containing 10 mM Tris, pH 7.2, 150 mM NaCl, 5 mM EDTA, 0.1% SDS, 0.5% Triton X-100, and 1% deoxycholic acid. For Western blots, 30 μg of protein samples were subjected to SDS-PAGE followed by transfer onto PVDF membranes. After blocking in 5% BSA in PBS, membranes were incubated with antibodies against IL-3 (1:1000), CCR3 (1:1000) and β-actin (1:1000) overnight at 4ºC followed by 1 h-incubation with secondary antibody (1:2000). Blots against β-actin served as loading control.
Statistical analysis
All data were analysed by SPSS (ver. 13.0) software and the results were showed by mean ± SD. Student’s t-test and two-way analysis of variance (ANOVA) were used to assess statistical significance, with p ≤ 0.05 being regarded as significant.
Results
LGG administration attenuates AR allergic responses
LGG has already been shown reduce allergic inflammation in airway within new-born mice [16]. To examine if LGG also have protective effects on OVA induced allergic rhinitis, we tested LGG administration on OVA induced AR model (Figure 1).
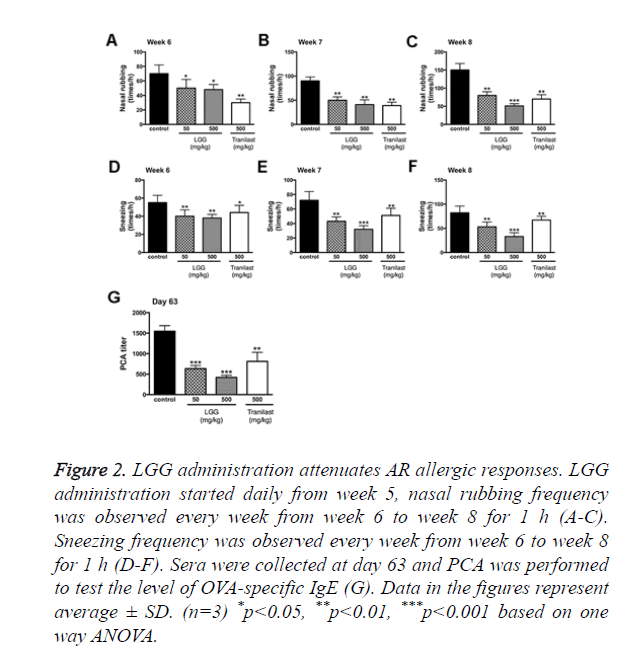
The established model showed rhinitis allergic response like nasal rubbing and sneezing after challenged with OVA intranasally. However, the symptoms were absent in the control group with no OVA treatments. The frequency of nasal rubbing and sneezing were observed and recorded (Figure 2).
Figure 2. LGG administration attenuates AR allergic responses. LGG administration started daily from week 5, nasal rubbing frequency was observed every week from week 6 to week 8 for 1 h (A-C). Sneezing frequency was observed every week from week 6 to week 8 for 1 h (D-F). Sera were collected at day 63 and PCA was performed to test the level of OVA-specific IgE (G). Data in the figures represent average ± SD. (n=3) *p<0.05, **p<0.01, ***p<0.001 based on one way ANOVA.
The results showed that the nasal rubbing and sneezing frequency started to show significant alterations from week 6. Groups treated with 500 mg/kg LGG had the best results after 3 weeks of administration (Week 8). We then performed PCA to examine the eosinophil infiltration rate and total allergic response. From the results we found that at day 63 both groups treated with 50 or 500 mg/kg LGG showed significant decrease in allergic response (p<0.001), groups treated with tranilast also showed significant change (p<0.01).
