Research Article - Biomedical Research (2017) Volume 28, Issue 3
A vascularized tissue-engineered trachea with ectopic pedicled muscle flap
Zhengcheng Liu and Xiaoying Zhang*Department of Thoracic and Cardiovascular Surgery, the First People’s Hospital of Changzhou, the Third affiliated Hospital of Soochow University, China
- *Corresponding Author:
- Xiaoying Zhang
Department of Thoracic and Cardiovascular Surgery
The First People’s Hospital of Changzhou, China
Accepted on July 28, 2016
Abstract
Objectives: Surgical treatment of segment tracheal reconstruction longer than 6 cm needs trachea substitutes, tissue engineering can provide alternatives for tracheal reconstruction. Constructs with blood supply are preferred for tracheal replacement. Here we describe an approach for engineering of vascularized trachea with ectopic pedicled muscle flap.
Methods: Poly (L-lactic-co-glycolic acid) scaffolds were seeded with chondrocytes, cell-scaffold constructs were cultured in vitro for 2 weeks. In experiment group, cleidomastoid muscle was dissected with pedicle to wrap the cell-scaffold constructs. In control group, the constructs were simply implanted subcutaneously in the dorsum. After 4 weeks of in vivo culture, neotrachea were used for reconstruction of segmental tracheal defects.
Results: A well-vascularized layer connecting with muscle flap was found in experiment group after in vivo culture, while in control group only connective tissue with fewer micro vascular could be found. After tracheal reconstruction, 3 animals in experiment group survived over six months, all the animals in control group died within one month. The mainly direct reason that caused animal death in control group was mucous impaction. Histological examinations revealed that poor epithelialization were observed in control group, the inner lumen surfaces were rough. Grafts in survival animals of experiment group showed a smooth inner lumen surface, covered by epithelium layer.
Conclusion: Tissue-engineered trachea with pedicled muscle flap was transferred to the defect as a tracheal replacement and yielded satisfactory results, indicating that this approach may enable longterm functional reconstruction of tracheal defect.
Keywords
Tissue engineer, Trachea, Vascularization, Ectopia, Muscle flap.
Introduction
Surgical treatment of segment tracheal reconstruction longer than 6 cm needs trachea substitutesit remains a great challenge due to lack of suitable autologous tissue donors [1], limited source and immuno-rejection of allogenous trachea [2], as well as inferior biological function of synthetic tracheal grafts [3]. Tissue-Engineered Trachea (TET) using autologous cells would be an ideal substitute, it has proper biological structure and function similar to native trachea and autologous availability [4,5], providing a promising strategy for reconstruction of long segmental tracheal defect.
Cartilage softening, overgrowth of granulation tissue at anastomosis site, lack of epithelium were major challenge of segmental tracheal defect reconstruction [6], leading to airway stenosis, airway collapse and mucous impaction. Chondrocytes has been employed as the cellular components of engineered tracheal cartilage. Chondrocytes produce cartilage similar to that found in the tracheal rings and can provide suitable mechanical strength. The neo-tracheal lining may rely on epithelial migration from adjacent portions of the host trachea into the lumen of the engineered construct or culture of respiratory epithelium in vitro with subsequent grafting into the lumen. Both methods require a stable vascular bed for epithelialization. Constructs from in vivo ectopic culture could produce a vascularized lamina to support the survival of chondrocytes and epithelial cells. Good vascularization may promote epithelial migration from native trachea to TET inner surface, it also has the ability to resist infection and diminish tissue necrosis and lumen stenosis [7]. Constructs with blood supply is preferred for tracheal replacement. Ectopic pedicled muscle flap could provide ideal blood supply during in vivo culture and after reconstruction. Thus, we developed a new strategy of tissue engineered trachea with pedicled muscle flap. After pre-cultured in vitro for 2 weeks, the cell-scaffold constructs were wrapped by pedicled muscular flap close to trachea as in vivo culture for 4 weeks to promote cartilage maturation and pre-vascularization, then replaced a 1 cm long segmental tracheal defect in rabbits. The feasibility and long-term efficacy of this strategy was evaluated.
