Research Article - Biomedical Research (2017) Volume 28, Issue 21
Agrin activates YAP in human skin fibroblast (HSF) through integrin mediated adhesion and Lrp4/MuSK receptor-mediated signaling pathways
Jia Yang*, Hongping Ju and Ping Li
Kunming University School of Medicine, Kunming City, Yunnan Province, China
- *Corresponding Author:
- Jia Yang
Kunming University School of Medicine
Kunming Economic and Technological Development Zone, PR China
Accepted date: November 2, 2017
Abstract
Objective: To investigate the effects of agrin on YAP expression in Human Skin Fibroblast (HSF), and the possible mechanism.
Methods: Expression of agrin in HSF was downregulated by siRNA transfection. Migration and invasion ability of HSF was determined by Transwell migration and invasion assay. Expression and phosphorylation levels of agrin, Yes-Associated Protein (YAP), Focal Adhesion Kinase (FAK) and Muscle Associated Receptor Tyrosine kinase (MuSK) were detected by Western blot.
Results: Expression of agrin was significantly downregulated after agrin siRNA transfection (p<0.01). Proliferation, migration and invasion ability of HSF cells was significantly reduced after agrin silencing (p<0.01). No significant differences in total expression levels of YAP, FAK and MuSK were found between cells with or without agrin silencing, while agrin silencing significantly increased phosphorylation level of YAP and significantly reduced phosphorylation level of FAK and MuSK (p<0.01).
Conclusion: Agrin can activate YAP in HSF possibly through the interactions with integrin mediated adhesion and Lrp4/MuSK receptor-mediated signaling pathways.
Keywords
Agrin, Human skin fibroblast, Yes-associated protein, Focal adhesion kinase, Muscle associated receptor tyrosine kinase
Introduction
Skin is an important protective barrier for the body against the harm from environment. Loss of skin integrity may cause illness, injury or even death [1]. Skin wound healing is a complex and interactive process with the involvement of various factors including parenchymal cells, blood cells, soluble mediators and Extracellular Matrix (ECM) components [2]. Repair of skin tissue damage can generally be divided into three overlapped stages including inflammatory phase, cell proliferation and tissue formation, and tissue remodeling [3]. During cell proliferation and tissue formation phase, a huge number of myofibroblasts will be accumulated to secrete collagen, Fibronectin (FN) and hyaluronic acid, which in turn leads to the formation of ECM [4]. Besides the secretion of ECM components, myofibroblasts can also release tissue inhibitors of metalloproteinases and matrix metalloproteinases to remodel newly formed ECM [4]. Myofibroblasts apoptosis will occur after secretion of ECM, but a portion of myofibroblasts will bypass apoptosis to transform into fibroblasts [4]. It's well accepted that interactions between ECM and intracellular factors such as growth factors play pivotal roles in skin wound healing [5,6]. Therefore, the understanding of interactions between ECM and cells will definitely accelerate clinical studies on treatment of skin injury. As a heparan sulfate proteoglycan of ECM, Agrin has been proved to be involved in various in vivo physiological processes including synaptogenesis and interactions between cells, which is important for tissue formation step of tissue damage repair [7]. A recent study has shown that agrin regulates focal adhesion integrity in patients with hepatocellular carcinoma to promote the progression of tumor [8]. In other studies, Agrin was found to activate Yes- Associated Protein (YAP) to regulate a variety of cellular processes including cell proliferation, differentiation and migration, which can seriously affect repair of skin tissue damage [3,9]. In addition, the functions of Agrin in those processes were proved to be related with its interactions with integrin mediated adhesion and low-density lipoprotein receptor-related protein-4 (Lrp4)/Muscle Associated Receptor Tyrosine Kinase (MuSK) receptor-mediated signaling pathways [9]. In view of the functionality of Agrin and the mechanism of skin wound healing, it will be reasonable to hypothesize that Agrin may also play important roles in skin damage repair through the interactions with the same signal transduction pathways. Therefore, understanding the role of Agrin in skin tissue damage repair may eventually improve its treatment.