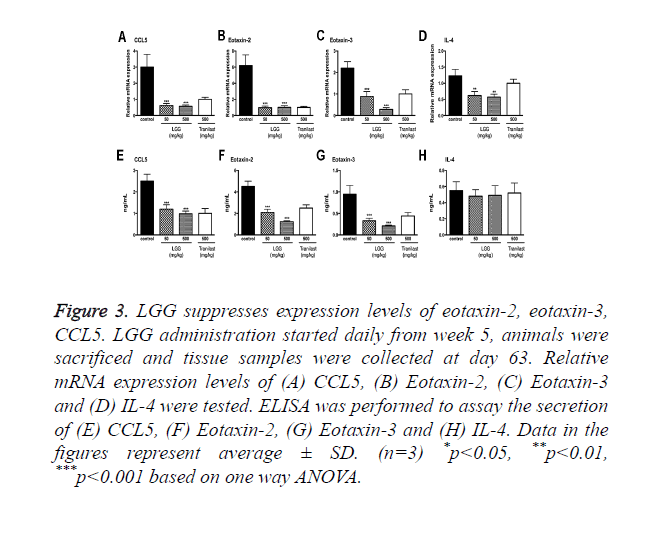
LGG suppresses expression levels of eotaxin-2, eotaxin-3, CCL5
Since OVA sensitization in murine model significantly increased both the total and anti-OVA IgEs as shown in previous results. It is indicated that LGG treatments can inhibit the immune response that lead to the allergic effects. To better understand the underlying mechanisms, we collected tissues and primary cells to test the expression of IL-4, CCL5, eotaxin-2 and eotaxin-3.
The expression of these cytokines was measured by qPCR and ELISA (Figure 3). The results showed that these chemokine’s were significantly suppressed by oral administration of LGG compared to the control group. Among the cytokines CCL5, eotaxin-2 and eotaxin-3 showed particular activity in attracting eosinophil in increasing its filtration rate.
Figure 3. LGG suppresses expression levels of eotaxin-2, eotaxin-3, CCL5. LGG administration started daily from week 5, animals were sacrificed and tissue samples were collected at day 63. Relative mRNA expression levels of (A) CCL5, (B) Eotaxin-2, (C) Eotaxin-3 and (D) IL-4 were tested. ELISA was performed to assay the secretion of (E) CCL5, (F) Eotaxin-2, (G) Eotaxin-3 and (H) IL-4. Data in the figures represent average ± SD. (n=3) *p<0.05, **p<0.01, ***p<0.001 based on one way ANOVA.
We also tested the expression of IL-4 finding that the mRNA expression level was decreased however the secretion of IL-4 assayed by ELISA showed no significant changes (Figures 3D and 3H).
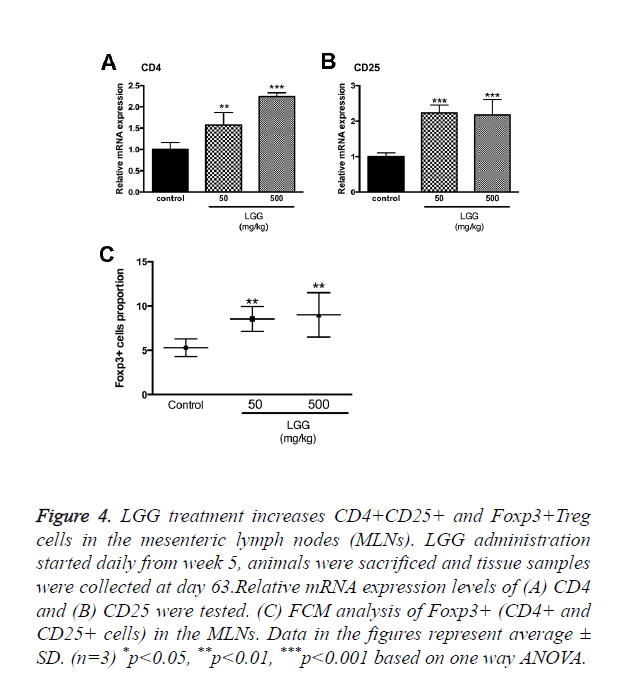
LGG treatment increases CD4+CD25+ and Foxp3+treg cells in the mesenteric lymph nodes (MLNs)
We assumed that LGG administration might function through altering the proportion and activities of Treg cells. Primary cells were isolated from the mesenteric lymph nodes in sacrificed rats and cells were assayed by FCM. The results showed that CD4+, CD25+ and Foxp3+ Tregs were significantly increased with administration of LGG compared to the control group (Figure 4C). We also tested the mRNA expression of CD4 and CD25 to make a confirmation. Results showed that CD4 expression was the highest in groups treated with 500 mg/kg LGG compared to other groups while CD25 expression was increased in LGG administration groups (50 and 500 mg/kg) (Figures 4A and 4B).