Materials and Methods
Twenty three months old New Zealand rabbits having average body weight 2.00 kg were divided into two groups in each group. Group-1: (Experimental Group, n=10, repaired by vascularized tissue-engineered trachea with pedicled muscle flap) and Group-2: (Control Group, n=10, repaired by tissueengineered trachea without pedicled muscle flap). All animals had received human care in compliance with the “Principles of Laboratory Animal Care” formulated by the National Society for Medical Research and the “Guide for the Care and Use of Laboratory Animals”.
Unwoven poly (L-lactic-co-glycolic acid) (PLGA, lactide: glycolide=75:25, Mn=80000) fibers were synthesized by DaiGang Biotechnologies (Jinan, China), sodium chloride, chloroform, Phosphate-Buffered Saline (PBS), collagenase II, 0.8 U/mg Dispase, Dulbecco’s Modified Eagle’s Medium (DMEM) and Foetal Bovine Serum (FBS) were purchased from Gibco BRL (Grand Island, NY), anti-Collagen Type II antibody was purchased from Acris Antibodies GmbH (Hiddenhausen, Germany), mouse anti-human cytokeratin monoclonal antibody, anti-CD31 antibody was purchased from Abcam (Cambridge, MA).
Scaffold preparation
80 milligrams of unwoven PLGA fibers were wrapped around a rubber tube (6 mm in outer diameter) to form a cylindrical scaffold. The scaffold was immersed in 75% ethanol solution for 60 min for disinfection, and rinsed with PBS twice.
Chondrocytes harvest and culture
According to previous described procedure, square cartilage slice was obtained from rabbits’ ear cartilage and rinsed with PBS, and the perichondrium was removed. Cartilage was minced into pieces with a size of about 1.0 × 1.0 × 1.0 mm3. Cartilage pieces were pre-treated with dispase for 60 min and washed with PBS, then digested with 0.25% collagenase type II for 600 min at 37˚C to isolate chondrocytes. The digest was filtered through a 70 mm nylon membrane, centrifuged at 1000 rpm for 5 min, and washed twice with PBS. The cell pellets were resuspended in expansion medium (DMEM containing 10% FBS and 1% penicillin) and plated in 10 cm tissue culture dishes. The cells were incubated in a 5% CO2 atmosphere at 37˚C. Each dish was replenished with 10 ml of the medium every 72 h. After one week of primary culture, the cells from a single dish were divided and replated onto five dishes. Chondrocytes in passage 1 were used for the construction of tubular cartilage.
Preparation of cell-scaffold constructs
The harvested chondrocytes were resuspended in expansion medium to a final concentration of 6.0 × 107 cells/ml. Cell-scaffold constructs were formed by seeding 1.0 ml cell suspension on the scaffold evenly. Constructs were flipped every 60 minutes and incubated for 4 h, allowing cells to adhere to scaffold fiber. Then 20 ml expansion medium was added, constructs were cultured in the condition with 5% CO2 at 37˚C for 2 weeks, each dish was replenished with 10 ml of the medium every 72 h.
In vivo implantation
All animal procedures complied with the animal research guidelines of Soochow University. The rabbits were anesthetized with intramuscular injection of ketamine (5 mg/kg) and xylazine (0.05 mg/kg). A 6-mm-diameter, 15-mm-long silicone tube was inserted into every construct as stent. In experiment group, a 4 cm incision in midline of neck was made; subcutaneous tissue and platysma were separated layer by layer. Cleidomastoid muscle of one side was anatomical dissected; great attention should be made that the muscle flap should be partly separated without hurting pedicle. Cell-scaffold constructs were wrapped by muscle flap with 6-0 silk suture. In control group, a 4-cm incision was made in the dorsum. Subcutaneous tissue was separated to form a pocket, the constructs were placed in the pocket, and the constructs were then sutured to the abdominal muscle to minimize movement. Then incision was closed in layers. Animals were allowed to breathe spontaneously and recover from anaesthesia.