In this study, expression of Agrin in Human Skin Fibroblast (HSF) was downregulated by siRNA transfection. Effects of agrin silencing on HSF proliferation, migration and invasion were investigated. In addition, effects of Agrin silencing on the expression and phosphorylation of YAP, Focal Adhesion Kinase (FAK) and MuSK were also explored.
Materials and Methods
Cell culture
HSF were purchased from American Type Culture Collection (ATCC, USA). Cells were cultured in 1640 medium (Hyclone, Logan, UT, USA) containing 10% FBS (Invitrogen, Carlsbad, CA, USA), 100 U/ml streptomycin and 100 mg/ml penicillin G (Invitrogen, Carlsbad, CA). Cell culture conditions were 37°C and 5% CO2.
siRNAs transfection
Agrin siRNA (L-031716-00-0050) was purchased from Dharmacon (Thermo Fisher Scientific). According to the instructions, 50 nmol siRNA was transfected into HSF cells using Lipofectamine 2000 transfection reagent (11668-019) (Invitrogen, Carlsbad, USA). Cells transfected with siRNA were harvested 48 h later for subsequent experiments.
Cell migration and invasion assay
To determine cell migration ability, 5 × 104 cells were inoculated into the upper chamber of Transwell chamber (BD Biosciences, San Jose, USA), and RPMI-1640 medium containing 20% FCS was used added to the lower chamber to serve as chemical attractant. After incubation for 24 h, the membrane was stained with 0.5% crystal violet for 20 min. After that, cells on membrane were counted under a light microscope (400X, Olympus, Tokyo, Japan). Cell invasion assay was performed in the same way of migration assay except that upper chamber was pre-coated with Matrigel (356234, Millipore, Billerica, USA).
Western blot
Cells were lysed with lysis buffer (P0013K, Beyotime), and total protein was extracted. Concentration of total protein was determined by BCA method. After that, 30 μg protein from each sample was subjected to 10% SDS-PAGE electrophoresis, followed by transmembrane to PVDF membrane (Bio-Rad, Hercules, USA). After blocking with 5% skim milk, membranes were incubated with primary antibodies of Agrin (1: 1000, sc-25528, Cruz Biotechnology Inc.), YAP (1: 1000, 14074, Cell Signaling Technology), phosphorylated YAP (Ser127) (1: 1000, 13008, Signaling Technology) and endogenous control β-actin (1: 1000, 4970, Cell Signaling Technology) overnight at 4°C. After washing, membranes were incubated with secondary antibody (1: 10000, 7074, Cell Signaling Technology) at 37°C for 1 h. Signal was detected by enhanced chemiluminescence method (Pierce SuperSignal, Thermo Scientific) and grayscale values were measured using Bio-Imaging System (Millipore, Billerica, USA). This experiment was repeated three times.
Statistical analysis
SPSS19.0 software was used to analyse the data. Comparisons between groups were performed by t test. p<0.05 was considered to be statistically significant.
Results
Effects of Agrin silencing on cell proliferation, migration and invasion
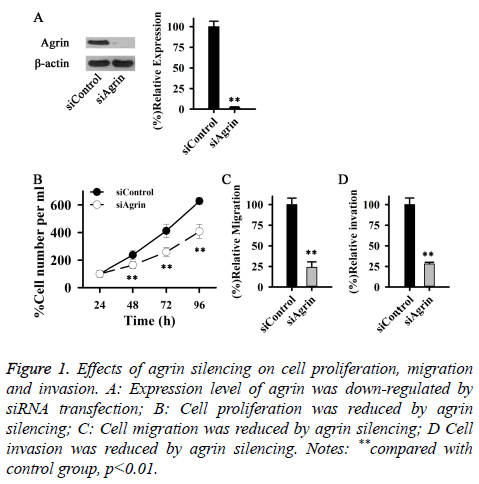
Agrin plays important roles in cell proliferation, differentiation, migration and invasion. In this study, Agrin expression was downregulated by siRNA transfection to study the effects of Agrin on cell proliferation, migration and invasion. Compared with control group, expression level of Agrin was significantly reduced after siRNA transfection, (p<0.01) (Figure 1A). Compared with control group, proliferation rate of cells transfected with siRNA was significantly reduced (Figure 1B). Besides cell proliferation, Agrin can also affect cell migration and invasion. In our study, migration and invasion ability of cells transfected with siRNA was significantly reduced (Figures 1C and 1D) compared with cells without siRNA transfection. Those data suggest that Agrin expression is essential for proliferation, migration and invasion of HSF.