Figure 4. LGG treatment increases CD4+CD25+ and Foxp3+Treg cells in the mesenteric lymph nodes (MLNs). LGG administration started daily from week 5, animals were sacrificed and tissue samples were collected at day 63.Relative mRNA expression levels of (A) CD4 and (B) CD25 were tested. (C) FCM analysis of Foxp3+ (CD4+ and CD25+ cells) in the MLNs. Data in the figures represent average ± SD. (n=3) *p<0.05, **p<0.01, ***p<0.001 based on one way ANOVA.
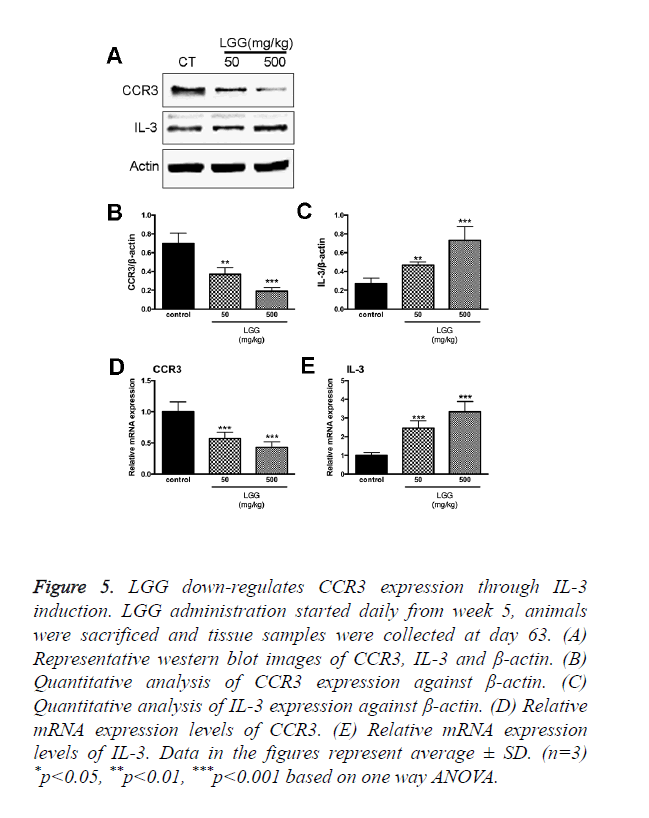
LGG down-regulates CCR3 expression through IL-3 induction
CCR3 responds to and interacts with a variety of chemokine’s such as eotaxin (CCL11), eotaxin-2 (CCL24), eotaxin-3 (CCL26), MCP-3 (CCL7), MCP-4 (CCL13), and RANTES (CCL5). The expression of CCR3 can be detected in Th1 and Th2 cell, epithelial cells contributing to the recruitment of activation of eosinophil’s during allergic pathogenesis. Since we discovered that LGG treatments had effects on eotaxin-2, eotaxin-3, CCL5 and CD4+CD25+ Foxp3+Tregs expression. Total RNA was extracted together with the specimen collection after the animals were sacrificed. We found that the expression of CCR3 was significantly increased in the control group treated with only OVA. However, the expression of CCR3 and CCR3+ cells were significantly down regulated with administration of LGG (Figure 5A).