Tracheal replacement
The reconstruction of segmental tracheal defects was performed after 4 weeks of in vivo culture. In experimental group, the same incision of neck was made; the construct with muscle flap was carefully dissected without injuring the pedicle. After removing the supporting tube, the ends of the engineered tubular cartilage were cut smoothly, the length was 1.0 cm. The implants of control group were harvested after 4 weeks of in vivo culture.
The trachea was exposed and dissected free; a 1.0 cm long trachea segment was removed. End-to-end anastomosis between the contructs and both the proximal and distal native trachea was performed by interrupted sutures using 3-0 vicryl (Ethicon, Somerville, NJ). A 15 mm long silicone tracheal catheter (Fr 3.5) was placed as a stent. Anastomosis was tested for pneumostasis by submerging it under saline, and then incision was closed layer by layer. In control group, constructs without muscle flap replaced a 1.0 cm long trachea segment identically to the experimental group.
Intramuscular injection of penicillin was given every day to prevent infection for 7 days postoperatively. Airway maintenance was performed daily to prevent sputum incarceration with obstruction. The breath situation of animals including dyspnoea, stridor, and hypoxia was monitored. Once the animals died, the survival period was recorded; the death causes were analysed according to the findings from autopsy. The animals survived more than 6 months were euthanized, the specimen was harvested for observation.
Morphology observation
Inverted phase contrast microscope was used to observe the growth of chondrocytes and Extracellular Matrix (ECM) secretion, Scanning Electron Microscopy (SEM) was used to observe cell adhesion to PLGA fibers in vitro culture. SEM was also used to observe maturation status of the ingrowth epithelium in the lumen of TET.
Gross morphology, histological and immunohistochemical analysis
After 4 weeks of in vivo implantation, the constructs were dissected carefully. The integrity, elasticity, and vascularization of engineered tubular cartilage were grossly evaluated. All the reconstructed specimens were harvested, the anastomosis healing, cartilage tissue, growth of epithelium at the inner surface of TET lumen was observed.
Samples were fixed in 4% paraformaldehyde, embedded in paraffin and sectioned; they were stained with hematoxylin and eosin and safranin-O to evaluate the structure and cartilage ECM deposition. The Sulfated Glycosaminoglycan (GAG) content of TET cartilage was quantified by Alcian Blue method. Expression of type II collagen was detected by immunohistochemical staining. The epithelium of the constructs was evaluated immunohistochemically with cytokeratin. The vasculature was evaluated immunohistochemically with the endothelial specific marker CD31.
Statistical analysis
The GAG content was recorded as mean ± standard deviation and evaluated by analysis of variance using Student’s t-test. A p-value less than 0.05 were considered as statistically significant.
Results
in vitro culture of TET
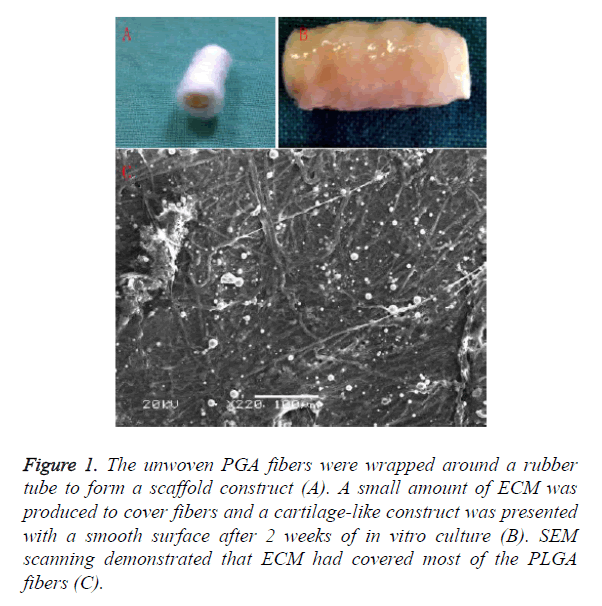
The unwoven PGA fibers were wrapped around a rubber tube to form a scaffold construct Figure 1A. After 2 weeks of in vitro culture, a small amount of ECM was produced to cover fibers and a cartilage-like construct was presented with a smooth surface as shown in Figure 1B. Histological examination showed cartilage formation with a certain amount of ECM deposition. SEM scanning demonstrated that ECM had covered most of the PLGA fibers as shown in Figure 1C.