Figure 1: Effects of agrin silencing on cell proliferation, migration and invasion. A: Expression level of agrin was down-regulated by siRNA transfection; B: Cell proliferation was reduced by agrin silencing; C: Cell migration was reduced by agrin silencing; D Cell invasion was reduced by agrin silencing. Notes: **compared with control group, p<0.01.
Effects of agrin silencing on YAP expression and phosphorylation
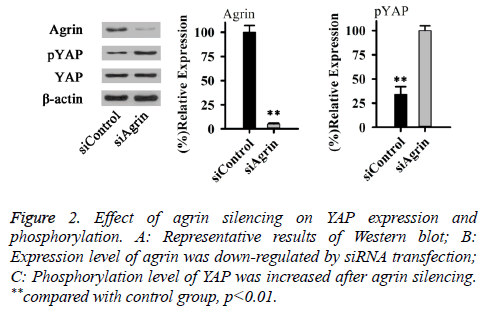
As an important intracellular effector, YAP can significantly affect cell structure and ECM rigidity. Therefore, effect of Agrin silencing on YAP expression and phosphorylation was explored in this study. As shown in Figure 2, no significant difference in total expression level of YAP was found between cells with or without Agrin silencing. However, phosphorylation level of YAP (Ser127) was significantly increased after Agrin silencing (p<0.01). Those results suggested that Agrin silencing can activate YAP.
Effects of agrin silencing on FAK expression and phosphorylation
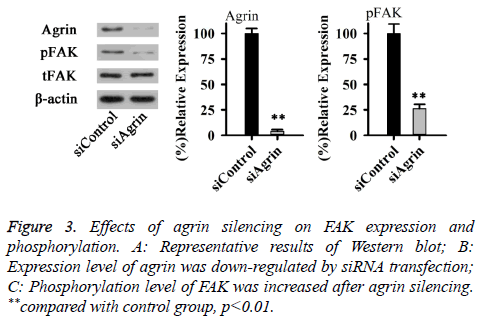
Effects of agrin on YAP depend on integrin mediated adhesion, where Focal Adhesion Kinase (FAK) plays an important role. Therefore, effects of Agrin silencing on FAK expression and phosphorylation were explored in this study. As shown in Figure 3, no significant difference in total expression level of FAK was found between cells with or without Agrin silencing. However, phosphorylation level of FAK was significantly decreased after Agrin silencing (p<0.01). Those results suggested that Agrin silencing can inactivate FAK.
Effects of agrin silencing on MuSK expression and phosphorylation
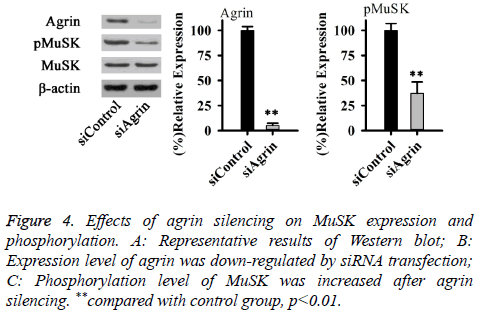
Effects of agrin on YAP may also depend on Lrp4/MuSK receptor-mediated signaling pathways. Therefore, effects of agrin silencing on MuSK expression and phosphorylation were explored in this study. As shown in Figure 4, no significant difference in total expression level of MuSK was found between cells with or without Agrin silencing. However, phosphorylation level of MuSK was significantly decreased after agrin silencing (p<0.01). Those results suggested that agrin silencing can inactivate MuSK.