Figure 5. LGG down-regulates CCR3 expression through IL-3 induction. LGG administration started daily from week 5, animals were sacrificed and tissue samples were collected at day 63. (A) Representative western blot images of CCR3, IL-3 and β-actin. (B) Quantitative analysis of CCR3 expression against β-actin. (C) Quantitative analysis of IL-3 expression against β-actin. (D) Relative mRNA expression levels of CCR3. (E) Relative mRNA expression levels of IL-3. Data in the figures represent average ± SD. (n=3) *p<0.05, **p<0.01, ***p<0.001 based on one way ANOVA.
To investigate the detailed mechanism of LGG administration inhibit CCR3 expression in vivo, we isolated OVA-sensitized rats spleen cells and treated with OVA with or without LGG presence in vitro. The results were consistent with our in vivo experiments; the expression of CCR3 was up regulated by OVA treatments while administration of LGG partially reversed the increase (Figure 5A). It reminded of us that IL-3 induction is associated with down regulation of CCR protein and mRNA in human eosinophil’s [17]. Moreover, reports have been made showing the relationship between LGG influences on the expression of IL-3 [18]. We then hypothesized that LGG administration might down regulate CCR3 expression via IL-3 induction. To test our hypothesis, we introduced IL-3 antibodies and specific primers. As expected, the transcription level and protein level were both elevated in cells treated with LGG and the results were adverse in cells treated with OVA only (Figure 5). Since we found that LGG could significantly reduce the CCR3 expression elevated by OVA treatments, we used IL-3 monoclonal antibody finding that the inhibitory activity was attenuated after the antibody treatment (Figure 5). We concluded that LGG inhibited OVA induced CCR3 overexpression through induction of IL-3 generation.
Discussion
Over expression of IgE is a major characterization of allergic rhinitis. In the allergic pathogenesis, expression of Th2 cytokines such as IL-4, IL-13 and IL-5 resulted in eosinophil infiltration in the nasal mucosa [19,20]. Patients with severe allergic rhinitis show infiltration of eosinophils, accumulation of mast cells and also basophils. The accumulation of mast cells, basophils and eosinophil’s is different in tissue location [21]. Histamine and other pro-inflammatory chemokine’s are expressed to maintain the allergic condition from the recruited immune cells like T cells and eosinophil’s [22]. In our study, we showed that OVA induction is associated with over expression of CCL5, CCL24 and CCL26. Each of the chemokine is important in eosinophils attraction and activation. Eosinophils are predominant in the allergic response of AR, the cell is characterized by a particular expression of cationic proteins like ECP and intracellular granules. It can also secrete a variety of cytokines and growth factors as well [23,24].
LGG belongs to probiotics have been shown to have abundant health beneficial effects in various diseases. Probiotic microorganisms were primarily discovered from the gut in which initially related with intestinal inflammation [25]. The applications of probiotics have been put into effect in various aspects among which the earliest one is treatment for gastrointestinal diseases [26]. LGG in particular was shown effective in alleviating rotavirus-induced diarrhoea [27,28]. The therapeutic function of LGG might due to the competing bind to microorganisms with epithelial cells and the potential activation of immune responses [29]. LGG presents activity on interacting with epithelial cells and immune cells directly. In addition, LGG is also capable involving in the secreted cytokines mediated recognition with host cells [30]. In our study, we showed that LGG is also protective against allergic rhinitis induced by ovalbumin.