Figure 1. The unwoven PGA fibers were wrapped around a rubber tube to form a scaffold construct (A). A small amount of ECM was produced to cover fibers and a cartilage-like construct was presented with a smooth surface after 2 weeks of in vitro culture (B). SEM scanning demonstrated that ECM had covered most of the PLGA fibers (C).
In vivo culture of TET
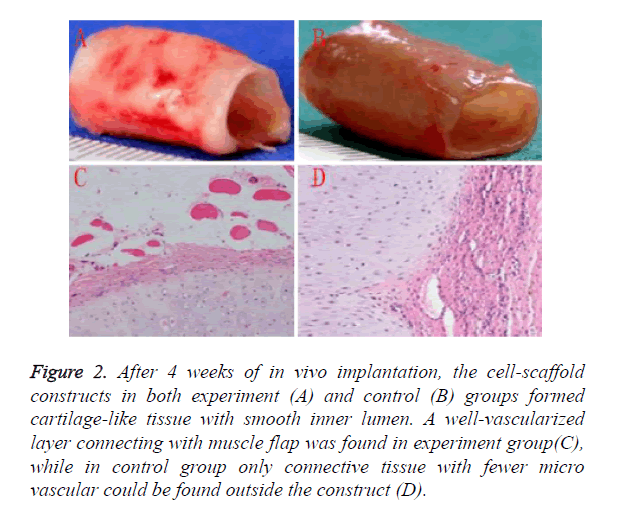
Regeneration and vascularization of TET graft was mainly achieved by in vivo culture. After 4 weeks of in vivo implantation, the cell-scaffold constructs in both experiment as shown in Figure 2 and control as shown in Figure 2B groups formed cartilage-like tissue with smooth inner lumen. GAG contents in experiment groups was higher than control group, however it did not reach a statistical significance as shown in Table 1. Histological examinations further revealed that some typical cartilage features with specific lacuna structures and cartilage ECM deposition could be found in experiment group, which were fewer in control group.
Figure 2. After 4 weeks of in vivo implantation, the cell-scaffold constructs in both experiment (A) and control (B) groups formed cartilage-like tissue with smooth inner lumen. A well-vascularized layer connecting with muscle flap was found in experiment group(C), while in control group only connective tissue with fewer micro vascular could be found outside the construct (D).
A well-vascularized layer connecting with muscle flap was found in experiment group as shown in Figure 2C, while in control group only connective tissue with fewer micro vascular could be found outside the construct as shown in Figure 2D. These results indicated that muscle-flap wrapping might be a more suitable way for cartilage regeneration and vascularization than subcutaneous implantation.
Reconstruction and postoperative evaluation of segmental tracheal defects
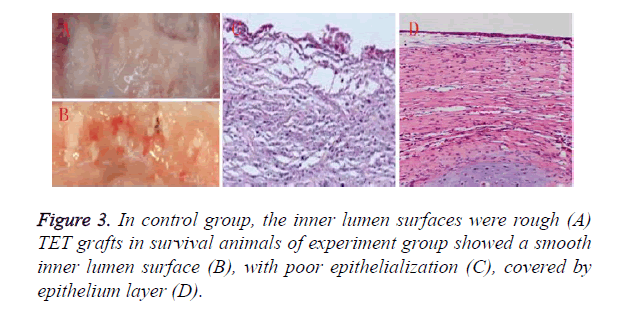
3 animals in experiment group survived over six months, all the animals in control group died within one month’s postoperatively. The main cause for animal death of control group was mucous impaction according to autopsy as shown in Figure 3A. Histological examinations revealed that poor epithelialization was observed in control group, the inner lumen surfaces was rough as shown in Figure 3C. 5 animals of experiment group died within one month, another 2 animals died within two month, mucous impaction was also the main reason for death. TET grafts in survival animals of experiment group showed a smooth inner surface as shown in Figure 3B covered by epithelium layer as shown in Figure 3D. Note worthily, airway collapse and granulation at anastomosis sites could be found in dead animals of both group, and were much more serious in control group.