Discussion
As a large proteoglycan, the primary role of agrin is involved in embryogenesis to regulate the development of the neuromuscular junction [10,11]. Besides that, functionality of agrin has been extensively investigated in a variety of human tumors including hepatocellular carcinoma [9,12], breast cancer [9], and oral squamous cell carcinoma [13] and so on. It has been reported that expression of Agrin significantly increases cell proliferation, differentiation and migration of both hepatocellular carcinoma cell line Hep3B 2.1 and breast cancer cell line MCF7 [9]. In the study of oral squamous cell carcinoma, Kawahara et al. reported that downregulation of Agrin expression could significantly reduce migration and adhesion ability of tumor cells, in addition, reduced expression level of Agrin protein also leaded to the increased chemosensitivity of tumor cells to cisplatin [13], which in turn promotes treatment efficacy of oral squamous cell carcinoma. Beside tumor cells, Agrin also plays a critical and nonredundant role in survival, development and functions of myeloid cells [14]. Consistent with previous studies, in this study, Agrin siRNA silencing was found to significantly reduce the proliferation, migration, and invasion abilities of HSF, indicating that Agrin may plays pivotal roles in cell proliferation and tissue formation after skin damage.
Yes-associated protein, or Yap, is a key player in hippo pathway-regulated cell differentiation and proliferation during the growth and development of adult animals including human [15]. In the study of skin development, activation of YAP was found to promote proliferation and inhibit differentiation and apoptosis of primary mouse keratinocytes, while inactivation of YAP reversed those processes [16], in addition, YAP can interact with the TEA domain of its target cysteine-rich angiogenic inducer 61 (CYR61) to balance the growth and differentiation of skin cells [16]. In kidney podocytes, YAP can interact with PPXY motifs of dendrin through its WW domains to reduce staurosporine-induced, dendrin-dependent apoptosis, in addition, downregulation of YAP in kidney podocytes can significantly increase podocyte apoptosis induced by adriamycin [17]. A recent study showed that, YAP, as a major player in hippo signaling pathway, could regulate tissue regeneration, organ size, and self-renewal of stem cells, indicating the essential roles of YAP in cutaneous wound healing [18]. All those previous studies indicate an opposite role of YAP to that of Agrin. In the study of liver cancer, Chakraborty et al. reported that Agrin played a role as a mechanotransduction signal to regulate the activation of YAP through hippo signaling pathway [8]. In our study, downregulation of Agrin was found to activate YAP, indicating the adverse effects of agrin on YAP activation in fibroblast.
A recent study has shown that effect of agrin on the activation of YAP is closely related to integrin-mediated focal adhesion [8]. As kind of cytoplasmic tyrosine kinase, FAK plays pivotal roles in transduction of various integrin-mediated signals [19]. FAK is usually localized in focal adhesions to recruit talin to nascent adhesions [20]. It has been reported that agrin confines hippo signaling to focal adhesions through its interactions with FAK-ILK-PAK1 pathway [21], and the axis formed by agrin and FAK drives EMT in hepatocellular carcinoma [22]. In our study, phosphorylation level of FAK was significantly reduced in cells with agrin silencing, indicating the important role of YAP in activating FAK protein in fibroblasts. Effects of agrin on the regulation of YAP activation can also be achieved through the interactions between agrin and Lrp4/MuSK pathway [21]. In motor neurons, agrin can promote the formation of neuromuscular junction by stimulating LRP4- MuSK receptor in muscles [23]. Previous studies also found that agrin in ECM could also interact with LRP4/MuSK pathway to maintain the activation of YAP, so as to promote the progression of hepatocellular carcinoma [21]. Consistent with previous studies, in our study, downregulation of agrin was found to significantly reduce the phosphorylation of MuSK protein, indicating the pivotal roles of agrin in the activation of MuSK.
In summary, agrin is essential for the proliferation, migration and invasion ability of HSF cells. Expression level of agrin is negatively correlated with the phosphorylation level of YAP but positively correlated with the phosphorylation level of FAK and MuSK. Therefore, we conclude that agrin can activate YAP in HSF possibly through the interactions with integrin mediated adhesion and Lrp4/MuSK receptor-mediated signaling pathways.