C-C chemokine ligand 5 (CCL5) or RANTES is a member of the β-chemokine family expressed by common T-cells considered to be chemo attractant for T-cells and also immune-regulatory. The function signalling of CCL5 is regarded related to the binding to G protein-coupled receptors as well as to proteoglycans [31]. CCL5 is considered to be a potential therapeutic target of diseases related to allergy, autoimmune response, virus infection and chronic inflammatory conditions [31]. Besides, CCL5 was considered playing important roles in the progression of cancer [32]. However, in our study, the eosinophils attractant activity of CCL5 is the main focus, it is also reported recently that CCL5 together with CCL11 and CCL24 induce circulating fibrocytes migration in patients with asthma [33]. In our study, the expression of CCL24 and CCL26 was also found elevated by OVA treatment. It is reported that all eotaxins are increased in patients with pulmonary tuberculosis [34]. The eotaxins regulate EVT function on vessel remodelling in the process of placentation. A more related study showed that the nasal lavage level of CCL24 is correlated with the eosinophils trafficking and eosinophils induced inflammation [35]. A very recent report explained the function of CCL26 from the proteomic perspective referring that eotaxins might contribute to innate host defence by activities modulated by mast cell proteases [36]. Interestingly, it is regarded that CCL26 is more efficient in recruiting eosinophils migration in allergic conditions than CCL11 and CCL24. CCL26 is still effective in inducing eosinophils even when CCR3 was blocked [37].
We focused on a particular important molecule IL-3 in our study. The results revealed that the induction of IL-3 by LGG treatment is associated with the down regulation of eotaxins receptor CCR3. A previous study supported our discovery explaining that CCR3 was specific down regulated by IL-3 induction although IL-3 is not a ligand [17]. Another study reported that absence of IL-3 is not related with enhance of local or systemic anaphylaxis, however eosinophils infiltration was significantly increased due to the absence of IL-3 [38]. The results were consistent with our findings, yet we showed that LGG treatment was correlated with the induction of IL-3. The detailed mechanism of IL-3 still needs to be elucidated. One possible mechanism is the involvement of RhoH through JAK-STAT pathway [39]. The limitation of this study also exists. We only evaluated CD4 and CD25 cell proportion together with a bunch of inflammatory and allergic cytokines; however, this can only represent partial allergic effects. More indexes should be included for a more comprehensive study.
In the present study, we showed that LGG might be a potential therapeutic strategy in treating allergic rhinitis. The anti-allergic effect of LGG is closely related with its ability to induce IL-3 expression which in turn down regulated the eotaxins. According to the above finding, LGG may be useful in developing novel pharmaceutical applications towards allergic rhinitis. However, the potential immune response and loss of efficacy caused by microbial variation should be concerned and studied further.
References
- Bauchau V, Durham SR. Durham, Prevalence and rate of diagnosis of allergic rhinitis in Europe. Eur Respir J 2004; 24: 758-764.
- Spinozzi F, Murgia N, Baldacci S, Maio S, Pala AP. Characteristics and predictors of allergic rhinitis undertreatment in primary care. Int J Immunopathol Pharmacol 2016; 29: 129-136.
- McCusker C, Chicoine M, Hamid Q, Mazer B. Site-specific sensitization in a murine model of allergic rhinitis: role of the upper airway in lower airways disease. J Allergy Clin Immunol 2002; 110: 891-898.
- Oh HA, Kim MJ, Shin TY, Kim HM, Jeong HJ. The antiallergic mechanisms of Citrus sunki and bamboo salt (K-ALL) in an allergic rhinitis model. Exp Biol Med 2014. 239: 83-93.
- Major T, Wohlleben G, Reibetanz B, Erb KJ. Application of heat killed Mycobacterium bovis-BCG into the lung inhibits the development of allergen-induced Th2 responses. Vacci 2002; 20: 1532-1540.
- Hattori H, Okano M, Yamamoto T, Yoshino T, Yamashita Y. Intranasal application of purified protein derivative suppresses the initiation but not the exacerbation of allergic rhinitis in mice. Clin Exp Allergy 2002; 32: 951-959.
- Smolensky MH, Lemmer B, Reinberg AE. Chronobiology and chronotherapy of allergic rhinitis and bronchial asthma. Adv Drug Deliv Rev 2007; 59: 852-882.