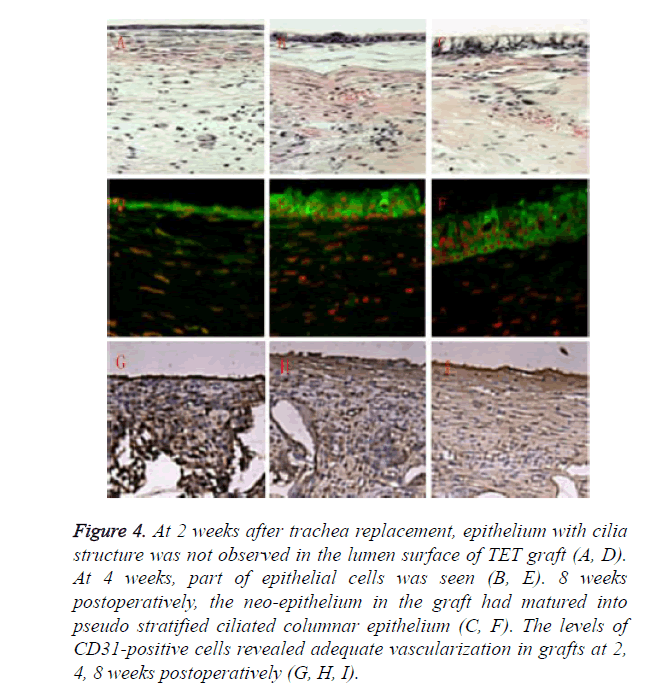
Relatively rare mucous impaction happened in experiment group. Inner surface of TET grafts in survival animals was smooth and covered by a continuous ciliated columnar epithelium layer. However, the epithelialization was a gradual process. At 2 weeks after trachea replacement, epithelium with cilia structure was not observed in the lumen surface of TET graft as shown in Figures 4A and 4D. At 4 weeks, part of epithelial cells was seen as shown in Figures 4B and 4E. 8 weeks postoperatively, the neo-epithelium in the graft had matured into pseudo stratified ciliated columnar epithelium as shown in Figures 4C and 4F. The features of tubular cartilage were different between groups. Cartilage in experiment group retained cartilage-like tissue during the observation period of six months, while samples in control group showed various degrees of cartilage structures and features lost during the survival period less than one month. The levels of CD31- positive cells revealed adequate vascularization in grafts at 2, 4, 8 weeks postoperatively, guaranteeing long-term survival of the animals as shown in Figures 4G-4I.
Figure 4. At 2 weeks after trachea replacement, epithelium with cilia structure was not observed in the lumen surface of TET graft (A, D). At 4 weeks, part of epithelial cells was seen (B, E). 8 weeks postoperatively, the neo-epithelium in the graft had matured into pseudo stratified ciliated columnar epithelium (C, F). The levels of CD31-positive cells revealed adequate vascularization in grafts at 2, 4, 8 weeks postoperatively (G, H, I).
Discussion
Tissue engineering provides a promising approach for the treatment of tracheal defects; however, no breakthrough has been achieved yet. Vacanti tried to repair tracheal defects in rats using tissue engineering approach, it was the first attempt [8], following by several research groups with extensively exploring the methods and strategies for tracheal reconstruction, but only achieved little. Macchiarini successfully repaired partial tracheal or bronchial defects swith tissue engineering approach using detergent-enzymatic tracheal tubular matrix from donor trachea, which might not be widely used due to lack of donors [9]. Up to date, the treatment of long segmental tracheal defect remains a great challenge in tracheal surgery. Ideal tracheal substitutes should have good biocompatibility, appropriate mechanical strength, and structure similar to normal trachea with fine vascularization and epithelium, guaranteeing stable ventilation and secretions discharge function [10].