References
- Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med 1999; 341: 738-746.
- Scott AN (Reviewer). The molecular and cellular biology of wound repair. Springer Sci Bus Media 2013.
- Guo S, Dipietro LA. Factors affecting wound healing. J Dent Res 2010; 89: 219-229.
- Darby IA, Laverdet B, Bonte F. Fibroblasts and myofibroblasts in wound healing. Clinic Cosm Investig Dermatol 2014; 7: 301.
- Schultz GS, Wysocki A. Interactions between extracellular matrix and growth factors in wound healing. Wound Rep Regene 2009; 17: 153-162.
- Martino MM, Briquez PS, Guc E. Growth factors engineered for super-affinity to the extracellular matrix enhance tissue healing. Science 2014; 343: 885-888.
- Cole GJ, Halfter W. Agrin: an extracellular matrix heparan sulfate proteoglycan involved in cell interactions and synaptogenesis. Persp Develop Neurobiol 1995; 3: 359-371.
- Chakraborty S, Lakshmanan M, Swa HLF. An oncogenic role of agrin in regulating focal adhesion integrity in hepatocellular carcinoma. Nat Commun 2015; 6.
- Chakraborty S, Njah K, Pobbati AV, Lim YB, Raju A. Agrin as a mechanotransduction signal regulating YAP through the hippo pathway. Cell Rep 2017; 18: 2464-2479.
- Butikofer L, Zurlinden A, Bolliger MF. Destabilization of the neuromuscular junction by proteolytic cleavage of agrin results in precocious sarcopenia. FASEB J 2011; 25: 4378-4393.
- Schmidt N, Basu S, Sladecek S. Agrin regulates CLASP2-mediated capture of microtubules at the neuromuscular junction synaptic membrane. J Cell Biol 2012; 201111130.
- Moeini A, Cornellà H, Villanueva A. Emerging signaling pathways in hepatocellular carcinoma. Liver Cancer 2012; 1: 83-93.
- Kawahara R, Granato DC, Carnielli CM, Cervigne NK, Oliveria CE. Agrin and perlecan mediate tumorigenic processes in oral squamous cell carcinoma. PLoS One 2014; 9: 115004.
- Mazzon C, Anselmo A, Soldani C. Agrin is required for survival and function of monocytic cells. Blood 2012; 119: 5502-5511.
- Zhao K, Shen C, Lu Y. Muscle Yap is a regulator of neuromuscular junction formation and regeneration. J Neurosci 2017; 37: 3465-3477.
- Zhang H, Pasolli HA, Fuchs E. Yes-associated protein (YAP) transcriptional coactivator functions in balancing growth and differentiation in skin. Proc Nat Acad Sci 2011; 108: 2270-2275.
- Campbell KN, Wong JS, Gupta R. Yes-associated protein (YAP) promotes cell survival by inhibiting proapoptotic dendrin signaling. J Biol Chem 2013; 288: 17057-17062.
- Lee MJ, Byun MR, Furutani-Seiki M. YAP and TAZ regulate skin wound healing. Journal of Investigative Dermatology 2014; 134: 518-525.
- Zhao X, Guan JL. Focal adhesion kinase and its signaling pathways in cell migration and angiogenesis. Adv Drug Deliv Rev 2011; 63: 610-615.
- Calderwood DA, Campbell ID, Critchley DR. Talins and kindlins: partners in integrin-mediated adhesion. Nat Rev Mol Cell Biol 2013; 14: 503-517.
- Xiong WC, Mei L. Agrin to YAP in cancer and neuromuscular junctions. Trends Cancer 2017; 3: 247-248.
- Frame MC, Patel H, Serrels B. The FERM domain: organizing the structure and function of FAK. Nature reviews. Mol Cell Biol 2010; 11: 802.
- Wu H, Xiong WC, Mei L. To build a synapse: signaling pathways in neuromuscular junction assembly. Development 2010; 137: 1017-1033.