- Iqbal MZ, Qadir MI, Hussain T, Janbaz KH, Khan YH, Ahmad B. Review: probiotics and their beneficial effects against various diseases. Pak J Pharm Sci 2014; 27: 405-415.
- Pelto L, Isolauri E, Lilius EM, Nuutila J, Salminen S. Probiotic bacteria down-regulate the milk-induced inflammatory response in milk-hypersensitive subjects but have an immunostimulatory effect in healthy subjects. Clin Exp Allergy 1998; 28: 1474-1479.
- Furrie E. Probiotics and allergy. Proc Nutr Soc 2005; 64: 465-469.
- West CE, Hammarström ML, Hernell O. Probiotics in primary prevention of allergic disease--follow-up at 8-9 years of age. Allergy 2013; 68: 1015-1020.
- Pant N, Marcotte H, Brüssow H, Svensson L, Hammarström L. Effective prophylaxis against rotavirus diarrhea using a combination of Lactobacillus rhamnosus GG and antibodies. BMC Microbiol 2007; 7: 86.
- Kannampalli P, Pochiraju S, Chichlowski M, Berg BM, Rudolph C. Probiotic Lactobacillus rhamnosus GG (LGG) and prebiotic prevent neonatal inflammation-induced visceral hypersensitivity in adult rats. Neurogastroenterol Motil 2014; 26: 1694-1704.
- Wu CT, Chen PJ, Lee YT, Ko JL, Lue KH. Effects of immunomodulatory supplementation with Lactobacillus rhamnosus on airway inflammation in a mouse asthma model. J Microbiol Immunol Infect 2014.
- Jeong HJ, Shin SY, Oh HA, Kim MH, Cho JS, Kim HM. IL-32 up-regulation is associated with inflammatory cytokine production in allergic rhinitis. J Pathol 2011; 224: 553-563.
- Harb H. Neonatal supplementation of processed supernatant from Lactobacillus rhamnosus GG improves allergic airway inflammation in mice later in life. Clin Exp Allergy 2013; 43: 353-364.
- Harb H, van Tol EA, Heine H, Braaksma M, Gross G. IL-3 induces down-regulation of CCR3 protein and mRNA in human eosinophils. J Immunol 2001; 167: 3443-3453.
- Liang QH, Zhang L, Duan SC, Wang P, Zhang YC. [Influence of intestinal dysbacteriosis on immune and hematopoietec function in mice]. Zhonghua Er Ke Za Zhi, 2004 42; 708-711.
- Okano M, Azuma M, Yoshino T, Hattori H, Nakada M. Differential role of CD80 and CD86 molecules in the induction and the effector phases of allergic rhinitis in mice. Am J Respir Crit Care Med 2001; 164: 1501-1507.
- Romagnani S. Immunologic influences on allergy and the TH1/TH2 balance. J Allergy Clin Immunol 2004; 113: 395-400.
- Fuentes-Beltrán A, Montes-Vizuet R, Valencia-Maqueda E, Negrete-García MC, García-Cruz Mde L, Teran LM. Chemokine CC-ligand 5 production and eosinophil activation into the upper airways of aspirin-sensitive patients. Clin Exp Allergy 2009; 39: 491-499.
- Imai T, Nagira M, Takagi S, Kakizaki M, Nishimura M. Selective recruitment of CCR4-bearing Th2 cells toward antigen-presenting cells by the CC chemokines thymus and activation-regulated chemokine and macrophage-derived chemokine. Int Immunol 1999; 11: 81-88.
- Hirai H, Tanaka K, Yoshie O, Ogawa K, Kenmotsu K. Prostaglandin D2 selectively induces chemotaxis in T helper type 2 cells, eosinophils, and basophils via seven-transmembrane receptor CRTH2. J Exp Med 2001; 193: 255-261.