By improvements in cartilage regeneration and vascularization with fine surgical techniques [11], the current study successfully repaired segmental tracheal defect in some rabbits, the structure and function of TET grafts were similar to native trachea, providing a promising approach for the treatment of long segmental tracheal defect. Tubular cartilage with certain mechanical strength was important for TET [12]. The leading cause affecting cartilage regeneration was the aseptic inflammation caused by degraded material of polymer scaffolds [13], which could be alleviated by in vitro pre-culture. ECM covered the polymer fibers and avoided direct contact between fibers and host tissue when in vivo implantation. After 2 weeks of in vitro culture, a small amount of ECM was produced to cover fibers; SEM scanning demonstrated that ECM had covered most of the PLGA fibers, indicating that 2 weeks of in vitro culture was long enough to produce sufficient ECM.
Constructs engineered by in vitro culture were not suitable to repair tracheal defect due to insufficient vascularization and nutrition supply [14]. This study proposed a two-step strategy combining in vitro and in vivo culture, in vitro pre-culture helped to alleviate inflammation, in vivo implantation with pedicled muscle flap wrapping promoted the generation of cartilage and vascularization. Typical cartilage features with specific lacuna structures and more cartilage ECM deposition could be observed in experiment group, GAG content was higher. More important, vascularization was well-developed, which may promote cartilage ECM production, enhance the mechanical strength, and accelerate the degradation of scaffolds and epithelialization.
Survival period was the most important issue to evaluate efficacy of segmental tracheal reconstruction, 3 animals in experiment group survived more than 6 months, indicating well vascularization might significantly enhance the survival time and rate of segmental tracheal reconstruction. There were still 7 animals in experiment group died in two month, mucous impaction, airway collapse and anastomosis stenosis caused by granulation were main reasons. Epithelialization was key to guarantee the stable ventilation function and long-term survival [15]. However, no breakthrough has been achieved in this field yet, even in some clinical successful cases, epithelialization was formed by recruitment of native epithelial cells to move onto graft’s inner surface [16]. In this study, inner surface of grafts in animals died within two month of both groups were all rough without complete epithelialization, which might be the leading reason for mucous impaction. The inner surface of graft in animals survived more than 6 months was smooth, indicating that two month might be long enough for development of epithelialization with sufficient blood supply; trachea stent should be applied during this period. Postoperative nurse was also quite important; especially before pseudo stratified ciliated columnar epithelium was formed. All animals in control group were died within one month, indicating that vascularization could promote epithelialization of TET graft.
Granulation was one of the reasons for animal death at the early stage after tracheal reconstruction [17], it might be mainly caused by the degradation materials of TET scaffold [18]. In current study, inflammatory reaction caused by the scaffolds had been basically avoided since the scaffolds had degraded during in vivo culture as mentioned above. A trachea stent might help effectively depress granulation overgrowth, prevent stenosis and maintain the patency of airway; it should be further examined [19]. Vascularization was a key factor for epithelium migration and regeneration [20]. As shown in this study, the TET graft could obtain enough blood supply by pre-vascularization and maintain vascularization after tracheal reconstruction with pedicled muscle flap, graft could easily integrate with adjacent native trachea, covered by a continuous ciliated columnar epithelium layer. Epithelial cells were rarely seen in control group without pedicled muscle flap wrapping, indicating that the pre-vascularization could promote epithelialization.
Conclusion
It is a promising approach for long-term functional reconstruction of tracheal defect with tissue-engineered trachea with pedicled muscle flap.
Acknowledgments
The authors appreciate the technical supports and other helps from colleges in the center laboratory of the third affiliated hospital of Soochow University and animals’ laboratory of Nanjing Medical University.
References
- Macchiarini P, Jungebluth P, Go T, Asnaghi MA, Rees LE, Cogan TA. Clinical transplantation of a tissue-engineered airway. Lancet 2008; 372: 2023-2030.