- Nagata K, Tanaka K, Ogawa K, Kemmotsu K, Imai T. Selective expression of a novel surface molecule by human Th2 cells in vivo. J Immunol 1999; 162: 1278-1286.
- DuPont AW, DuPont HL. The intestinal microbiota and chronic disorders of the gut. Nat Rev Gastroenterol Hepatol 2011; 8: 523-531.
- Isolauri E, Juntunen M, Rautanen T, Sillanaukee P, Koivula T. A human Lactobacillus strain (Lactobacillus casei sp strain GG) promotes recovery from acute diarrhea in children. Pediatrics 1991; 88: 90-97.
- Van Niel CW, Feudtner C, Garrison MM, Christakis DA. Lactobacillus therapy for acute infectious diarrhea in children: a meta-analysis. Pediatrics 2002; 109: 678-684.
- Canani RB, Cirillo P, Terrin G, Cesarano L, Spagnuolo MI. Probiotics for treatment of acute diarrhoea in children: randomised clinical trial of five different preparations. BMJ 2007; 335: 340-345.
- Zhang L, Xu YQ, Liu HY, Lai T, Ma JL. Evaluation of Lactobacillus rhamnosus GG using an Escherichia coli K88 model of piglet diarrhoea: Effects on diarrhoea incidence, faecal microflora and immune responses. Vet Microbiol 2010; 141: 142-148.
- Gómez-Llorente C, Muñoz S, Gil A. Role of Toll-like receptors in the development of immunotolerance mediated by probiotics. Proc Nutr Soc 2010; 69: 381-389.
- Marques RE, Guabiraba R, Russo RC, Teixeira MM. Targeting CCL5 in inflammation. Expert Opin Ther Targets 2013; 17: 1439-1460.
- Aldinucci D, Colombatti A. The inflammatory chemokine CCL5 and cancer progression. Mediators Inflamm 2014; 2014: 292376.
- Isgrò M, Bianchetti L, Marini MA, Bellini A, Schmidt M, Mattoli S. The C-C motif chemokine ligands CCL5, CCL11, and CCL24 induce the migration of circulating fibrocytes from patients with severe asthma. Mucosal Immunol 2013; 6: 718-727.
- Sharifabadi AR, Hassanshahi G, Ghalebi SR, Arababadi MK, Khorramdelazad H. All eotaxins CCL11, CCL24 and CCL26 are increased but to various extents in pulmonary tuberculosis patients. Clin Lab 2014; 60: 93-97.
- De Corso E, Baroni S, Romitelli F, Luca L, Di Nardo W, Passali GC, Paludetti G. Nasal lavage CCL24 levels correlate with eosinophils trafficking and symptoms in chronic sino-nasal eosinophilic inflammation. Rhinology 2011; 49: 174-179.
- Gela A, Kasetty G, Jovic S, Ekoff M, Nilsson G. Eotaxin-3 (CCL26) exerts innate host defense activities that are modulated by mast cell proteases. Allergy 2014; 70: 161-170.
- Provost V, Larose MC, Langlois A, Rola-Pleszczynski M, Flamand N, Laviolette M. CCL26/eotaxin-3 is more effective to induce the migration of eosinophils of asthmatics than CCL11/eotaxin-1 and CCL24/eotaxin-2. J Leukoc Biol 2013; 94: 213-222.
- Neel NF, Creasy BM, Rankin JN, Pierce EM, McCoy ME. Absence of interleukin-3 does not affect the severity of local and systemic anaphylaxis but does enhance eosinophil infiltration in a mouse model of allergic peritonitis. Immunol Lett 2004; 95: 37-44.
- Gündogdu MS, Liu H, Metzdorf D, Hildebrand D, Aigner M. The haematopoietic GTPase RhoH modulates IL3 signalling through regulation of STAT activity and IL3 receptor expression. Mol Cancer 2010; 9: 225-237.