- Jungebluth P, Haag JC, Sjqvist S, Gustafsson Y, Beltran Rodriguez A, Del Gaudio C. Tracheal tissue engineering in rats. Nat Protoc 2014; 9: 2164-2179.
- Shin YS, Choi JW, Park JK, Kim YS, Yang SS, Min BH. Tissue-engineered tracheal reconstruction using mesenchymal stem cells seeded on a porcine cartilage powder scaffold. Ann Biomed Eng 2015; 43: 1003-1013.
- Burghartz M, Gehrke T, Storck K, Staudenmaier R, Mandlik V, Schurr C. Vascularization of engineered cartilage constructs in a mouse model. Cell Tissue Res 2015; 359: 479-487.
- Batioglu-Karaaltin A, Karaaltin MV, Ovali E, Yigit O, Kongur M, Inan O. In vivo tissue-engineered allogenic trachea transplantation in rabbits: a preliminary report. Stem Cell Rev 2015; 11: 347-356.
- Steinke M, Dally I, Friedel G, Walles H, Walles T. Host-integration of a tissue-engineered airway patch: two-year follow-up in a single patient. Tissue Eng 2015; 21: 573-579.
- Dikina AD, Strobel HA, Lai BP, Rolle MW. Alsberg E. Engineered cartilaginous tubes for tracheal tissue replacement via self-assembly and fusion of human mesenchymal stem cell constructs. Biomater 2015; 52: 452-462.
- Vacanti CA, Paige KT, Kim WS, Sakata J, Upton J, Vacanti JP. Experimental tracheal replacement using tissue-engineered cartilage. J Pediatr Surg 1994; 29: 201.
- Jungebluth P, Alici E, Baiguera S. Tracheobronchial transplantation with a stem-cell-seeded bioartificial nanocomposite-a proof-of-concept study. Lancet 2011; 378: 1997-2004.
- Steinke M, Dally I, Friedel G, Walles H, Walles T. Host-integration of a tissue-engineered airway patch: two-year follow-up in a single patient. Tissue Eng 2015; 21: 573-579.
- Hoffman B, Martin M, Brown BN, Bonassar LJ, Cheetham J. Biomechanical and biochemical characterization of porcine tracheal cartilage. Laryngoscope 2016.
- Baiguera S, Gonfiotti A, Jaus M. Development of bioengineered human larynx. Biomater 2011; 32: 4433-4442.
- Nakamura T, Ohmori K, Kanemaru S. Tissue-engineered airway and in situ tissue engineering. Gen Thorac Cardiovasc Surg 2011; 59: 91-97.
- Zhang H, Fu W, Xu Z. Re-epithelialization-a key element in tracheal tissue engineering. Regen Med 2015; 10: 1005-1023.
- Butler CR, Hynds RE, Gowers KH, Lee DD, Brown JM, Crowley C. Rapid expansion of human epithelial stem cells suitable for airway tissue engineering. Am J Respir Crit Care Med 2016.
- Elliott MJ, De Coppi P, Speggiorin S. Stem-cell-based, tissue engineered tracheal replacement in a child-a 2-year follow-up study. Lancet 2012; 380: 994-1000.
- Yoshioka T, Kawazoe N, Tateishi T, Chen G. In vitro evaluation of biodeg-radation of poly (lactic-co-glycolic acid) sponges. Biomater 2008; 29: 3438-3443.
- Park JH, Hong JM, Ju YM, Jung JW, Kang HW, Lee SJ. A novel tissue-engineered trachea with a mechanical behavior similar to native trachea. Biomater 2015; 62: 106-115.
- Zhang H, Fu W, Xu Z. Re-epithelialization-a key element in tracheal tissue engineering. Regen Med 2015; 10: 1005-1023.
- Berg M, Ejnell H, Kovacs A. Replacement of a tracheal stenosis with a tissue-engineered human trachea using autologous stem cells-a case report. Tissue Eng 2014; 20: 389-397.